Abstract
Ischemic heart disease (IHD) is common in women, and cardiovascular disease is a leading cause of morbidity and mortality. While obstructive coronary artery disease is the most common form of IHD, millions of women suffer from angina with nonobstructive coronary arteries (ANOCA), an umbrella term encompassing multiple nonatherosclerotic disorders of the coronary tree. The underlying pathology leading to ischemia in these syndromes may be challenging to diagnose, leaving many women without a diagnosis despite persistent symptoms that impact quality of life and adversely affect long-term cardiovascular prognosis. In the last decade, there have been significant advances in the recognition and diagnostic evaluation of ANOCA. Despite these advances, the standard approach to evaluating suspected IHD in women continues to focus predominantly on the assessment of atherosclerotic coronary artery disease, leading to missed opportunities to accurately diagnose and treat underlying coronary vasomotor disorders. The goal of this review is to describe advances in diagnostic testing that can be used to evaluate angina in women and present a pragmatic diagnostic algorithm to guide evaluation of ANOCA in symptomatic patients. The proposed approach for the assessment of ANOCA is consistent with prior expert consensus documents and guidelines but is predicated on the medical interview and pretest probability of disease to inform a personalized diagnostic strategy.
Keywords: angina, coronary artery disease, coronary microvascular dysfunction, coronary vasospasm, ischemic heart disease, myocardial bridge
Introduction
Every year, more than 10 million people in the United States seek evaluation for suspected ischemic heart disease (IHD).1 Cardiovascular disease is a leading cause of morbidity and mortality in women, but significant disparities exist in evaluation and management.2,3 In the last 60 years, the overall population mortality rate due to atherosclerotic coronary artery disease (CAD) has declined by almost 50%; however, in women the rate of decline has been substantially lower. Furthermore, the frequency of hospitalizations for acute myocardial infarction in young women (35-54 years) has increased from 21% in 1995-1999 to 31% in 2010-2014, compared to a relatively stable frequency in young men (from 30% to 33%).4
Historically, the evaluation of suspected cardiac chest pain, or angina pectoris, has focused on identifying myocardial ischemia caused by obstructive CAD; however, nearly 50% of patients undergoing invasive coronary angiography for suspected IHD are found to have no obstructive CAD or no coronary arteries with ≥50% stenosis.5,6 Instead, prior studies have suggested that 3 to 4 million people in the United States suffer from angina with nonobstructive coronary arteries (ANOCA).5,7 ANOCA is an umbrella term defined by the presence of anginal chest pain with either normal epicardial coronal arteries or coronary arteries with nonobstructive CAD.5,8 It encompasses coronary vasomotor disorders including coronary microvascular dysfunction (CMD), coronary vasospasm, endothelial dysfunction, symptomatic myocardial bridging (MB), and other nonatherosclerotic disorders.9,10 ANOCA endotypes may occur in isolation or as mixed syndromes, and they may exist in combination with CAD, which can be diffuse and missed with invasive angiography without intravascular imaging.11 Additionally, atherosclerosis of any degree can impair endothelial function and contribute to development of ANOCA.12 Although CAD is the leading cause of mortality in both men and women, women are more likely than men to suffer from ANOCA.5,13
Routine cardiovascular testing, which focuses on the evaluation of obstructive CAD, is less likely to define the underlying diagnosis in ANOCA, creating a diagnostic disparity for women with stable IHD. According to the 2021 American Heart Association and American College of Cardiology guideline for the evaluation and diagnosis of chest pain, assessment of patients with angina should start with an estimation of the pretest probability of atherosclerotic CAD, and additional diagnostic testing may be advised to rule out obstructive CAD. Although there is a growing array of testing options that can shed light on ANOCA, including invasive coronary function testing (CFT) and positron emission tomography (PET) myocardial perfusion imaging (MPI), evaluation of ANOCA is usually considered only after CAD is excluded.14,15 Additionally, these tests remain out of reach of many practicing clinicians, with variation in clinician awareness, site availability, insurance coverage, and patient preference regarding exposure to radiation or contrast dye, additional noninvasive tests, or repeat invasive procedures. As a result, many women with underlying ANOCA or mixed CAD/coronary vasomotor disorders are likely to be misdiagnosed, have a delayed diagnosis, and experience persistent symptoms.8,16 For example, in one study of 112 women with nonobstructive CAD entering a multidisciplinary women’s health center, 64% of patients did not have a specific diagnosis prior to entering the center, and 71% received a new or changed diagnosis after undergoing further testing.17 In another study of 297 women with a self-reported diagnosis of ANOCA, almost 78% were told that their symptoms were noncardiac prior to receiving the correct diagnosis.18
Testing that can elucidate the specific subtype of ANOCA is important given the prevalence and increased morbidity and mortality associated with these disorders in women.5,19, 20, 21, 22, 23 A study of 297 women with ANOCA found that after symptom onset, women reported lower functional capacity and adverse effects on home life, social life, mental health, interpersonal relationships, and work. Furthermore, a meta-analysis of 6631 patients with and without CMD described a 4-fold increase in mortality and a 5-fold increase in major cardiovascular events in patients with CMD.24 Recent studies such as the randomized CORonary MICrovascular Angina (CorMicA) trial have demonstrated significant improvement in symptom burden and quality of life with stratified medical therapy based on the identification of the underlying vasomotor disorder. Although it seems intuitive that an accurate diagnosis allows patients to understand their condition and is essential for guiding therapy, stratified medical therapy rarely occurs in clinical practice.25
The objective of this review is to introduce subtypes of ANOCA as commonly encountered syndromes in women with angina and to provide a practical diagnostic algorithm for ANOCA.
