Abstract
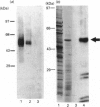
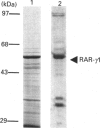
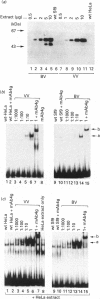
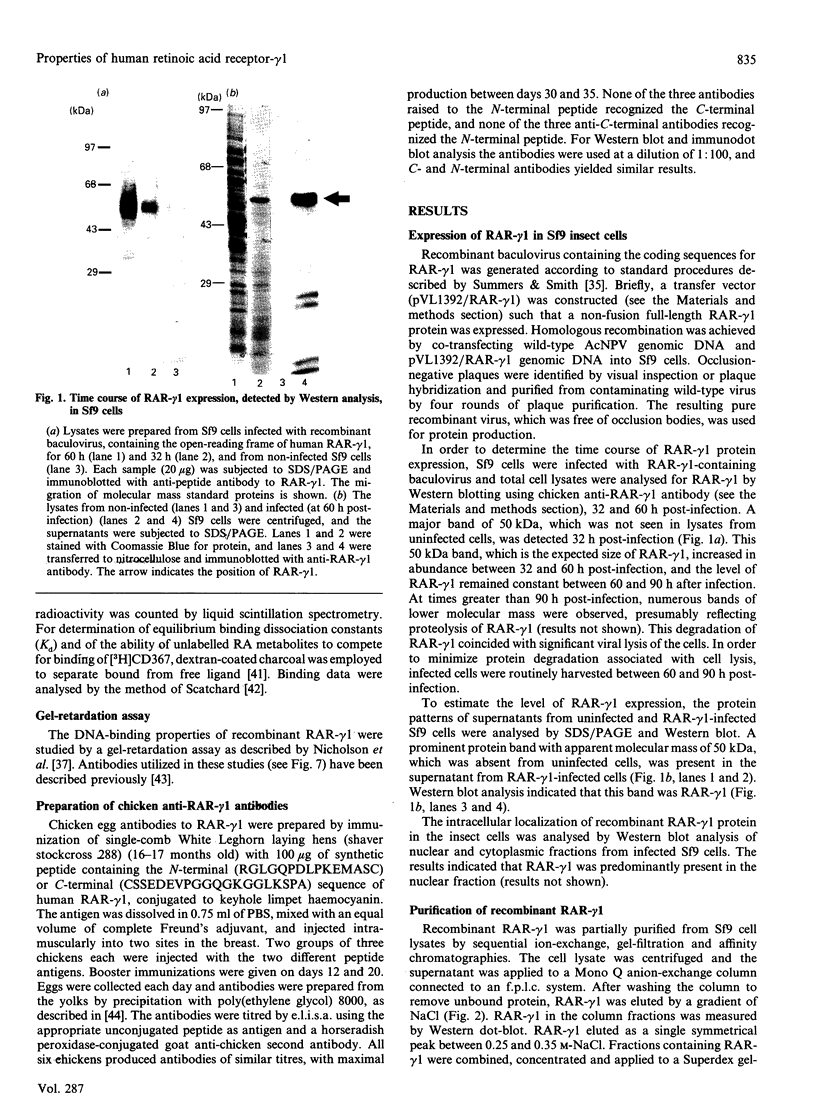
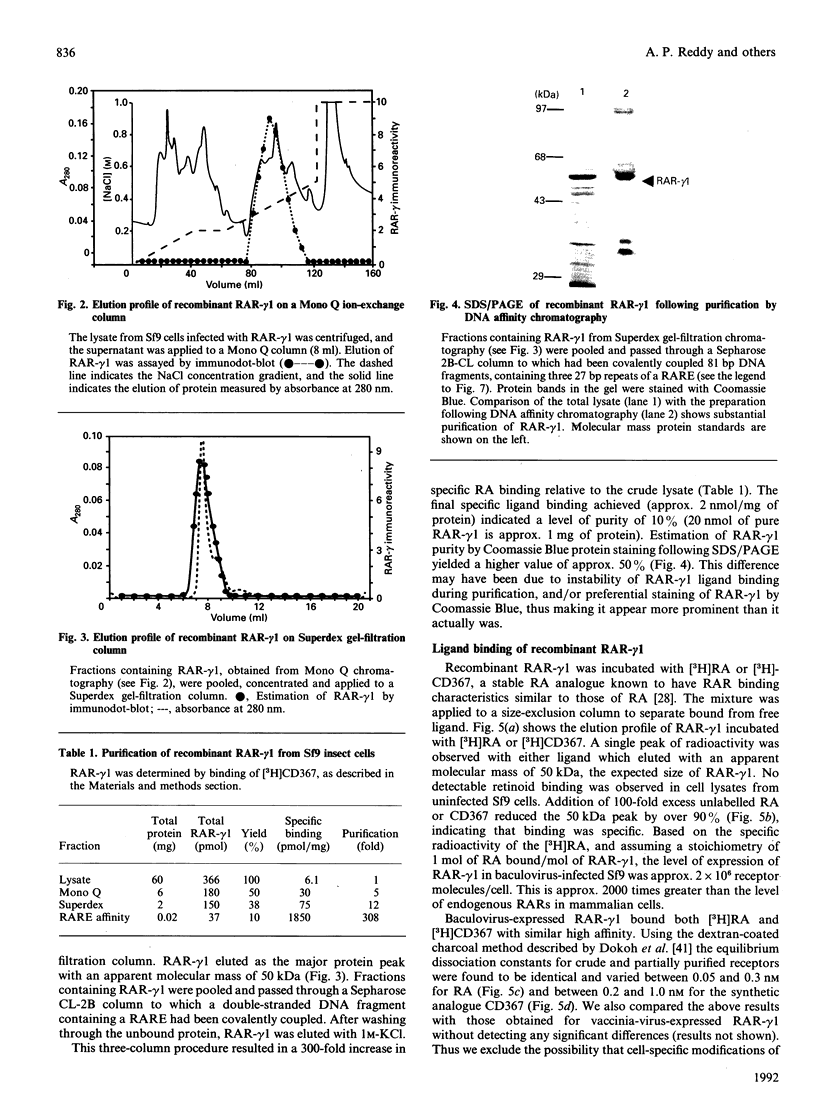
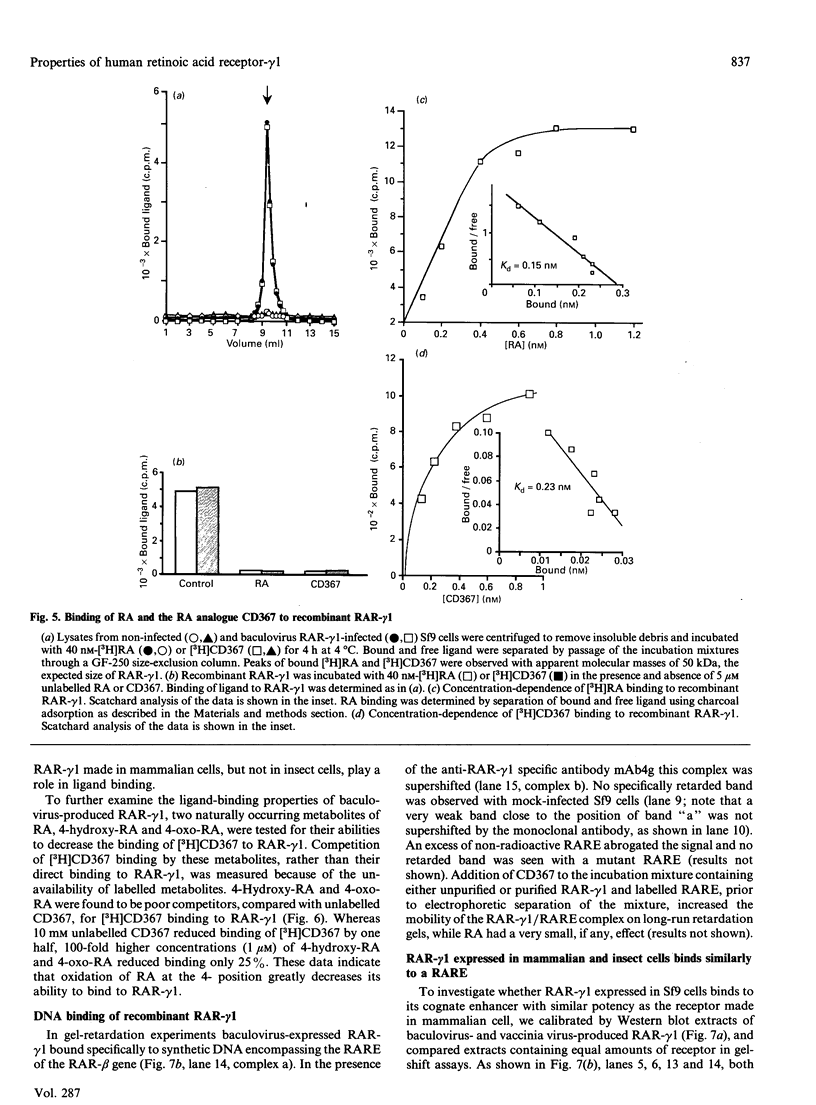
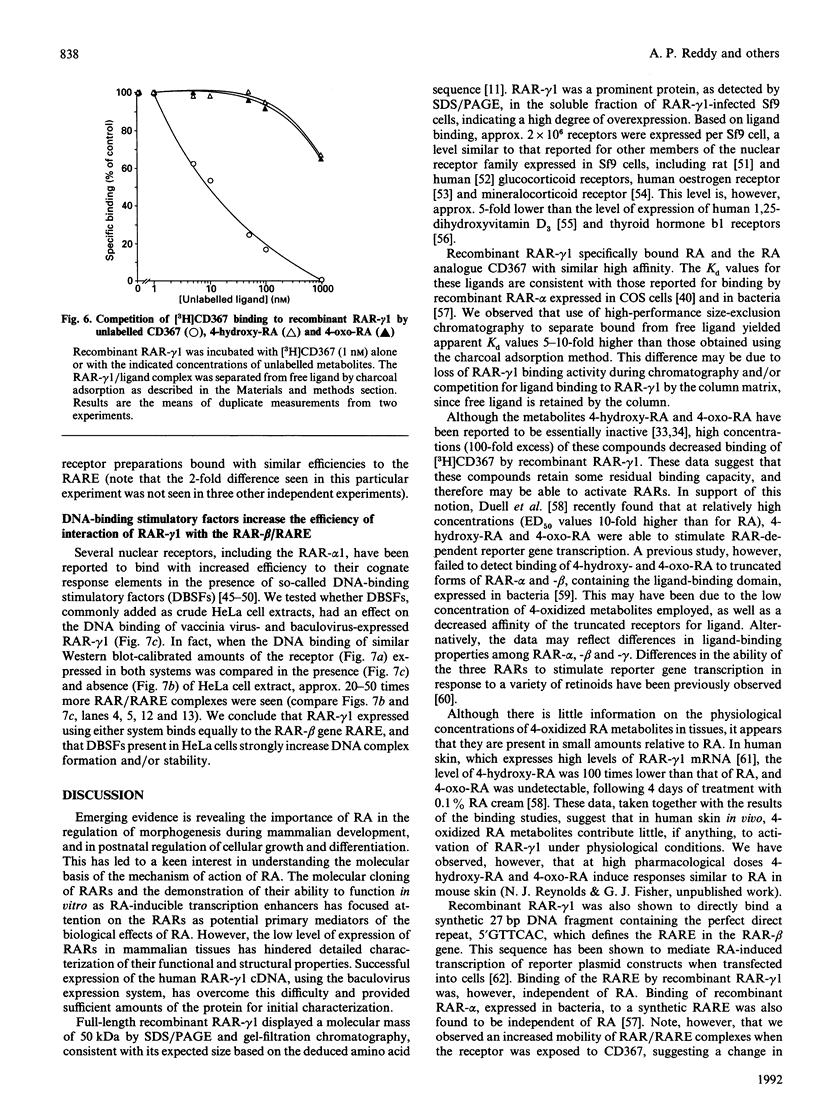
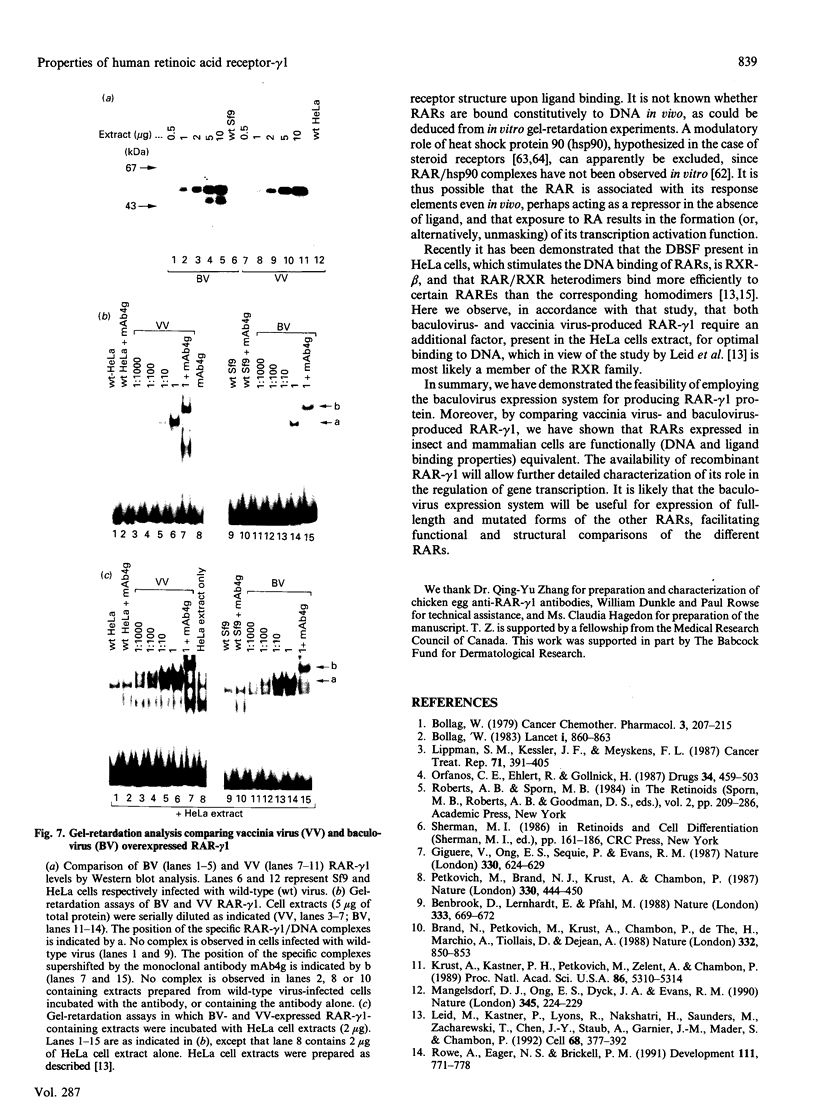
The full-length cDNA for the human retinoic acid receptor-gamma 1 (RAR-gamma 1) has been expressed to high levels in Spodoptera frugiferda (Sf9) cells using the baculovirus expression system. Western blot analysis revealed that RAR-gamma 1 expression increased between 32 and 60 h post-infection. The recombinant receptor was expressed primarily as a nuclear protein and displayed a molecular mass of 50 kDa as determined by SDS/PAGE and gel-filtration chromatography, consistent with its cDNA-deduced size. Based on ligand binding, 2 x 10(6) RAR-gamma 1 molecules were expressed per Sf9 cell, a level approx. 2000 times greater than in mammalian cells. The receptor was partially purified 300-fold by sequential anion-exchange, gel-filtration and DNA affinity chromatographies. The overexpressed receptor specifically bound all-trans-retinoic acid (RA) and the synthetic retinoid CD367 with high affinity (Kd 0.15 nM and 0.23 nM respectively). The RA metabolites 4-hydroxy-RA and 4-oxo-RA were poor competitors for [3H]CD367 binding to recombinant RAR-gamma 1 (K(i) > 1 microM), indicating that 4-oxidation of RA greatly reduces its affinity for RAR-gamma 1. Gel-retardation analysis demonstrated that RAR-gamma 1 specifically bound the RA response element of the mouse RAR-beta gene. RAR-gamma 1 species expressed from recombinant baculovirus (in Sf9 cells) and vaccinia virus (in HeLa cells) exhibited similar affinities for RA and CD367 and had comparable DNA-binding properties in gel-retardation experiments. Moreover, a similar requirement for additional DNA-binding stimulatory factor(s) was observed in both cases. These results provide a basis for the use of baculovirus-expressed RAR-gamma 1 in further functional and structural studies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnemri E. S., Maksymowych A. B., Robertson N. M., Litwack G. Characterization and purification of a functional rat glucocorticoid receptor overexpressed in a baculovirus system. J Biol Chem. 1991 Feb 25;266(6):3925–3936. [PubMed] [Google Scholar]
