Abstract
In hepatocytes cultured in the presence of oleate (initial concn. 0.75 mM), the secretion of very-low-density lipoprotein (VLDL) triacylglycerol and, to a lesser extent, apoprotein B (apoB) increased with time, whereas there was a large decline in the extracellular concentration of fatty acid. There was thus no synchronous relationship between the extracellular fatty acid concentration and the secretion of VLDL. Rather, the appearance of VLDL in the medium was dependent on the intracellular triacylglycerol concentration. At a given concentration of extracellular fatty acid, cells depleted of triacylglycerol secreted less VLDL triacylglycerol and apoB than did control cells. A similar pattern was observed for triacylglycerol newly synthesized from extracellular [3H]oleate. By contrast, the synthesis and output of ketone bodies were directly dependent on the fatty acid concentration of the medium. These results suggest that, at least for oleic acid, extracellular fatty acids are not utilized directly for VLDL assembly, but first enter a temporary intracellular storage pool of triacylglycerol, which is the immediate precursor of secreted triacylglycerol. The size of this pool then determines the rate of secretion of VLDL triacylglycerol apoB. Ketogenesis, on the other hand, relies mainly on the direct utilization of extracellular fatty acids.
Full text
PDF
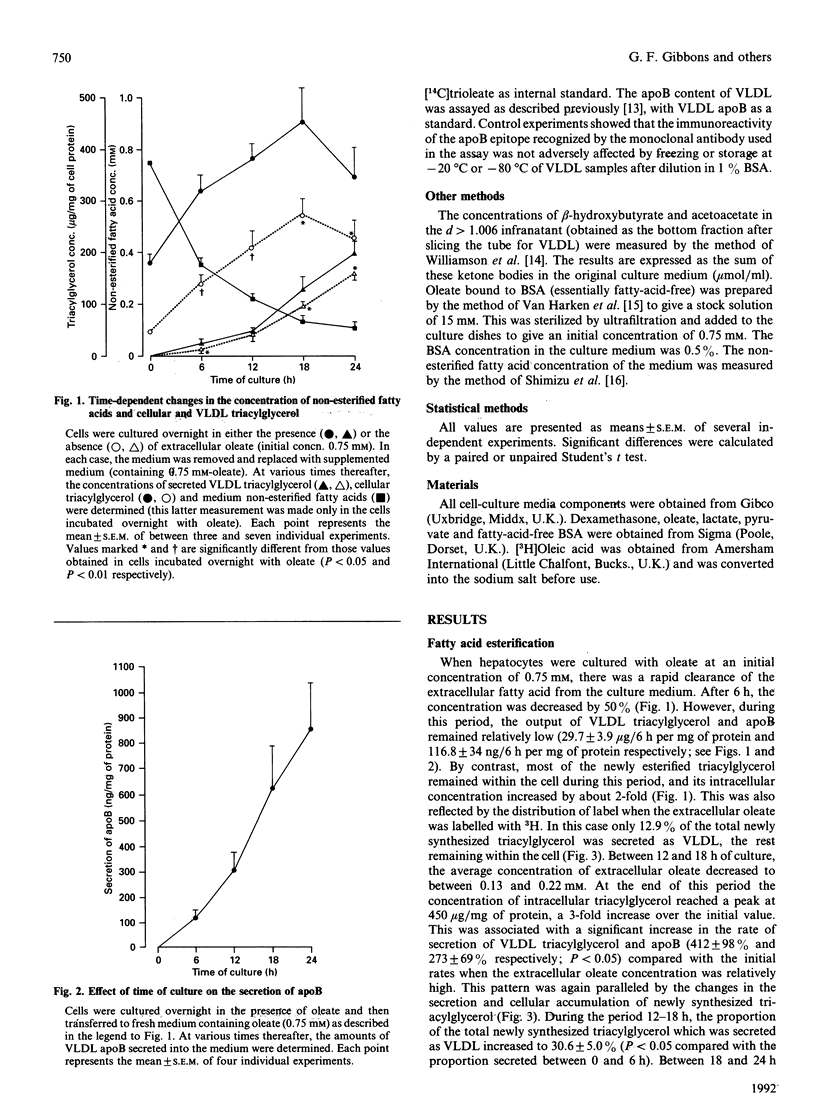
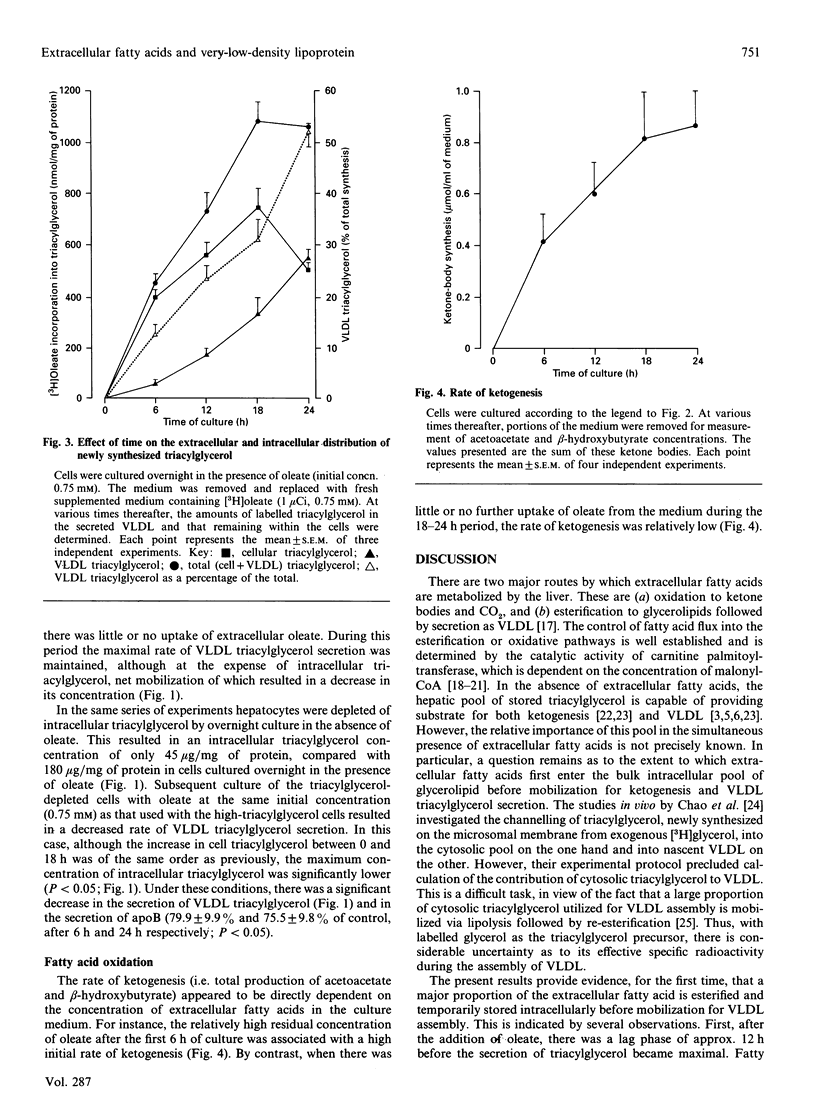


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti K. G., Johnston D. G., Burrin J., Blesa-Malpica G., McCulloch A., Nosadini R., Walker M. Ketogenesis: regulatory factors in vivo. Biochem Soc Trans. 1981 Feb;9(1):8–9. doi: 10.1042/bst0090008. [DOI] [PubMed] [Google Scholar]
- Azain M. J., Fukuda N., Chao F. F., Yamamoto M., Ontko J. A. Contributions of fatty acid and sterol synthesis to triglyceride and cholesterol secretion by the perfused rat liver in genetic hyperlipemia and obesity. J Biol Chem. 1985 Jan 10;260(1):174–181. [PubMed] [Google Scholar]
- Bamberger M. J., Lane M. D. Assembly of very low density lipoprotein in the hepatocyte. Differential transport of apoproteins through the secretory pathway. J Biol Chem. 1988 Aug 25;263(24):11868–11878. [PubMed] [Google Scholar]