Terminology
The purpose of this review is to identify and review biological differences that may impact diagnostic testing based on sex, which is a biological construct based on anatomy, physiology, genetics, and hormones.26 The words “woman” and “women” are used throughout the manuscript referring to a specific gender. The authors acknowledge that gender is a multidimensional construct that encompasses gender identity and expression, as well as social and cultural expectations about status, characteristics, and behaviors and that gender can have complex interactions with biological sex throughout life that can impact cardiovascular health.27
Subtypes of ANOCA
There is considerable variation between studies regarding the prevalence of ANOCA subtypes. However, a meta-analysis conducted on 56 studies and a total of 14,427 patients found the pooled prevalence of CMD, epicardial vasospasm, microvascular spasm, and combined CMD and vasospastic angina to be 41%, 40%, 24%, and 23%, respectively.28
Within the coronary vascular system, the larger epicardial arteries function as conductance channels that offer minimal resistance to coronary blood flow, and the microvascular network is the primary determinant of coronary vascular resistance and regulator of coronary blood flow. In response to increased myocardial oxygen demand, the microvascular bed dilates to ensure adequate cardiac perfusion. The larger prearterioles predominantly maintain smooth muscle tone and alter vessel diameter through an endothelium-dependent mechanism that involves the release of vasoactive mediators that act on vascular smooth muscle. The medium-sized arterioles mainly regulate tone through a myogenic mechanism in which stretch receptors in the vessel wall respond to changes in intraluminal pressure. The tone and diameter of the smallest distal arterioles are mainly regulated by vasoactive metabolic byproducts during periods of increased cardiac activity.29 Abnormalities in any of these vasoregulatory mechanisms may contribute to the development of CMD.
Coronary microvascular dysfunction
CMD is defined as an impaired ability of the coronary microvasculature to autoregulate coronary blood flow due to varying degrees of structural, functional, and extravascular abnormalities. This leads to a mismatch between oxygen supply and demand. Mechanisms include vascular remodeling, vessel wall infiltration, extrinsic compression from surrounding myocardium, altered intracellular signaling, or increased production of reactive oxygen species. Additionally, there may be functional abnormalities from impaired vasodilation, enhanced vasoconstriction, or a combination of both. Autonomic dysfunction may also contribute to abnormal vasodilation.30
As part of the diagnostic evaluation of ANOCA, intracoronary or intravenous adenosine can be administered to assess the integrity of the endothelium-independent vasodilatory pathway. Adenosine binds to A2 receptors, which are concentrated in the vascular smooth muscle of the microvascular vessels, leading to an increase in coronary flow or hyperemia. Clinically, the coronary flow reserve (CFR), the index of microcirculatory resistance (IMR), and hyperemic microvascular resistance (hMR) can be measured after the onset of hyperemia. Reduced adenosine-mediated vasodilation, defined as low CFR (<2.5) or high hMR (≥2.5), can be indicative of endothelium-independent microvascular dysfunction.10,31
Endothelial dysfunction
The endothelium is essential for maintenance of vascular homeostasis. It releases vasodilation-promoting factors such as nitric oxide, prostacyclin, and endothelium-derived hyperpolarizing factor, and vasoconstriction-promoting factors such as endothelin-1 and thromboxane. Endothelial dysfunction develops when there is diminished production or reduced sensitivity to vasodilatory mediators and/or overproduction or hypersensitivity to substances that cause vasoconstriction.32,33
When performing CFT for evaluation of ANOCA, acetylcholine (ACh) can be administered to assess the integrity of the endothelium-dependent vasodilatory pathway.34 Under physiologic circumstances, ACh binds to muscarinic receptors on endothelial cells to induce nitric oxide release, which leads to vasodilation. However, a paradoxical response occurs when there is endothelial dysfunction. Endothelial dysfunction allows ACh to bind to the underlying vascular smooth muscle, causing either vasospasm or blunted vasodilation.33
Coronary vasospasm
Coronary vasospasm may involve the epicardial arteries or the coronary microcirculation, and the resulting clinical presentation is referred to as vasospastic angina. The terms Prinzmetal or variant angina refer to both epicardial and microvascular spasms.10 In the Acetylcholine Testing in Patients with Stable Angina Pectoris and Unobstructed Coronary Arteries (ACOVA) study, two-thirds of patients with no obstructive CAD were found to have coronary vasospasm.35 Single-center registries have reported a prevalence of vasospastic angina as high as 97% in patients undergoing invasive CFT.36 Coronary vasospasm can be attributed to endothelial dysfunction, which allows ACh to bind to muscarinic ACh receptors on vascular smooth muscle and induce vasospasm, or to vascular hypercontractility secondary to vascular smooth muscle cell hypersensitivity to vasoconstrictive stimuli.25,37 Anginal symptoms are often triggered in response to a precipitant, such as emotional stress, cold weather, cigarette smoking, or cocaine use.34
Myocardial bridge
MB is a congenital variant, estimated to be present in up to 30% of the population.14 In MB, a portion of an epicardial coronary artery takes an intramuscular route and undergoes dynamic compression during systole, leading to ischemia.38 However, this mechanism alone is usually insufficient to account for the ischemia associated with this condition, and the mechanism of ischemia in patients with MB frequently includes CMD, endothelial dysfunction, and spasm.39 Beyond the hemodynamic effects of extrinsic compression, MB is associated with impaired endothelial-dependent epicardial vasorelaxation and vascular smooth muscle hyperreactivity.38,40 Notably, the recently described Acute presentation, Bridge, C-reactive protein, Dyslipidemia risk score identified the presence of MB as a key predictor of inducible vasospasm in patients undergoing provocative testing with ACh.41
Evaluation of angina in women
In clinical practice, the approach to the evaluation of chest pain with suspected IHD focuses almost exclusively on the evaluation of atherosclerotic CAD. In the 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR chest pain guideline, there are no sex-specific recommendations for the diagnostic evaluation of chest pain, and evaluation for ANOCA is recommended only after obstructive CAD is excluded. For patients with known CAD and stable chest pain, these guidelines recommend intensification of guideline-directed medical therapy. For persistent symptoms in patients with known nonobstructive CAD, coronary computed tomography angiography (CCTA) with fractional flow reserve (FFR) or stress testing is recommended. Evaluation for ANOCA is recommended only after these tests yield negative results and the patient remains symptomatic. For patients with stable chest pain and no known CAD, evaluation for ANOCA is only recommended for intermediate/high-risk patients whose CCTA shows no obstructive CAD and who have evidence of ischemia on stress testing.15
Women with chest pain in all age groups are less likely to have obstructive CAD and more likely to have ANOCA compared to men.42 As a result, women are at a diagnostic disadvantage as the cause of their symptoms is less likely to be determined when they are evaluated using routine diagnostic strategies. There are biological differences in the pathophysiology of IHD, and an approach that relegates diagnostic evaluation of ANOCA to a last resort can expose women to misdiagnosis and delays in care.
