Abstract
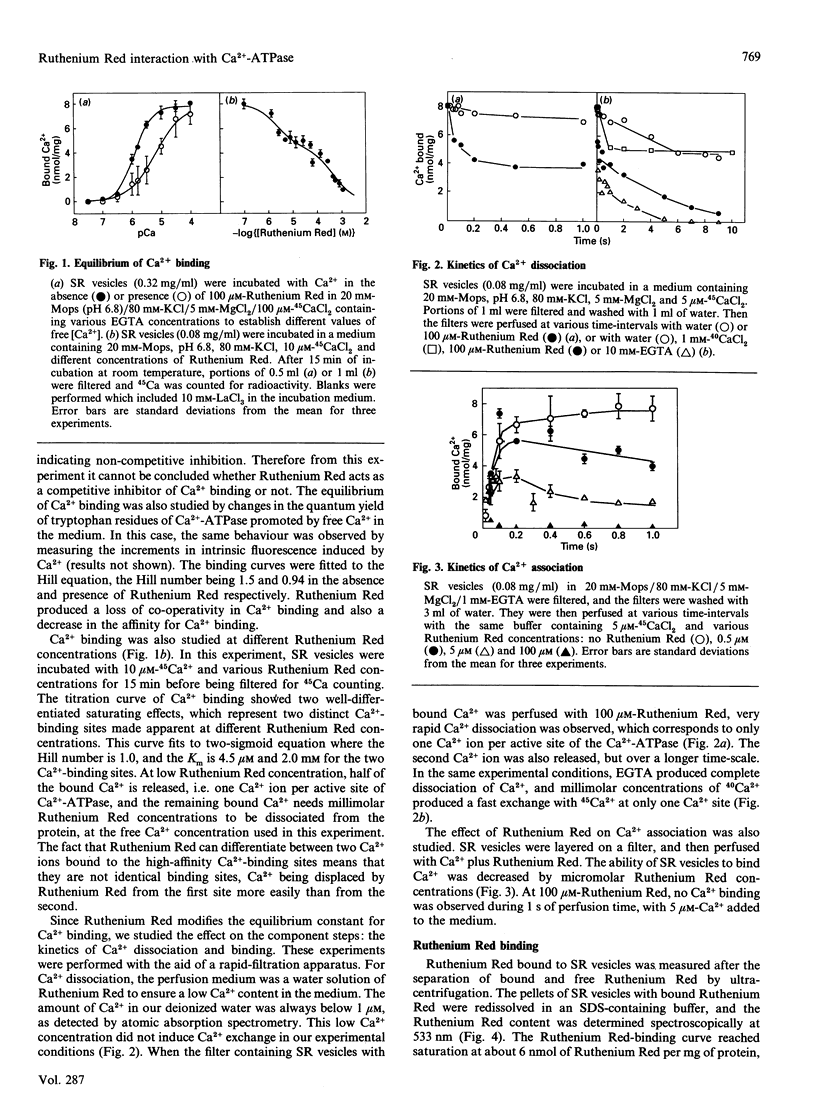
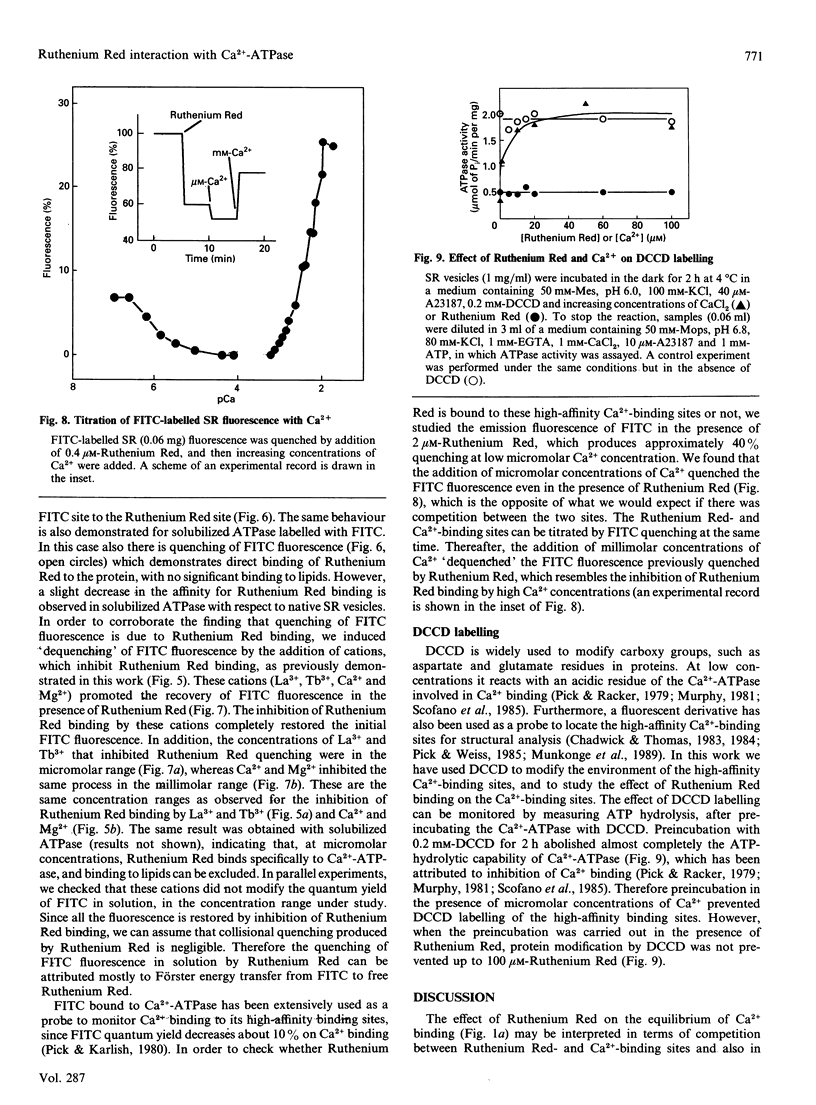
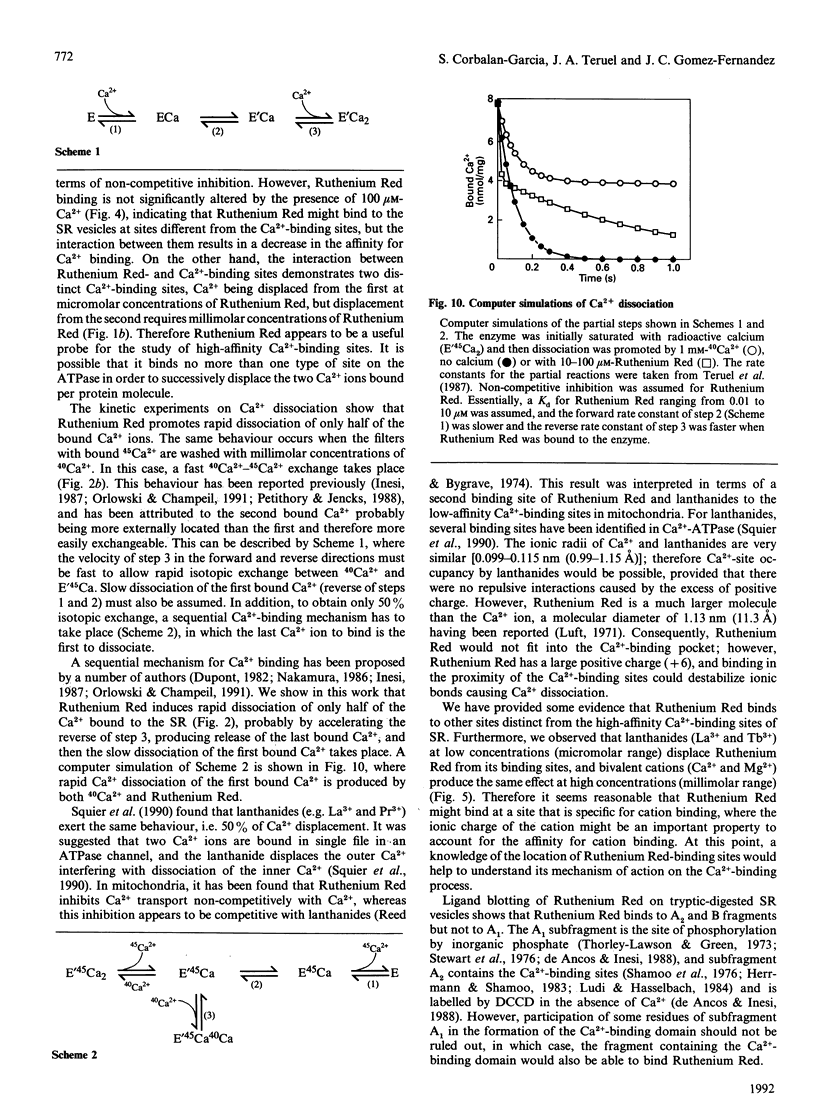
Sarcoplasmic reticulum Ca(2+)-ATPase has previously been shown to bind and dissociate two Ca2+ ions in a sequential mode. This behaviour is confirmed here by inducing sequential Ca2+ dissociation with Ruthenium Red. Ruthenium Red binds to sarcoplasmic reticulum vesicles (6 nmol/mg) with a Kd = 2 microM, producing biphasic kinetics of Ca2+ dissociation from the Ca(2+)-ATPase, decreasing the affinity for Ca2+ binding. Studies on the effect of Ca2+ on Ruthenium Red binding indicate that Ruthenium Red does not bind to the high-affinity Ca(2+)-binding sites, as suggested by the following observations: (i) micromolar concentrations of Ca2+ do not significantly alter Ruthenium Red binding to the sarcoplasmic reticulum; (ii) quenching of the fluorescence of fluorescein 5'-isothiocyanate (FITC) bound to Ca(2+)-ATPase by Ruthenium Red (resembling Ruthenium Red binding) is not prevented by micromolar concentrations of Ca2+; (iii) quenching of FITC fluorescence by Ca2+ binding to the high-affinity sites is achieved even though Ruthenium Red is bound to the Ca(2+)-ATPase; and (iv) micromolar Ca2+ concentrations prevent inhibition of the ATP-hydrolytic capability by dicyclohexylcarbodi-imide modification, but Ruthenium Red does not. However, micromolar concentrations of lanthanides (La3+ and Tb3+) and millimolar concentrations of bivalent cations (Ca2+ and Mg2+) inhibit Ruthenium Red binding as well as quenching of FITC-labelled Ca(2+)-ATPase fluorescence by Ruthenium Red. Studies of Ruthenium Red binding to tryptic fragments of Ca(2+)-ATPase, as demonstrated by ligand blotting, indicate that Ruthenium Red does not bind to the A1 subfragment. Our observations suggest that Ruthenium Red might bind to a cation-binding site in Ca(2+)-ATPase inducing fast release of the last bound Ca2+ by interactions between the sites.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandl C. J., Green N. M., Korczak B., MacLennan D. H. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986 Feb 28;44(4):597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- Chadwick C. C., Thomas E. W. Inactivation of sarcoplasmic reticulum (Ca2+ + Mg2+)-ATPase by N-cyclohexyl-N'-(4-dimethylamino-alpha-naphthyl)carbodiimide. Biochim Biophys Acta. 1983 May 5;730(2):201–206. doi: 10.1016/0005-2736(83)90334-6. [DOI] [PubMed] [Google Scholar]