- Alnemri E. S., Maksymowych A. B., Robertson N. M., Litwack G. Overexpression and characterization of the human mineralocorticoid receptor. J Biol Chem. 1991 Sep 25;266(27):18072–18081. [PubMed] [Google Scholar]
- Aström A., Pettersson U., Krust A., Chambon P., Voorhees J. J. Retinoic acid and synthetic analogs differentially activate retinoic acid receptor dependent transcription. Biochem Biophys Res Commun. 1990 Nov 30;173(1):339–345. doi: 10.1016/s0006-291x(05)81062-9. [DOI] [PubMed] [Google Scholar]
- Barkhem T., Carlsson B., Simons J., Möller B., Berkenstam A., Gustafsson J. A., Nilsson S. High level expression of functional full length human thyroid hormone receptor beta 1 in insect cells using a recombinant baculovirus. J Steroid Biochem Mol Biol. 1991 Jun;38(6):667–675. doi: 10.1016/0960-0760(91)90077-i. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E. On the mechanism of action of RU486. Ann N Y Acad Sci. 1991;626:545–560. doi: 10.1111/j.1749-6632.1991.tb37946.x. [DOI] [PubMed] [Google Scholar]
- Benbrook D., Lernhardt E., Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988 Jun 16;333(6174):669–672. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- Bollag W. Retinoids and cancer. Cancer Chemother Pharmacol. 1979;3(4):207–215. doi: 10.1007/BF00254733. [DOI] [PubMed] [Google Scholar]
- Bollag W. Vitamin A and retinoids: from nutrition to pharmacotherapy in dermatology and oncology. Lancet. 1983 Apr 16;1(8329):860–863. doi: 10.1016/s0140-6736(83)91394-6. [DOI] [PubMed] [Google Scholar]
- Brand N., Petkovich M., Krust A., Chambon P., de Thé H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988 Apr 28;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Brown M., Sharp P. A. Human estrogen receptor forms multiple protein-DNA complexes. J Biol Chem. 1990 Jul 5;265(19):11238–11243. [PubMed] [Google Scholar]