An updated approach for evaluation of IHD in women
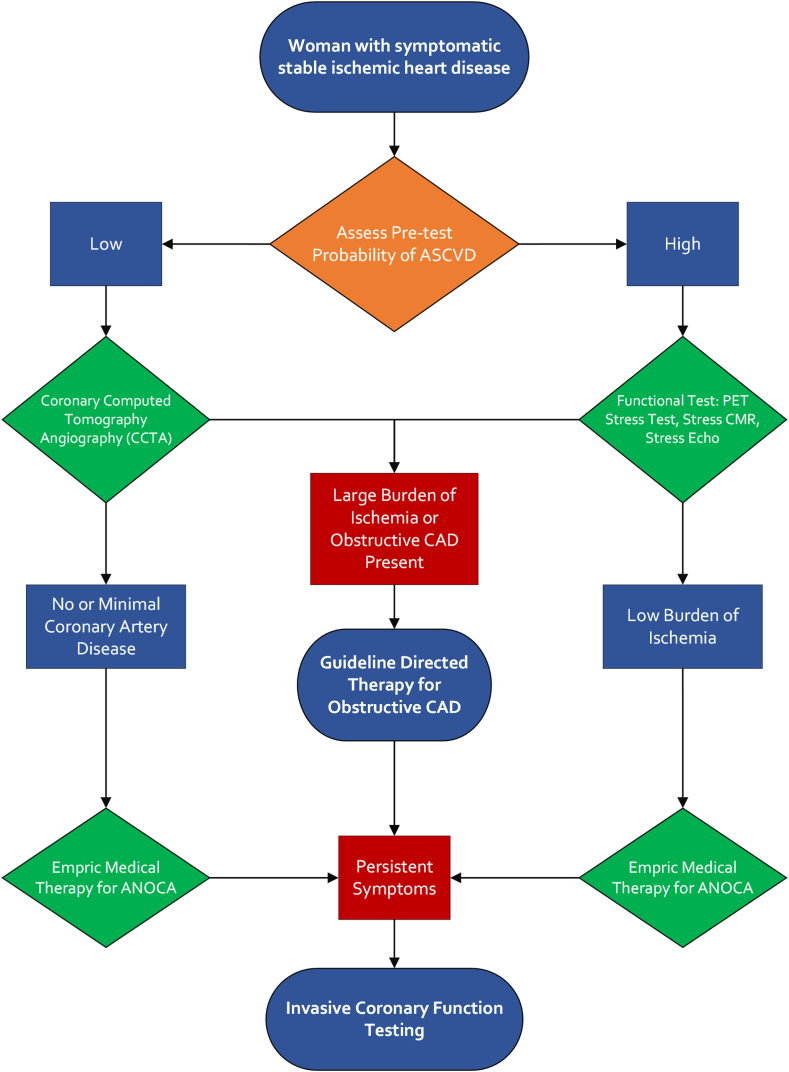
We propose consideration of a diagnostic approach that can streamline the evaluation of IHD in women and can also be employed in men with ischemia for whom there is a low probability of CAD (Central Illustration).
Central Illustration.
Proposed approach for the evaluation and management of suspected ischemic heart disease in women. Our proposed approach for evaluating women with suspected ischemic heart disease begins with assessing the pretest probability of atherosclerotic cardiovascular disease (ASCVD), including an evaluation of anginal symptoms and symptoms associated with angina with nonobstructive coronary arteries (ANOCA). Next, we recommend coronary computed tomography angiography (CCTA) for low-risk patients and functional testing for high-risk patients. Patients with a large burden of ischemia on imaging should be placed on goal-directed therapy for obstructive coronary artery disease (CAD), and those with a low burden of ischemia can begin empiric medical therapy for ANOCA. Finally, in patients with persistent symptoms despite appropriate medical therapy, invasive coronary function testing can be pursued for a definitive diagnosis. CMR, cardiac magnetic resonance imaging; PET, positron emission tomography.
Assessing pretest probability
Our proposed approach begins with assessing the pretest probability of atherosclerotic cardiovascular disease (ASCVD). In women with persistent symptoms and no evidence of ischemia on traditional cardiac testing, there should be a high index of suspicion for ANOCA. In assessing the pretest probability of CAD, it is important to consider a patient’s age, and risk factors and use existing standardized risk scores, such as the ASCVD score, to determine the likelihood of atherosclerotic CAD.
