Abstract
Several key papers published in 2015 highlight important emerging trends in endoscopic imaging that promise to improve patient diagnosis and guidance of therapy. These studies reflect the future role for ‘smart’ contrast agents and fluorescence endoscopes to provide a molecular basis for disease detection, identify precancerous lesions and determine optimal choice of therapy.
Molecular changes that occur in gastrointestinal diseases are now being assessed in vivo with new tools for endoscopic imaging (FIG. 1). Emerging technologies show great promise for more precise decisions to be made in many areas of patient care. Conventional whole-body imaging methods — such as CT, MRI and ultrasonography — are well-established for detecting disease by visualizing changes in tissue architecture. Increased knowledge of genomic and proteomic biomarkers have created new opportunities for imaging to be performed at a molecular level, which can be combined with anatomical imaging. We review several key manuscripts published in 2015 that report the introduction of these technologies into the clinic. These methods could dramatically increase the efficiency and effectiveness of endoscopic imaging and improve our understanding of the natural history of disease in the digestive tract.
Figure 1 |. Diseases throughout the digestive tract are being evaluated in vivo with new endoscopic imaging technologies that identify molecular changes.
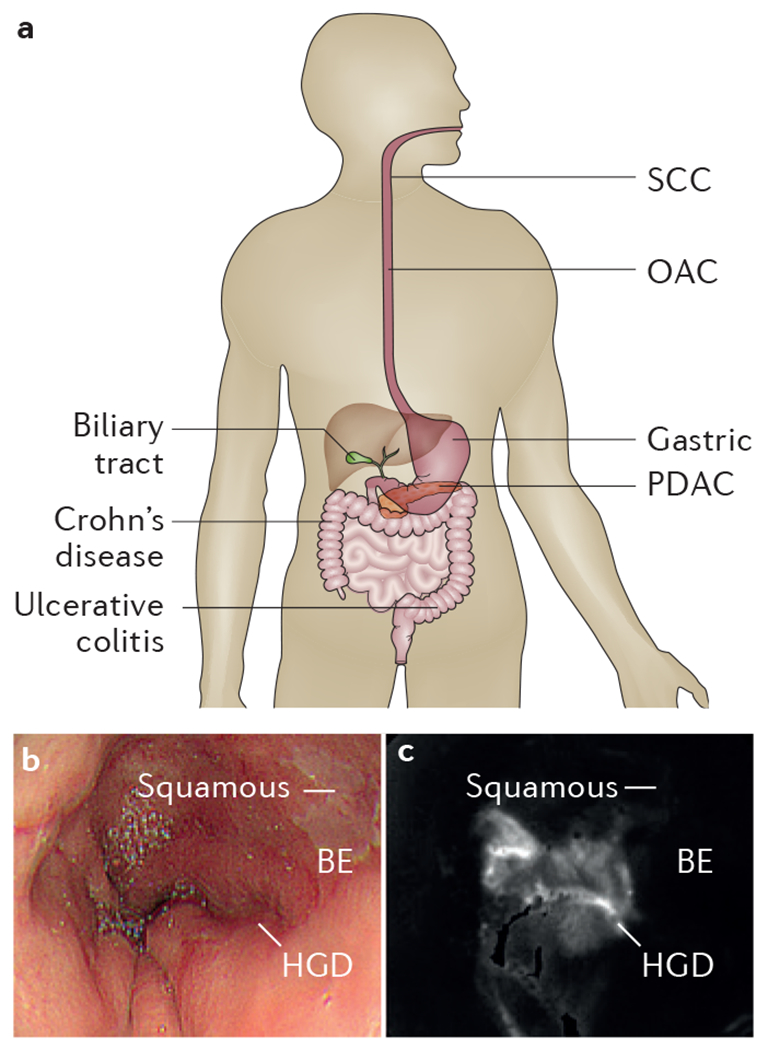
a | Key areas and conditions that are being examined by emerging endoscopic technology. b | White-light endoscopic image shows squamous and BE but no distinguishing features for HGD from human oesophagus in vivo. c | After topical administration of molecular probe, ratio image collected with multimodal endoscope shows region of increased intensity (arrow) from HGD5. BE, Barrett oesophagus; HGD, high-grade dysplasia; OAC, oesophageal adenocarcinoma; PDAC, pancreatic adenocarcinoma; SCC, squamous cell carcinoma.