- Chadwick C. C., Thomas E. W. Ligand binding properties of the sarcoplasmic reticulum (Ca2+ + Mg2+)-ATPase labelled with N-cyclohexyl-N'-(4-dimethylamino-alpha-naphthyl)carbodiimide. Biochim Biophys Acta. 1984 Jan 25;769(2):291–296. doi: 10.1016/0005-2736(84)90309-2. [DOI] [PubMed] [Google Scholar]
- Chamberlain B. K., Volpe P., Fleischer S. Inhibition of calcium-induced calcium release from purified cardiac sarcoplasmic reticulum vesicles. J Biol Chem. 1984 Jun 25;259(12):7547–7553. [PubMed] [Google Scholar]
- Champeil P., Guillain F., Vénien C., Gingold M. P. Interaction of magnesium and inorganic phosphate with calcium-deprived sarcoplasmic reticulum adenosinetriphosphatase as reflected by organic solvent induced perturbation. Biochemistry. 1985 Jan 1;24(1):69–81. doi: 10.1021/bi00322a012. [DOI] [PubMed] [Google Scholar]
- Charuk J. H., Pirraglia C. A., Reithmeier R. A. Interaction of ruthenium red with Ca2(+)-binding proteins. Anal Biochem. 1990 Jul;188(1):123–131. doi: 10.1016/0003-2697(90)90539-l. [DOI] [PubMed] [Google Scholar]
- Clarke D. M., Loo T. W., Inesi G., MacLennan D. H. Location of high affinity Ca2+-binding sites within the predicted transmembrane domain of the sarcoplasmic reticulum Ca2+-ATPase. Nature. 1989 Jun 8;339(6224):476–478. doi: 10.1038/339476a0. [DOI] [PubMed] [Google Scholar]
- De Meis L., Suzano V. A., Caldeira T., Mintz E., Guillain F. Ca2+ gradient and drugs reveal different binding sites for Pi and Mg2+ in phosphorylation of the sarcoplasmic reticulum ATPase. Eur J Biochem. 1991 Aug 15;200(1):209–213. doi: 10.1111/j.1432-1033.1991.tb21069.x. [DOI] [PubMed] [Google Scholar]
- Dupont Y. Low-temperature studies of the sarcoplasmic reticulum calcium pump. Mechanisms of calcium binding. Biochim Biophys Acta. 1982 May 21;688(1):75–87. doi: 10.1016/0005-2736(82)90580-6. [DOI] [PubMed] [Google Scholar]