- Burnside J., Darling D. S., Chin W. W. A nuclear factor that enhances binding of thyroid hormone receptors to thyroid hormone response elements. J Biol Chem. 1990 Feb 15;265(5):2500–2504. [PubMed] [Google Scholar]
- Cavey M. T., Martin B., Carlavan I., Shroot B. In vitro binding of retinoids to the nuclear retinoic acid receptor alpha. Anal Biochem. 1990 Apr;186(1):19–23. doi: 10.1016/0003-2697(90)90565-q. [DOI] [PubMed] [Google Scholar]
- Crettaz M., Baron A., Siegenthaler G., Hunziker W. Ligand specificities of recombinant retinoic acid receptors RAR alpha and RAR beta. Biochem J. 1990 Dec 1;272(2):391–397. doi: 10.1042/bj2720391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollé P., Ruberte E., Leroy P., Morriss-Kay G., Chambon P. Retinoic acid receptors and cellular retinoid binding proteins. I. A systematic study of their differential pattern of transcription during mouse organogenesis. Development. 1990 Dec;110(4):1133–1151. doi: 10.1242/dev.110.4.1133. [DOI] [PubMed] [Google Scholar]
- Duester G., Shean M. L., McBride M. S., Stewart M. J. Retinoic acid response element in the human alcohol dehydrogenase gene ADH3: implications for regulation of retinoic acid synthesis. Mol Cell Biol. 1991 Mar;11(3):1638–1646. doi: 10.1128/mcb.11.3.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. P., Kühnel B., Estes P. A., Nordeen S. K. Human progesterone receptor binding to mouse mammary tumor virus deoxyribonucleic acid: dependence on hormone and nonreceptor nuclear factor(s). Mol Endocrinol. 1989 Feb;3(2):381–391. doi: 10.1210/mend-3-2-381. [DOI] [PubMed] [Google Scholar]
- Elder J. T., Fisher G. J., Zhang Q. Y., Eisen D., Krust A., Kastner P., Chambon P., Voorhees J. J. Retinoic acid receptor gene expression in human skin. J Invest Dermatol. 1991 Apr;96(4):425–433. doi: 10.1111/1523-1747.ep12469889. [DOI] [PubMed] [Google Scholar]