- Bates M. W., Krebs H. A., Williamson D. H. Turnover rates of ketone bodies in normal, starved and alloxan-diabetic rats. Biochem J. 1968 Dec;110(4):655–661. doi: 10.1042/bj1100655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird M. I., Saggerson E. D. Binding of malonyl-CoA to isolated mitochondria. Evidence for high- and low-affinity sites in liver and heart and relationship to inhibition of carnitine palmitoyltransferase activity. Biochem J. 1984 Sep 15;222(3):639–647. doi: 10.1042/bj2220639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnsson O. G., Duerden J. M., Bartlett S. M., Sparks J. D., Sparks C. E., Gibbons G. F. The role of pancreatic hormones in the regulation of lipid storage, oxidation and secretion in primary cultures of rat hepatocytes. Short- and long-term effects. Biochem J. 1992 Jan 15;281(Pt 2):381–386. doi: 10.1042/bj2810381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchardt R. A., Davis R. A. Intrahepatic assembly of very low density lipoproteins. Rate of transport out of the endoplasmic reticulum determines rate of secretion. J Biol Chem. 1987 Dec 5;262(34):16394–16402. [PubMed] [Google Scholar]
- Chao F. F., Stiers D. L., Ontko J. A. Hepatocellular triglyceride synthesis and transfer to lipid droplets and nascent very low density lipoproteins. J Lipid Res. 1986 Nov;27(11):1174–1181. [PubMed] [Google Scholar]
- Coleman R., Bell R. M. Evidence that biosynthesis of phosphatidylethanolamine, phosphatidylcholine, and triacylglycerol occurs on the cytoplasmic side of microsomal vesicles. J Cell Biol. 1978 Jan;76(1):245–253. doi: 10.1083/jcb.76.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. A., Boogaerts J. R. Intrahepatic assembly of very low density lipoproteins. Effect of fatty acids on triacylglycerol and apolipoprotein synthesis. J Biol Chem. 1982 Sep 25;257(18):10908–10913. [PubMed] [Google Scholar]
- Debeer L. J., Thomas J., De Schepper P. J., Mannaerts G. P. Lysosomal triacylglycerol lipase and lipolysis in isolated rat hepatocytes. J Biol Chem. 1979 Sep 25;254(18):8841–8846. [PubMed] [Google Scholar]
- Dixon J. L., Furukawa S., Ginsberg H. N. Oleate stimulates secretion of apolipoprotein B-containing lipoproteins from Hep G2 cells by inhibiting early intracellular degradation of apolipoprotein B. J Biol Chem. 1991 Mar 15;266(8):5080–5086. [PubMed] [Google Scholar]
- Duerden J. M., Bartlett S. M., Gibbons G. F. Long-term maintenance of high rates of very-low-density-lipoprotein secretion in hepatocyte cultures. A model for studying the direct effects of insulin and insulin deficiency in vitro. Biochem J. 1989 Nov 1;263(3):937–943. doi: 10.1042/bj2630937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden J. M., Bartlett S. M., Gibbons G. F. Regulation of