- Eletr S., Inesi G. Phospholipid orientation in sarcoplasmic membranes: spin-label ESR and proton MNR studies. Biochim Biophys Acta. 1972 Sep 1;282(1):174–179. doi: 10.1016/0005-2736(72)90321-5. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Guillain F., Gingold M. P., Champeil P. Direct fluorescence measurements of Mg2+ binding to sarcoplasmic reticulum ATPase. J Biol Chem. 1982 Jul 10;257(13):7366–7371. [PubMed] [Google Scholar]
- Herrmann T. R., Shamoo A. E. Ionophorous properties of the 13 000-Da fragment from sarcoplasmic reticulum (Ca2+ + Mg2+)-ATPase. Biochim Biophys Acta. 1983 Aug 10;732(3):647–650. doi: 10.1016/0005-2736(83)90242-0. [DOI] [PubMed] [Google Scholar]
- Highsmith S. R., Head M. R. Tb3+ binding to Ca2+ and Mg2+ binding sites on sarcoplasmic reticulum ATPase. J Biol Chem. 1983 Jun 10;258(11):6858–6862. [PubMed] [Google Scholar]
- Highsmith S. Evidence that the ATP binding site of sarcoplasmic reticulum CaATPase has a Mg(2+) ion binding sub-site. Biochem Biophys Res Commun. 1984 Oct 15;124(1):183–189. doi: 10.1016/0006-291x(84)90934-3. [DOI] [PubMed] [Google Scholar]
- Inesi G. Sequential mechanism of calcium binding and translocation in sarcoplasmic reticulum adenosine triphosphatase. J Biol Chem. 1987 Dec 5;262(34):16338–16342. [PubMed] [Google Scholar]
- Inesi G., Sumbilla C., Kirtley M. E. Relationships of molecular structure and function in Ca2(+)-transport ATPase. Physiol Rev. 1990 Jul;70(3):749–760. doi: 10.1152/physrev.1990.70.3.749. [DOI] [PubMed] [Google Scholar]
- Kosk-Kosicka D., Kurzmack M., Inesi G. Kinetic characterization of detergent-solubilized sarcoplasmic reticulum adenosinetriphosphatase. Biochemistry. 1983 May 10;22(10):2559–2567. doi: 10.1021/bi00279a037. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin T. I., Morales M. F. Application of a one-step procedure for measuring inorganic phosphate in the presence of proteins: the actomyosin ATPase system. Anal Biochem. 1977 Jan;77(1):10–17. doi: 10.1016/0003-2697(77)90284-6. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Lüdi H., Hasselbach W. Separation of the tryptic fragments of sarcoplasmic reticulum ATPase with high performance liquid chromatography. Identification of the calcium binding site. FEBS Lett. 1984 Feb 13;167(1):33–36. doi: 10.1016/0014-5793(84)80827-3. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- Madeira V. M., Antunes-Madeira M. C. Interaction of ruthenium red with isolated sarcolemma. J Membr Biol. 1974;17(1):41–50. doi: 10.1007/BF01870171. [DOI] [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Moutin M. J., Dupont Y. Interaction of potassium and magnesium with the high affinity calcium-binding sites of the sarcoplasmic reticulum calcium-ATPase. J Biol Chem. 1991 Mar 25;266(9):5580–5586. [PubMed] [Google Scholar]
- Munkonge F., East J. M., Lee A. G. Positions of the sites labeled by N-cyclohexyl-N'-(4-dimethylamino-1-naphthyl)carbodiimide on the (Ca2+ + Mg2+)-ATPase. Biochim Biophys Acta. 1989 Feb 13;979(1):113–120. doi: 10.1016/0005-2736(89)90530-0. [DOI] [PubMed] [Google Scholar]
- Murphy A. J. Kinetics of the inactivation of the ATPase of sarcoplasmic reticulum by dicyclohexylcarbodiimide. J Biol Chem. 1981 Dec 10;256(23):12046–12050. [PubMed] [Google Scholar]
- Nakamura J. Calcium-dependent non-equivalent characteristics of calcium binding sites of the sarcoplasmic reticulum Ca2+-ATPase. Biochim Biophys Acta. 1986 Apr 22;870(3):495–501. doi: 10.1016/0167-4838(86)90258-x. [DOI] [PubMed] [Google Scholar]
- Orlowski S., Champeil P. Kinetics of calcium dissociation from its high-affinity transport sites on sarcoplasmic reticulum ATPase. Biochemistry. 1991 Jan 15;30(2):352–361. doi: 10.1021/bi00216a007. [DOI] [PubMed] [Google Scholar]
- Petithory J. R., Jencks W. P. Binding of Ca2+ to the calcium adenosinetriphosphatase of sarcoplasmic reticulum. Biochemistry. 1988 Nov 15;27(23):8626–8635. doi: 10.1021/bi00423a018. [DOI] [PubMed] [Google Scholar]
- Pick U., Karlish S. J. Indications for an oligomeric structure and for conformational changes in sarcoplasmic reticulum Ca2+-ATPase labelled selectively with fluorescein. Biochim Biophys Acta. 1980 Nov 20;626(1):255–261. doi: 10.1016/0005-2795(80)90216-0. [DOI] [PubMed] [Google Scholar]