- Frolik C. A., Roberts A. B., Tavela T. E., Roller P. P., Newton D. L., Sporn M. B. Isolation and identification of 4-hydroxy- and 4-oxoretinoic acid. In vitro metabolites of all-trans-retinoic acid in hamster trachea and liver. Biochemistry. 1979 May 15;18(10):2092–2097. doi: 10.1021/bi00577a039. [DOI] [PubMed] [Google Scholar]
- Gassmann M., Thömmes P., Weiser T., Hübscher U. Efficient production of chicken egg yolk antibodies against a conserved mammalian protein. FASEB J. 1990 May;4(8):2528–2532. doi: 10.1096/fasebj.4.8.1970792. [DOI] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Giguère V., Ong E. S., Evans R. M., Tabin C. J. Spatial and temporal expression of the retinoic acid receptor in the regenerating amphibian limb. Nature. 1989 Feb 9;337(6207):566–569. doi: 10.1038/337566a0. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Devary O. V., Rosenfeld M. G. Multiple cell type-specific proteins differentially regulate target sequence recognition by the alpha retinoic acid receptor. Cell. 1990 Nov 16;63(4):729–738. doi: 10.1016/0092-8674(90)90139-6. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H. Transcription activation by estrogen and progesterone receptors. Annu Rev Genet. 1991;25:89–123. doi: 10.1146/annurev.ge.25.120191.000513. [DOI] [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P., Krust A., Mendelsohn C., Garnier J. M., Zelent A., Leroy P., Staub A., Chambon P. Murine isoforms of retinoic acid receptor gamma with specific patterns of expression. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2700–2704. doi: 10.1073/pnas.87.7.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krust A., Kastner P., Petkovich M., Zelent A., Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Leroy P., Krust A., Zelent A., Mendelsohn C., Garnier J. M., Kastner P., Dierich A., Chambon P. Multiple isoforms of the mouse retinoic acid receptor alpha are generated by alternative splicing and differential induction by retinoic acid. EMBO J. 1991 Jan;10(1):59–69. doi: 10.1002/j.1460-2075.1991.tb07921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy P., Nakshatri H., Chambon P. Mouse retinoic acid receptor alpha 2 isoform is transcribed from a promoter that contains a retinoic acid response element. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10138–10142. doi: 10.1073/pnas.88.22.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin A. A., Sturzenbecker L. J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Kratzeisen C., Rosenberger M., Lovey A. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992 Jan 23;355(6358):359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- Lippman S. M., Kessler J. F., Meyskens F. L., Jr Retinoids as preventive and therapeutic anticancer agents (Part I). Cancer Treat Rep. 1987 Apr;71(4):391–405. [PubMed] [Google Scholar]
- Lucas P. C., O'Brien R. M., Mitchell J. A., Davis C. M., Imai E., Forman B. M., Samuels H. H., Granner D. K. A retinoic acid response element is part of a pleiotropic domain in the phosphoenolpyruvate carboxykinase gene. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2184–2188. doi: 10.1073/pnas.88.6.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. High level expression of nonfused foreign genes with Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1989 May;170(1):31–39. doi: 10.1016/0042-6822(89)90348-6. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990 May 17;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- Mukherjee R., Chambon P. A single-stranded DNA-binding protein promotes the binding of the purified oestrogen receptor to its responsive element. Nucleic Acids Res. 1990 Oct 11;18(19):5713–5716. doi: 10.1093/nar/18.19.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. B., Towle H. C. Identification of nuclear factors that enhance binding of the thyroid hormone receptor to a thyroid hormone response element. Mol Endocrinol. 1989 Sep;3(9):1434–1442. doi: 10.1210/mend-3-9-1434. [DOI] [PubMed] [Google Scholar]
- Nervi C., Grippo J. F., Sherman M. I., George M. D., Jetten A. M. Identification and characterization of nuclear retinoic acid-binding activity in human myeloblastic leukemia HL-60 cells. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5854–5858. doi: 10.1073/pnas.86.15.5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson R. C., Mader S., Nagpal S., Leid M., Rochette-Egly C., Chambon P. Negative regulation of the rat stromelysin gene promoter by retinoic acid is mediated by an AP1 binding site. EMBO J. 1990 Dec;9(13):4443–4454. doi: 10.1002/j.1460-2075.1990.tb07895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfanos C. E., Ehlert R., Gollnick H. The retinoids. A review of their clinical pharmacology and therapeutic use. Drugs. 1987 Oct;34(4):459–503. doi: 10.2165/00003495-198734040-00003. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C., Lutz Y., Saunders M., Scheuer I., Gaub M. P., Chambon P. Retinoic acid receptor gamma: specific immunodetection and phosphorylation. J Cell Biol. 1991 Oct;115(2):535–545. doi: 10.1083/jcb.115.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross T. K., Prahl J. M., DeLuca H. F. Overproduction of rat 1,25-dihydroxyvitamin D3 receptor in insect cells using the baculovirus expression system. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6555–6559. doi: 10.1073/pnas.88.15.6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe A., Eager N. S., Brickell P. M. A member of the RXR nuclear receptor family is expressed in neural-crest-derived cells of the developing chick peripheral nervous system. Development. 1991 Mar;111(3):771–778. doi: 10.1242/dev.111.3.771. [DOI] [PubMed] [Google Scholar]
- Silva D. P., Jr, Valliere C. R., DeLuca H. F. Lack of biological activity of physiological metabolites of all-trans-retinoic acid on vaginal epithelial differentiation. Arch Biochem Biophys. 1987 Dec;259(2):391–401. doi: 10.1016/0003-9861(87)90505-4. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Nakshatri H., Leroy P., Rees J., Chambon P. A retinoic acid response element is present in the mouse cellular retinol binding protein I (mCRBPI) promoter. EMBO J. 1991 Aug;10(8):2223–2230. doi: 10.1002/j.1460-2075.1991.tb07758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan G., Thompson E. B. Overexpression of full-length human glucocorticoid receptor in Spodoptera frugiperda cells using the baculovirus expression vector system. Mol Endocrinol. 1990 Feb;4(2):209–216. doi: 10.1210/mend-4-2-209. [DOI] [PubMed] [Google Scholar]