It is important to recognize that the clinical presentation of ANOCA may not be distinct from that of ischemia due to CAD. Patients with ANOCA often experience anginal symptoms such as chest discomfort and shortness of breath. These symptoms may be exertional, as in CAD, but can also occur with emotional stress or without any provocation.43, 44, 45 Additionally, each subtype of ANOCA may present with differing symptoms. Patients with CMD experience exertional fatigue or dyspnea that limits usual exercise capacity, persistent chest pain after cessation of exercise, postexertional fatigue, as well as other symptoms such as shortness of breath, jaw pain, and profound weakness.34,44 Patients with vasospastic angina describe a sudden tightness in the chest that has a discreet onset, occurs at rest, and resolves quickly or persists at a lower level throughout the day, especially between night and early morning. Marked diurnal variation in exercise tolerance has been described. Symptoms are ameliorated with nitrate medications.46 Patients with MB are typically asymptomatic but may experience exertional or resting angina47,48 and less commonly, arrhythmias such as atrioventricular block, supraventricular tachycardia, or ventricular tachycardia.49
Women who have symptoms consistent with or suggestive of ANOCA are often perimenopausal or in the early postmenopausal period, and this presentation may be related to reduction in estrogen or other cardiovascular risk factors.50 Assessment of extracardiac factors not directly associated with ANOCA, but still risk-enhancing, is also important. These factors include the presence of chronic inflammatory disease (eg, rheumatoid arthritis; systemic lupus erythematosus); obesity51; elevated C-reactive protein ≥2 mg/L; peripheral arterial disease; premature menopause (ovarian failure before the age of 40 years); a history of preeclampsia; and a family history of premature atherosclerotic disease (men aged <55 years or women aged <65 years).52 Additionally, although ANOCA can occur as an independent disease, it can also occur comorbidly with CAD. Thus, traditional cardiovascular risk factors, including hypertension, diabetes mellitus, and obesity should be assessed.53
To quantify a patient’s risk of ASCVD, we recommend the use of a standardized risk assessment tool in the form of a validated risk score for the population being studied. The most commonly used score in the United States is the ASCVD risk score, a pooled cohort equation that estimates the 10-year risk of cardiovascular events.52 The Reynold’s risk score (which additionally includes high-sensitivity C-reactive protein and family history of myocardial infarction in first-degree relatives prior to the age of 60 years), developed and validated in women, may also be appropriate.54
Noninvasive diagnostic testing
Technological advances have led to the widespread availability of both functional (ie, stress testing) and anatomic diagnostic test modalities for the investigation of IHD. Overall, functional testing is most appropriate for documenting ischemia or assessing the symptom response to exercise. In patients with a low pretest probability of CAD, anatomic tests such as CCTA or invasive coronary angiography have a high negative predictive value for excluding CAD.
The exercise tolerance test (ETT) or treadmill test is one of the most common initial tests used for the assessment of CAD. Historically, ETT was thought to have low sensitivity and specificity in women due to a higher rate of presumed false positive stress tests compared with men. However, more contemporary evidence based on invasive coronary Doppler assessment has shown that when CMD is added to the reference standard in addition to obstructive CAD, the specificity of ETT is excellent at 100%, and the false positive rate of ETT decreases from 31% to 0%.55 The misconception that ETT has poor diagnostic value in women highlights a disparity that has existed and been perpetuated because obstructive CAD was used as the reference.
Stress echocardiography with either exercise or pharmacologic agents can be used in the evaluation of angina. In contrast to radionuclide stress testing, stress echocardiography does not expose the patient to radiation and has been shown to have a high specificity for obstructive CAD (Table 1).56, 57, 58, 59, 60 Pharmacologic stress echocardiography using vasodilators has been used to measure CFR, which may be helpful in the diagnosis of CMD, but this technique has not been widely adopted due to the expertise required for image acquisition.61,62
Table 1.
Sensitivity and specificity of noninvasive diagnostic modalities for the evaluation of obstructive CAD in women58, 59, 60
| Test modality | Sensitivity | Specificity |
|---|---|---|
| Exercise tolerance test (ETT) | 62% | 68% |
| Single photon emission computed tomography (SPECT) myocardial perfusion imaging | 84.2% | 78.7% |
| Stress positron emission tomography (PET) myocardial perfusion imaging | 90%-92% | 81%-88% |
| Coronary computed tomography angiography (CCTA) | 93.5% | 80.6% |
| Stress cardiac MRI | 86.5% | 83.4% |
| Dobutamine stress echocardiography | 70.4% | 94.6% |
CAD, coronary artery disease; MRI, magnetic resonance imaging.