Burggraaf et al.1 demonstrated clinical use of a peptide that binds to c-Met that was labelled with Cy5 (a near-infrared dye) for early detection of colorectal cancer (CRC), a step forward in endoscopy screening in 2015. A transmembrane tyrosine kinase with proto-oncogene function, c-Met is over-expressed in adenomas, known pre-cursors to CRC2. Safety of this imaging agent was first established in animals; probe biodistribution was studied in rats and monkeys with single and repeated doses. No adverse effects were seen in any of these studies, and these safety results motivated regulatory approval for human use with intravenous administration. A clinical study was performed in 15 patients at high risk of CRC. Back-to-back examinations consisted of white light alone followed by white light with fluorescent peptide. A clear increase in fluorescence intensity was observed from adenomas with either polypoid or flat morphology. Some hyperplastic polyps and normal mucosa also showed an increase in signal, but to a lesser degree. The fluorescence positively correlated with c-Met levels in these polyps. A total of 101 lesions were detected with white light alone, and 22 additional lesions were detected on a second look with peptide-based fluorescence. Of these, 17 were visible with fluorescence only. These additional lesions tended to be small (<6 mm) and have non-polypoid morphology (Paris 0–IIa and 0–IIb)3. A limitation of this study is that a fibreoptic instrument was used that has noticeably worse image resolution than current video endoscopes. This technology could enhance conventional white-light colonoscopy, which has been shown to miss polyps that can result in unexplained interval CRC. In previous studies, >28% of common polyps have gone undetected on tandem examination with white-light colonoscopy4. In the future, this integrated methodology might be clinically useful in high-risk patients, such as those with Lynch syndrome and history of multiple polyps, to increase the yield of adenoma detection and to extend the interval between colonoscopy examinations.
In 2015, we demonstrated clinical use of a video molecular endoscope5 to localize early neoplasia (high-grade dysplasia and early adenocarcinoma) in patients with Barrett oesophagus using a peptide labelled with fluorescein isothiocyanate (FITC)6. Early cancer detection in patients with Barrett oesophagus is currently performed with white-light endoscopy and random biopsies. However, early neoplasia is often difficult to detect because of its flat morphology and patchy distribution. The appearance and handling of this instrument, including the endoscope and video processor, is almost identical to that of a standard endoscope. Registered fluorescence and reflectance images were collected sequentially and then used as a ratio to correct for differences in distance to the mucosa over the field-of-view. This feature enables the fluorescence intensities over the entire image field-of-view to be quantified so that ‘red flag’ regions associated with increased risk of cancer progression can be displayed to guide tissue biopsy. This molecular endoscope was used safely in 50 patients undergoing endoscopic mucosal resection and, on targeted imaging, early neoplasia was detected with 94% specificity and 96% positive predictive value. Included in the analysis were 28 flat lesions (Paris 0–IIb) that were poorly visualized with white light. The peptide was topically administered to the distal oesophagus to minimize the quantity (and hence cost) of the imaging agent needed and to avoid probe biodistribution to non-target tissues for increased safety. Binding occurred within 5 min, which resulted in minimal time added to the procedure. No adverse events associated with either the endoscope or peptide occurred. Limitations of this study included the use of an endoscope with a standard rather than high-definition detector and collection of visible rather than near-infrared fluorescence, resulting in increased background autofluorescence. By avoiding random biopsies, this molecular endoscope can minimize procedure time and reduce risks from sedation, bleeding and perforation, and could be used in the future for image-guided resection, surveillance, risk stratification and monitoring of therapy in patients with Barrett oesophagus.