- Sucov H. M., Murakami K. K., Evans R. M. Characterization of an autoregulated response element in the mouse retinoic acid receptor type beta gene. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5392–5396. doi: 10.1073/pnas.87.14.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasios G. W., Gold J. D., Petkovich M., Chambon P., Gudas L. J. A retinoic acid-responsive element is present in the 5' flanking region of the laminin B1 gene. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9099–9103. doi: 10.1073/pnas.86.23.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasios G., Mader S., Gold J. D., Leid M., Lutz Y., Gaub M. P., Chambon P., Gudas L. The late retinoic acid induction of laminin B1 gene transcription involves RAR binding to the responsive element. EMBO J. 1991 May;10(5):1149–1158. doi: 10.1002/j.1460-2075.1991.tb08055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Schüle R., Mangelsdorf D. J., Evans R. M. Characterization of DNA binding and retinoic acid binding properties of retinoic acid receptor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3559–3563. doi: 10.1073/pnas.88.9.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zelent A., Krust A., Petkovich M., Kastner P., Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989 Jun 29;339(6227):714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- Zelent A., Mendelsohn C., Kastner P., Krust A., Garnier J. M., Ruffenach F., Leroy P., Chambon P. Differentially expressed isoforms of the mouse retinoic acid receptor beta generated by usage of two promoters and alternative splicing. EMBO J. 1991 Jan;10(1):71–81. doi: 10.1002/j.1460-2075.1991.tb07922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de The H., Marchio A., Tiollais P., Dejean A. Differential expression and ligand regulation of the retinoic acid receptor alpha and beta genes. EMBO J. 1989 Feb;8(2):429–433. doi: 10.1002/j.1460-2075.1989.tb03394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]