very-low-density-lipoprotein lipid secretion in hepatocyte cultures derived from diabetic animals. Biochem J. 1989 Aug 15;262(1):313–319. doi: 10.1042/bj2620313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden J. M., Gibbons G. F. Secretion and storage of newly synthesized hepatic triacylglycerol fatty acids in vivo in different nutritional states and in diabetes. Biochem J. 1988 Nov 1;255(3):929–935. doi: 10.1042/bj2550929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden J. M., Gibbons G. F. Storage, mobilization and secretion of cytosolic triacylglycerol in hepatocyte cultures. The role of insulin. Biochem J. 1990 Dec 15;272(3):583–587. doi: 10.1042/bj2720583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovson J., Chatterton J. E., Bell G. T., Schumaker V. N., Reuben M. A., Puppione D. L., Reeve J. R., Jr, Young N. L. Plasma very low density lipoproteins contain a single molecule of apolipoprotein B. J Lipid Res. 1988 Nov;29(11):1461–1473. [PubMed] [Google Scholar]
- Francone O. L., Kalopissis A. D., Griffaton G. Contribution of cytoplasmic storage triacylglycerol to VLDL-triacylglycerol in isolated rat hepatocytes. Biochim Biophys Acta. 1989 Mar 14;1002(1):28–36. doi: 10.1016/0005-2760(89)90060-x. [DOI] [PubMed] [Google Scholar]
- Fukuda N., Azain M. J., Ontko J. A. Altered hepatic metabolism of free fatty acids underlying hypersecretion of very low density lipoproteins in the genetically obese Zucker rats. J Biol Chem. 1982 Dec 10;257(23):14066–14072. [PubMed] [Google Scholar]
- Fungwe T. V., Cagen L., Wilcox H. G., Heimberg M. Regulation of hepatic secretion of very low density lipoprotein by dietary cholesterol. J Lipid Res. 1992 Feb;33(2):179–191. [PubMed] [Google Scholar]
- Gibbons G. F. Assembly and secretion of hepatic very-low-density lipoprotein. Biochem J. 1990 May 15;268(1):1–13. doi: 10.1042/bj2680001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons G. F., Burnham F. J. Effect of nutritional state on the utilization of fatty acids for hepatitic triacylglycerol synthesis and secretion as very-low-density lipoprotein. Biochem J. 1991 Apr 1;275(Pt 1):87–92. doi: 10.1042/bj2750087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb S., Barber T. A., Pariza M. W., Pugh T. D. Lipid synthesis and ultrastructure of adult rat hepatocytes during their first twenty-four hours in culture. Exp Cell Res. 1978 Nov;117(1):39–46. doi: 10.1016/0014-4827(78)90425-1. [DOI] [PubMed] [Google Scholar]