Radionuclide stress testing options include single photon emission computed tomography (SPECT) and PET-MPI imaging. Unlike PET stress testing, SPECT stress testing can be combined with an ETT to provide additional information regarding functional capacity.58 However, PET stress testing has increasingly been used in the assessment of ischemic symptoms given its advantages over SPECT imaging, particularly for women, including lower radiation exposure, superior imaging quality, accurate attenuation correction, and detection of regional perfusion defects. It can also measure rest and stress myocardial blood flow (MBF) and myocardial flow reserve (MFR), which can be used as a global measure of CFR.63, 64, 65, 66, 67 Notably, MFR has been shown to be predictive of all-cause mortality and major adverse cardiovascular events independent of angiographic stenosis severity.13,68 The use of PET-MPI has become more common in clinical practice,67 and it is an excellent noninvasive first step in assessing MFR and perfusion for the diagnosis of CMD.69
Stress cardiac magnetic resonance imaging (CMR) has higher resolution compared to SPECT or PET-MPI. Additionally, stress CMR has high diagnostic accuracy and can yield MBF and myocardial perfusion reserve index, providing valuable information regarding the presence of CMD.70 Both stress CMR with MBF and PET-MPI have a class 2A recommendation for the evaluation of ANOCA and are appropriate for initial assessment.15 However, stress CMR is not widely available in the United States.56
Anatomic testing with CCTA can be considered as an alternative to functional testing as an initial noninvasive investigation due to its higher accuracy in the detection of stenosis and nonobstructive lesions. In the 2021 chest pain guideline, CCTA has a class 1a recommendation for the evaluation of chest pain in patients with intermediate-risk and no known CAD.15 Trials including the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) and the Scottish Computed Tomography of the Heart trial found CCTA to have a high specificity for the detection of CAD.71 The PROMISE trial, which compared the strategy of initial functional testing versus initial CCTA, showed that clinical outcomes at the end of a 2-year follow-up period did not differ significantly between the 2 groups. CCTA can provide additional prognostic information in women regarding the burden of atherosclerosis at a similar cost to that of stress testing. Importantly, CCTA is associated with significantly less exposure to radiation compared to nuclear stress testing.69,72 Furthermore, a unique advantage of CCTA in patients with suspected ANOCA is the ability to detect and characterize MB.73
Invasive CFT
Although noninvasive diagnostic testing may be the initial test option for the assessment of patients who present with suspected IHD, CFT can definitively assess for CAD and characterize functional abnormalities within ANOCA. In 1997, the initial Women’s Ischemia Syndrome Evaluation (WISE) trial was initiated to investigate IHD in women, including women with and without obstructive CAD, and a novel coronary reactivity testing protocol was developed using Doppler assessment.74,75 The WISE protocol raised awareness regarding the prevalence and prognostic implications of coronary vasomotor dysfunction, but the protocol could not be scaled to routine practice.76 Coronary thermodilution using a pressure- and temperature-sensor–tipped coronary guide wire has emerged as a readily available method for assessing CFR and IMR with high interoperator reproducibility.77 Historically, invasive coronary Doppler was the gold standard for the assessment of CFR and hMR, but this modality is currently off the market in the United States.78, 79, 80 Beyond FFR or nonhyperemic pressure ratios to assess translesional pressure gradients, invasive assessment of CFR and IMR can be used clinically to comprehensively assess the coronary circulation for causes of myocardial ischemia.31,61
In addition to wire-based testing, intracoronary ACh can be safely used to assess the endothelium-dependent vasodilatory pathway and identify coronary vasospasm and endothelial dysfunction. This test is performed by serially infusing ACh into the coronary arteries and evaluating for changes in coronary artery diameter, electrocardiographic changes, and patient symptoms.81 In healthy vessels with preserved endothelial function, ACh injection should lead to vasodilatation. However, if there is endothelial dysfunction or underlying coronary vasospasm, ACh administration will lead to vasoconstriction.29,80,81 A summary of the indices of invasive CFT can be found in Table 2.58,63, 64, 65, 66
Table 2.
Indices of invasive coronary function testing.
| Definition | Abnormal findings | Considerations | |
|---|---|---|---|
| Coronary flow reserve (CFR) | Ratio of coronary flow during hyperemia vs rest | Low CFR with no visualized angiographic obstruction may indicate CMD | Cannot be used independently to diagnose CMD because it reflects both epicardial and microvascular disease and is dependent on hemodynamic factors58 |
| Fractional flow reserve (FFR) | Pressure difference proximal and distal to a given stenosis in response to intracoronary dilation with adenosine or papaverine | FFR > 0.80 and CFR ≤ 2.5 is consistent with CMD; FFR < 0.80 and CFR ≤ 2.5 is consistent with epicardial disease alone or mixed epicardial obstruction and CMD. | In comparison to FFR, CFR has greater prognostic value for adverse cardiac events in the absence of obstructive CAD and may be more useful for risk stratification in the setting of suspected CMD.63,64 |
| Index of microvascular resistance (IMR) | Minimal achievable microvascular resistance | IMR ≥ 25 is consistent with CMD | Specific to microcirculation and independent of hemodynamic factors and epicardial stenosis,65 making it a specific and reproducible measure of CMD.66 |
| Acetylcholine (ACh) testing | Injection of intracoronary ACh followed by assessment of coronary artery diameter, ECG changes, and patient symptoms | Reduction of coronary artery diameter <90% with ACh is consistent with endothelial dysfunction; ≥90% is consistent with epicardial vasospasm; ECG changes and symptoms only is consistent with microvascular spasm | Safe method of assessing endothelial dysfunction and coronary vasospasm |
CAD, coronary artery disease; CMD, coronary microvascular dysfunction; ECG, electrocardiogram.
Definitions of ANOCA endotypes based on invasive CFT results can be found in Table 3.10,81 These were reviewed extensively in a recent publication.10
Table 3.
Definitions for endotypes of ANOCA based on invasive coronary function testing.
| ANOCA endotypes | Definitions based on invasive coronary function testing10,81 | Treatment |
|---|---|---|
| Coronary microvascular dysfunction | Based on coronary Doppler or thermodilution techniques:
|
|
| Microvascular spasm | Based on acetylcholine provocation testing:
|
|
| Endothelial dysfunction | Based on acetylcholine provocation testing:
|
|
| Epicardial coronary spasm | Based on acetylcholine provocation testing:
|
|
| Myocardial bridging |
|
|
ACE, angiotensin converting enzyme; ANOCA, angina with nonobstructive coronary arteries; ARB, angiotensin receptor blocker; CFR, coronary flow reserve; dFFR, diastolic FFR; ECG, electrocardiogram; hMR, hyperemic microvascular resistance; iFR, instantaneous wave-free ratio; IMR, index of microcirculatory resistance; IVUS, intravascular ultrasound; RFR, resting full-cycle ratio.