Finally, in 2015, clinical use of confocal endomicroscopy with intravenous fluorescein to collect fluorescence images from colonic mucosa with subcellular resolution to distinguish between Crohn’s disease and ulcerative colitis was demonstrated7. A total of 79 patients were studied, including 40 with Crohn’s disease and 39 with ulcerative colitis. Unique image features, including architectural distortion, irregular surface, crypt density, discontinuous crypt abnormality, focal cryptitis and discontinuous inflammation, were identified. The imaging results were compared with histology results and clinical history. Based on these criteria, sensitivity, specificity and positive predictive values of 90%, 97.4% and 97.3%, respectively, for Crohn’s disease and 97.4%, 90.0% and 90.5%, respectively, for ulcerative colitis was determined. An overall accuracy in diagnosis of 93.7% was found for both conditions. This distinction could be useful for determining the best choice of therapy for patients with IBD. Limitations of this study included no difference in diffuse lamina propria cells and mucin preservation and no visible granulomas. Confocal endomicroscopy is an emerging imaging technique that can be can be used as an accessory to standard medical endoscopes to collect histology-like images to provide an instantaneous ‘optical biopsy’. Currently, medical decisions are made based on pathology of biopsied tissues that are not processed until after the procedure is over and the patient has left the clinic. Multiple tissue biopsies are often taken at random sites, and key sites of disease might be missed. Confocal imaging can also be used to monitor patient response to therapy, and was used to image a FITC-labelled anti-TNF antibody to assess likelihood of patient response to adalimumab in Crohn’s disease8. A high number of TNF-positive cells predicted sustained clinical response to anti-TNF therapy for over 1 year and was associated with presence of mucosal healing on endoscopy.
The clinical studies published in 2015 and highlighted in this Year-in-Review reflect the enormous potential of novel molecular probes and imaging instruments being developed for targeted endoscopy to have future importance in patient care. In addition to c-Met, probes are being developed for a broad range of molecular targets that are overexpressed in disease. Multiplexed methods that use flexible optical fibres can visualize multiple targets simultaneously to address genetic heterogeneity found in disease9. Wide-field endoscopes are increasing in image resolution, and confocal endomicroscopes are achieving greater tissue imaging depths compared with current instruments being used in the clinic10. These technologies are being integrated for in vivo use, and can be generalized to hollow organs either within or outside of the digestive tract.
Key advances.
Fluorescently labelled probes that are specific for cancer biomarkers can visualize precancerous lesions in vivo that are otherwise difficult to see with conventional white-light endoscopes1
Multimodal endoscopes that are sensitive to fluorescence can quantify image intensities and provide a map of high-risk mucosal regions to guide tissue biopsy5
Real-time fluorescence images with subcellular resolution can perform instantaneous ‘optical biopsy’ to distinguish mucosal features to determine choice of therapy7
Acknowledgements
T.D.W. is funded in part by NIH U54 CA163059, U01 CA189291, R01 CA142750, R01 CA200007, and R01 EB020644 grants.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Burggraaf J et al. Detection of colorectal polyps in humans using an intravenously administered fluorescent peptide targeted against c-Met. Nat. Med 21, 955–961 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Di Renzo MF et al. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin. Cancer Res 1, 147–154 (1995). [PubMed] [Google Scholar]
- 3.Participants in the Paris Workshop. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest. Endosc 58, S3–S43 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Heresbach D. et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy 40, 284–290 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Joshi BP et al. Multimodal endoscope can quantify wide-field fluorescence detection of Barrett’s neoplasia. Endoscopy 48, 1–15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturm MB et al. Targeted imaging of esophageal neoplasia with a fluorescently labeled peptide: first-in-human results. Sci. Transl. Med 5, 184ra61 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tontini GE et al. Confocal laser endomicroscopy for the differential diagnosis of ulcerative colitis and Crohn’s disease: a pilot study. Endoscopy 47, 437–443 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Atreya R. et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat. Med 20, 313–318 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshi BP et al. Multispectral endoscopic imaging of colorectal dysplasia in vivo. Gastroenterology 143, 1435–1437 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu Z. et al. Vertical cross-sectional imaging of colonic dysplasia in vivo with multi-spectral dual axes confocal endomicroscopy. Gastroenterology 146, 615–617 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