- Hopp J. F., Palmer W. K. Effect of glucose and insulin on triacylglycerol metabolism in isolated normal and diabetic skeletal muscle. Metabolism. 1991 Mar;40(3):223–225. doi: 10.1016/0026-0495(91)90100-b. [DOI] [PubMed] [Google Scholar]
- Kondrup J., Damgaard S. E., Fleron P. Metabolism of palmitate in perfused rat liver. Computer models of subcellular triacylglycerol metabolism. Biochem J. 1979 Oct 15;184(1):73–81. doi: 10.1042/bj1840073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes P. A. Control of hepatic triacylglycerol metabolism. Biochem Soc Trans. 1976;4(4):575–580. doi: 10.1042/bst0040575. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Meier J. M., Foster D. W. The effects of starvation and refeeding on carbohydrate and lipid metabolism in vivo and in the perfused rat liver. The relationship between fatty acid oxidation and esterification in the regulation of ketogenesis. J Biol Chem. 1973 Jan 10;248(1):270–278. [PubMed] [Google Scholar]
- Mooney R. A., Lane M. D. Formation and turnover of triglyceride-rich vesicles in the chick liver cell. Effects of cAMP and carnitine on triglyceride mobilization and conversion to ketones. J Biol Chem. 1981 Nov 25;256(22):11724–11733. [PubMed] [Google Scholar]
- Olofsson S. O., Bjursell G., Boström K., Carlsson P., Elovson J., Protter A. A., Reuben M. A., Bondjers G. Apolipoprotein B: structure, biosynthesis and role in the lipoprotein assembly process. Atherosclerosis. 1987 Nov;68(1-2):1–17. doi: 10.1016/0021-9150(87)90088-8. [DOI] [PubMed] [Google Scholar]
- Palmer J. F., Cooper C., Shipley R. A. Rate of release of hepatic triacylglycerol into serum in the starved rat. Biochem J. 1978 May 15;172(2):219–226. doi: 10.1042/bj1720219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch W., Tamai T., Schonfeld G. Effect of fatty acids on lipid and apoprotein secretion and association in hepatocyte cultures. J Clin Invest. 1983 Jul;72(1):371–378. doi: 10.1172/JCI110977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease R. J., Harrison G. B., Scott J. Cotranslocational insertion of apolipoprotein B into the inner leaflet of the endoplasmic reticulum. Nature. 1991 Oct 3;353(6343):448–450. doi: 10.1038/353448a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustan A. C., Nossen J. O., Christiansen E. N., Drevon C. A. Eicosapentaenoic acid reduces hepatic synthesis and secretion of triacylglycerol by decreasing the activity of acyl-coenzyme A:1,2-diacylglycerol acyltransferase. J Lipid Res. 1988 Nov;29(11):1417–1426. [PubMed] [Google Scholar]