Although invasive coronary angiography alone lacks sensitivity for the detection of MB, detection may be enhanced through intravascular imaging such as optical coherence tomography (OCT) and intravascular ultrasound (IVUS).82 Generally, IVUS is preferred over OCT for the diagnosis of MB due to the ability to visualize systolic compression in real-time. In addition to OCT and IVUS, characterization with pressure-wire testing during intravenous dobutamine infusion may be performed to simulate physiologic stress. Pressure indices that focus on diastole are best for determining the functional significance of a bridge due to delayed diastolic expansion. These indices include diastolic FFR (must be calculated manually), instantaneous wave-free ratio (Philips), and resting full-cycle ratio (Abbott Vascular).48
Treatment
Overall, the evidence base for pharmacologic treatment of ANOCA is limited.83 Currently, the only pharmacologic interventions with moderate-quality evidence on quality of life improvement are ACE inhibitors and ranolazine, which are only effective in patients with a low CFR.84, 85, 86 If CMD is suspected, other medications to consider include calcium-channel blockers,84,85 beta-blockers, and statins (given that inflammation and thrombi are hypothesized to preferentially affect the small microvasculature of the heart in CMD).87 However, there are few large randomized control trials to guide treatment.88,89
If vasospasm is suspected, calcium-channel blockers can improve symptoms and long-term prognosis, but beta-blockers should be avoided as beta blockade may result in unopposed alpha-mediated vasoconstriction.90 Although epicardial vasospasm may be responsive to short-acting nitrates, there are concerns over the safety and effectiveness of long-term nitrate use.91 Of note, nitrates are generally ineffective in improving symptoms in CMD.92 For patients with symptomatic MB, beta-blockers are the first-line treatment, and calcium-channel blockers may be used. Short-acting nitrates may be helpful, especially in patients with vasospasm related to endothelial dysfunction in the bridge segment. Surgical unroofing is reserved for patients with MB who fail medical treatment and have refractory symptoms, and these patients should continue calcium-channel blockers postoperatively.83,93
Guidelines on pharmacologic therapy for ANOCA remain limited. The Trial on Reversing ENdothelial Dysfunction (TREND trial) was an early randomized controlled study from 1996 that found that quinalapril improves endothelial function in patients with CAD undergoing CFT.94 The recent CHAMP-CMD trial, in which 87 CMD and control patients were randomized to either amlodipine or ranolazine, showed significant improvement of symptoms in patients with CMD and reduced CFR with ranolazine compared to amlodipine.95 However, the EDIT-CMD trial, with 126 patients with CMD, randomized patients to diltiazem or placebo, showed no significant symptom improvement with diltiazem.96 Several WISE cohort subgroups were developed over the years, including WISE-CVD, WISE-HFpEF, and the ongoing Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD, which is evaluating the impact of intensive medical therapy with aspirin, statins, and ACE inhibitors versus usual care.88 In addition to medical therapy, lifestyle changes and risk factor modification including exercise, dietary adjustment, and cardiac rehabilitation may alleviate symptom burden and enhance quality of life in patients with ANOCA.5,79
It has been suggested that hormone replacement therapy can provide symptom relief in patients with ANOCA. However, the association between declining estrogen levels in the perimenopausal period and development of ANOCA is not clearly established,97 and there is limited evidence to support the routine use of hormone replacement therapy in this population.98
Establishing a definitive diagnosis with invasive CFT can enable targeted treatment and improve angina burden and quality of life.5,14 Additionally, studies suggest that a diagnosis may in and of itself be therapeutic by providing patients with both an understanding of the cause of their symptoms and the most appropriate medical therapy. In the CorMicA trial, patients who underwent invasive CFT were randomized to either disclosure of the results to the patient and care team or blinding of the results. Participants who received medical therapy guided by the results of CFT had improved angina and quality of life.25
In addition to improved symptom control, stratified medical treatment for ANOCA based on findings from invasive coronary angiography and adjunctive function testing has been found to be cost-effective. A study conducted by the United Kingdom’s National Health Service based on the CorMicA trial determined a 96% probability of cost-effectiveness for invasive testing that defines ANOCA subtypes and guides medical management, suggesting significant utility for such testing in women with suspected ANOCA.99 Another study published by the Mayo Clinic in the United States yielded similar results. This economic impact study evaluated 200 patients with ANOCA who underwent CFT and 200 patients with ANOCA who underwent standard coronary angiography and found that those who underwent CFT had significantly lower annual health care costs at $13,679 vs $37,804 at 2 years.100
Proposed approach for the evaluation and management of suspected IHD in women
Our proposed approach to the evaluation of IHD in women (Central Illustration) begins with assessment of the pretest probability of ASCVD. The initial evaluation should also include an assessment of anginal symptoms such as exertional chest pain and shortness of breath, and symptoms associated with ANOCA such as resting angina, exertional fatigue, and weakness. For diagnostic testing, we recommend functional testing with stress echocardiography, stress CMR, or PET stress testing for patients with a high pretest probability for IHD, and anatomic testing with CCTA for patients with a low pretest probability. If noninvasive testing shows a large burden of ischemia or obstructive CAD, patients should proceed with guideline-directed care for obstructive CAD. However, if noninvasive testing reveals no or minimal CAD or a low burden of ischemia, symptomatic patients should be started on empiric therapy for ANOCA and followed closely to assess for symptom relief. Patients with persistent symptoms despite goal-directed medical therapy for obstructive CAD or empiric therapy for ANOCA should undergo invasive coronary angiography and CFT to definitively determine the underlying diagnosis.