- Pick U., Racker E. Inhibition of the (Ca2+)ATPase from sarcoplasmic reticulum by dicyclohexylcarbodiimide: evidence for location of the Ca2+ binding site in a hydrophobic region. Biochemistry. 1979 Jan 9;18(1):108–113. doi: 10.1021/bi00568a017. [DOI] [PubMed] [Google Scholar]
- Pick U., Weiss M. Spectral and catalytical properties of the sarcoplasmic reticulum Ca-ATPase labeled with N-cyclohexyl-N'-(4-dimethylamino-1-naphthyl)-carbodiimide. Eur J Biochem. 1985 Oct 1;152(1):83–89. doi: 10.1111/j.1432-1033.1985.tb09166.x. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Bygrave F. L. The inhibition of mitochondrial calcium transport by lanthanides and ruthenium red. Biochem J. 1974 May;140(2):143–155. doi: 10.1042/bj1400143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofano H. M., Barrabin H., Lewis D., Inesi G. Specific dicyclohexylcarbodiimide inhibition of the E-P + H2O equilibrium E + Pi reaction and ATP equilibrium Pi exchange in sarcoplasmic reticulum adenosinetriphosphatase. Biochemistry. 1985 Feb 12;24(4):1025–1029. doi: 10.1021/bi00325a033. [DOI] [PubMed] [Google Scholar]
- Shamoo A. E., Ryan T. E., Stewart P. S., MacLennan D. H. Localization of ionophore activity in a 20,000-dalton fragment of the adenosine triphosphatase of Sarcoplasmic reticulum. J Biol Chem. 1976 Jul 10;251(13):4147–4154. [PubMed] [Google Scholar]
- Squier T. C., Bigelow D. J., Fernandez-Belda F. J., deMeis L., Inesi G. Calcium and lanthanide binding in the sarcoplasmic reticulum ATPase. J Biol Chem. 1990 Aug 15;265(23):13713–13720. [PubMed] [Google Scholar]
- Stephens E. M., Grisham C. M. Lithium-7 nuclear magnetic resonance, water proton nuclear magnetic resonance, and gadolinium electron paramagnetic resonance studies of the sarcoplasmic reticulum calcium ion transport adenosine triphosphatase. Biochemistry. 1979 Oct 30;18(22):4876–4885. doi: 10.1021/bi00589a016. [DOI] [PubMed] [Google Scholar]
- Stewart P. S., MacLennan D. H. Isolation and characterization of tryptic fragments of the adenosine triphosphatase of sarcoplasmic reticulum. J Biol Chem. 1976 Feb 10;251(3):712–719. [PubMed] [Google Scholar]
- Sumbilla C., Cantilina T., Collins J. H., Malak H., Lakowicz J. R., Inesi G. Structural perturbation of the transmembrane region interferes with calcium binding by the Ca2+ transport ATPase. J Biol Chem. 1991 Jul 5;266(19):12682–12689. [PubMed] [Google Scholar]