- Saddik M., Lopaschuk G. D. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J Biol Chem. 1991 May 5;266(13):8162–8170. [PubMed] [Google Scholar]
- Salam W. H., Wilcox H. G., Heimberg M. Effects of oleic acid on the biosynthesis of lipoprotein apoproteins and distribution into the very-low-density lipoprotein by the isolated perfused rat liver. Biochem J. 1988 May 1;251(3):809–816. doi: 10.1042/bj2510809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Inoue K., Tani Y., Yamada H. Enzymatic microdetermination of serum free fatty acids. Anal Biochem. 1979 Oct 1;98(2):341–345. doi: 10.1016/0003-2697(79)90151-9. [DOI] [PubMed] [Google Scholar]
- Sparks J. D., Bolognino M., Trax P. A., Sparks C. E. The production and utility of monoclonal antibodies to rat apolipoprotein B lipoproteins. Atherosclerosis. 1986 Sep;61(3):205–211. doi: 10.1016/0021-9150(86)90139-5. [DOI] [PubMed] [Google Scholar]
- Van Harken D. R., Dixon C. W., Heimberg M. Hepatic lipid metabolism in experimental diabetes. V. The effect of concentration of oleate on metabolism of triglycerides and on ketogenesis. J Biol Chem. 1969 May 10;244(9):2278–2285. [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettesten M., Boström K., Bondjers G., Jarfeldt M., Norfeldt P. I., Carrella M., Wiklund O., Borén J., Olofsson S. O. Pulse-chase studies of the synthesis of apolipoprotein B in a human hepatoma cell line, Hep G2. Eur J Biochem. 1985 Jun 18;149(3):461–466. doi: 10.1111/j.1432-1033.1985.tb08947.x. [DOI] [PubMed] [Google Scholar]
- Wiggins D., Gibbons G. F. The lipolysis/esterification cycle of hepatic triacylglycerol. Its role in the secretion of very-low-density lipoprotein and its response to hormones and sulphonylureas. Biochem J. 1992 Jun 1;284(Pt 2):457–462. doi: 10.1042/bj2840457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodside W. F., Heimberg M. Effects of anti-insulin serum, insulin, and glucose on output of triglycerides and on ketogenesis by the perfused rat liver. J Biol Chem. 1976 Jan 10;251(1):13–23. [PubMed] [Google Scholar]
- Zammit V. A., Corstorphine C. G., Kolodziej M. P. Target size analysis by radiation inactivation of carnitine palmitoyltransferase activity and malonyl-CoA binding in outer membranes from rat liver mitochondria. Biochem J. 1989 Oct 1;263(1):89–95. doi: 10.1042/bj2630089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierler K. L. Fatty acids as substrates for heart and skeletal muscle. Circ Res. 1976 Jun;38(6):459–463. doi: 10.1161/01.res.38.6.459. [DOI] [PubMed] [Google Scholar]