Challenges and future outlook
Even when using a systematic method for evaluating ANOCA, there may be challenges to determining a definitive diagnosis. Symptoms of ANOCA can overlap with those of obstructive CAD. Additionally, risk factors for ANOCA are not unique or well understood. Once ANOCA is suspected, there may be institutional, financial, and health care provider or patient-related barriers that could preclude additional testing. Furthermore, ACh for coronary administration remains an off-label use of an ophthalmic preparation, and no FDA-approved formulation exists.101, 102 Women are also more likely to have differences in pain perception103 and anatomical variations that can influence the diagnostic accuracy of cardiac testing modalities.58 Finally, even if ANOCA is diagnosed, there is limited guidance and awareness regarding pharmacologic treatment.
Despite these challenges, it is critical to recognize ANOCA given the prevalence and increased morbidity and mortality associated with the diagnosis.5,19, 20, 21, 22 The Determining the Cause of Coronary Vasomotor Disorders in Ischemia and No Obstructive Coronary Artery Disease (DISCOVER INOCA) prospective multicenter registry, which is currently enrolling, will determine the phenotypes found during invasive CFT and the long-term prognosis of each specific phenotype.104 It is essential to recognize the gender disparity that exists in the pathophysiology and disease course of IHD, and diagnostic guidelines and treatment strategies should be sex-specific for optimal patient outcomes.105
Conclusion
Many women suffer from persistent symptoms due to undiagnosed ANOCA, impacting quality of life and potentially leading to an increased risk for long-term cardiovascular events. Although there has been increasing awareness of ANOCA and advancement in diagnostic testing, the diagnostic approach to IHD in clinical practice focuses predominantly on obstructive CAD, and ANOCA is not routinely considered. Our proposed approach includes equal consideration of ANOCA, which involves assessment of pretest probability, strategic noninvasive testing, empiric treatment for CAD or ANOCA, and a recommendation for invasive CFT at the time of coronary angiography in persistently symptomatic patients. This strategy is consistent with existing expert guidelines and provides an algorithm that can be adopted by all clinicians caring for women with suspected IHD.
Declaration of competing interest
Samit M. Shah receives investigator-initiated research support from Abbott Vascular and the United States Food and Drug Administration.
Funding sources
Samit M. Shah is supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a financial assistance award (U01FD005938).
References
- 1.Reinhardt S.W., Lin C.-J., Novak E., Brown D.L. Noninvasive cardiac testing vs clinical evaluation alone in acute chest pain: a secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018;178(2):212–219. doi: 10.1001/jamainternmed.2017.7360s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan S.U., Yedlapati S.H., Lone A.N., et al. A comparative analysis of premature heart disease- and cancer-related mortality in women in the USA, 1999-2018. Eur Heart J Qual Care Clin Outcomes. 2022;8(3):315–323. doi: 10.1093/ehjqcco/qcaa099. [DOI] [PubMed] [Google Scholar]
- 3.Bybee K.A., Stevens T.L. Matters of the heart: cardiovascular disease in U.S. Women. Mo Med. 2013;110(1):65–70. [PMC free article] [PubMed] [Google Scholar]
- 4.Arora S., Stouffer G.A., Kucharska-Newton A.M., et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation. 2019;139(8):1047–1056. doi: 10.1161/CIRCULATIONAHA.118.037137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bairey Merz C.N.B., Pepine C.J., Walsh M.N., Fleg J.L. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135(11):1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mintz G.S., Matsumura M., Ali Z., Maehara A. Clinical utility of intravascular imaging: past, present, and future. JACC Cardiovasc Imaging. 2022;15(10):1799–1820. doi: 10.1016/j.jcmg.2022.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Herscovici R., Sedlak T., Wei J., Pepine C.J., Handberg E., Bairey Merz C.N. Ischemia and no obstructive coronary artery disease (INOCA): what is the risk? J Am Heart Assoc. 2018;7(17) doi: 10.1161/JAHA.118.008868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds H.R., Bairey Merz C.N., Berry C., et al. Coronary arterial function and disease in women with no obstructive coronary arteries. Circ Res. 2022;130(4):529–551. doi: 10.1161/CIRCRESAHA.121.319892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee B.-K., Lim H.-S., Fearon W.F., et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131(12):1054–1060. doi: 10.1161/CIRCULATIONAHA.114.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuels B.A., Shah S.M., Widmer R.J., et al. Comprehensive management of ANOCA, part 1-definition, patient population, and diagnosis: JACC state-of-the-art review. J Am Coll Cardiol. 2023;82(12):1245–1263. doi: 10.1016/j.jacc.2023.06.043. [DOI] [PubMed] [Google Scholar]
- 11.Khuddus M.A., Pepine C.J., Handberg E.M., et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) J Interv Cardiol. 2010;23(6):511–519. doi: 10.1111/j.1540-8183.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeiher A.M., Drexler H., Wollschläger H., Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83(2):391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]