- Teruel J. A., Inesi G. Roles of phosphorylation and nucleotide binding domains in calcium transport by sarcoplasmic reticulum adenosinetriphosphatase. Biochemistry. 1988 Aug 9;27(16):5885–5890. doi: 10.1021/bi00416a010. [DOI] [PubMed] [Google Scholar]
- Teruel J. A., Kurzmack M., Inesi G. Kinetic and thermodynamic control of ATP synthesis by sarcoplasmic reticulum adenosinetriphosphatase. J Biol Chem. 1987 Sep 25;262(27):13055–13060. [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Green N. M. Studies on the location and orientation of proteins in the sarcoplasmic reticulum. Eur J Biochem. 1973 Dec 17;40(2):403–413. doi: 10.1111/j.1432-1033.1973.tb03209.x. [DOI] [PubMed] [Google Scholar]
- Vale M. G., Carvalho A. P. Effects of ruthenium red on Ca2+ uptake and ATPase of sarcoplasmic reticulum of rabbit skeletal muscle. Biochim Biophys Acta. 1973 Oct 19;325(1):29–37. doi: 10.1016/0005-2728(73)90147-3. [DOI] [PubMed] [Google Scholar]
- Vasington F. D., Gazzotti P., Tiozzo R., Carafoli E. The effect of ruthenium red on Ca 2+ transport and respiration in rat liver mitochondria. Biochim Biophys Acta. 1972 Jan 21;256(1):43–54. doi: 10.1016/0005-2728(72)90161-2. [DOI] [PubMed] [Google Scholar]
- Volpe P., Salviati G., Chu A. Calcium-gated calcium channels in sarcoplasmic reticulum of rabbit skinned skeletal muscle fibers. J Gen Physiol. 1986 Feb;87(2):289–303. doi: 10.1085/jgp.87.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson E. L., Vincenzi F. F., Davis P. W. Ca 2+ -activated membrane ATPase: selective inhibition by ruthenium red. Biochim Biophys Acta. 1971 Dec 3;249(2):606–610. doi: 10.1016/0005-2736(71)90140-4. [DOI] [PubMed] [Google Scholar]
- de Ancos J. G., Inesi G. Patterns of proteolytic cleavage and carbodiimide derivatization in sarcoplasmic reticulum adenosinetriphosphatase. Biochemistry. 1988 Mar 8;27(5):1793–1803. doi: 10.1021/bi00405a061. [DOI] [PubMed] [Google Scholar]
- de Meis L. Fast efflux of Ca2+ mediated by the sarcoplasmic reticulum Ca2(+)-ATPase. J Biol Chem. 1991 Mar 25;266(9):5736–5742. [PubMed] [Google Scholar]