- 13.Shaw L.J., Bairey Merz C.N., Pepine C.J., et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) study: part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47(3 Suppl):S4–S20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 14.Ford T.J., Yii E., Sidik N., et al. Ischemia and no obstructive coronary artery disease: prevalence and correlates of coronary vasomotion disorders. Circ Cardiovasc Interv. 2019;12(12) doi: 10.1161/CIRCINTERVENTIONS.119.008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulati M., Levy P.D., Mukherjee D., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;144(22):e368–e454. doi: 10.1161/CIR.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 16.Dalsgaard J.L., Hansen M.S., Thrysoee L., et al. Self-reported health and adverse outcomes among women living with symptoms of angina or unspecific chest pain but no diagnosis of obstructive coronary artery disease-findings from the DenHeart study. Eur J Cardiovasc Nurs. 2023;22(5):506–515. doi: 10.1093/eurjcn/zvac085. [DOI] [PubMed] [Google Scholar]
- 17.Parvand M., Cai L., Ghadiri S., et al. One-year prospective follow-up of women with INOCA and MINOCA at a Canadian women’s Heart Centre. Can J Cardiol. 2022;38(10):1600–1610. doi: 10.1016/j.cjca.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Gulati M., Khan N., George M., et al. Ischemia with no obstructive coronary artery disease (INOCA): a patient self-report quality of life survey from INOCA international. Int J Cardiol. 2023;371:28–39. doi: 10.1016/j.ijcard.2022.09.047. [DOI] [PubMed] [Google Scholar]
- 19.Widmer R.J., Samuels B., Samady H., et al. The functional assessment of patients with non-obstructive coronary artery disease: expert review from an international microcirculation working group. EuroIntervention. 2019;14(16):1694–1702. doi: 10.4244/EIJ-D-18-00982. [DOI] [PubMed] [Google Scholar]
- 20.Maddox T.M., Stanislawski M.A., Grunwald G.K., et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312(17):1754–1763. doi: 10.1001/jama.2014.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniel M., Agewall S., Caidahl K., et al. Effect of myocardial infarction with nonobstructive coronary arteries on physical capacity and quality-of-life. Am J Cardiol. 2017;120(3):341–346. doi: 10.1016/j.amjcard.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Hemingway H., Shipley M., Britton A., Page M., Macfarlane P., Marmot M. Prognosis of angina with and without a diagnosis: 11 year follow up in the Whitehall II prospective cohort study. BMJ. 2003;327(7420):895. doi: 10.1136/bmj.327.7420.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sedlak T.L., Lee M., Izadnegahdar M., Merz C.N.B., Gao M., Humphries K.H. Sex differences in clinical outcomes in patients with stable angina and no obstructive coronary artery disease. Am Heart J. 2013;166(1):38–44. doi: 10.1016/j.ahj.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Gdowski M.A., Murthy V.L., Doering M., Monroy-Gonzalez A.G., Slart R., Brown D.L. Association of isolated coronary microvascular dysfunction with mortality and major adverse cardiac events: a systematic review and meta-analysis of aggregate data. J Am Heart Assoc. 2020;9(9) doi: 10.1161/JAHA.119.014954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ford T.J., Stanley B., Good R., et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol. 2018;72(23 Pt A):2841–2855. doi: 10.1016/j.jacc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 26.National Academies of Sciences, Engineering, and Medicine, Division of Behavioral and Social Sciences and Education, Committee on National Statistics, Committee on Measuring Sex, Gender Identity, and Sexual Orientation . National Academies Press; 2022. Measuring Sex, Gender Identity, and Sexual Orientation.http://www.ncbi.nlm.nih.gov/books/NBK578625/ [PubMed] [Google Scholar]
- 27.O’Neil A., Scovelle A.J., Milner A.J., Kavanagh A. Gender/sex as a social determinant of cardiovascular risk. Circulation. 2018;137(8):854–864. doi: 10.1161/CIRCULATIONAHA.117.028595. [DOI] [PubMed] [Google Scholar]
- 28.Mileva N., Nagumo S., Mizukami T., et al. Prevalence of coronary microvascular disease and coronary vasospasm in patients with nonobstructive coronary artery disease: systematic review and meta-analysis. J Am Heart Assoc. 2022;11(7) doi: 10.1161/JAHA.121.023207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vancheri F., Longo G., Vancheri S., Henein M. Coronary microvascular dysfunction. J Clin Med. 2020;9(9):2880. doi: 10.3390/jcm9092880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Buono MG., Montone R.A., Camilli M., et al. Coronary microvascular dysfunction across the spectrum of cardiovascular diseases: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78(13):1352–1371. doi: 10.1016/j.jacc.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feenstra R.G.T., Boerhout C.K.M., Woudstra J., et al. Presence of coronary endothelial dysfunction, coronary vasospasm, and adenosine-mediated vasodilatory disorders in patients with ischemia and nonobstructive coronary arteries. Circ Cardiovasc Interv. 2022;15(8) doi: 10.1161/CIRCINTERVENTIONS.122.012017. [DOI] [PubMed] [Google Scholar]
- 32.Godo S., Takahashi J., Yasuda S., Shimokawa H. Endothelium in coronary macrovascular and microvascular diseases. J Cardiovasc Pharmacol. 2021;78(Suppl 6):S19–S29. doi: 10.1097/FJC.0000000000001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rehan R., Yong A., Ng M., Weaver J., Puranik R. Coronary microvascular dysfunction: a review of recent progress and clinical implications. Front Cardiovasc Med. 2023;10 doi: 10.3389/fcvm.2023.1111721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konst R.E., Meeder J.G., Wittekoek M.E., et al. Ischaemia with no obstructive coronary arteries. Neth Heart J. 2020;28(Suppl 1):66–72. doi: 10.1007/s12471-020-01451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ong P., Athanasiadis A., Borgulya G., Mahrholdt H., Kaski J.C., Sechtem U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA Study (Abnormal COronary vasomotion in patients with stable angina and unobstructed coronary arteries) J Am Coll Cardiol. 2012;59(7):655–662. doi: 10.1016/j.jacc.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 36.Konst R.E., Damman P., Pellegrini D., et al. Vasomotor dysfunction in patients with angina and nonobstructive coronary artery disease is dominated by vasospasm. Int J Cardiol. 2021;333:14–20. doi: 10.1016/j.ijcard.2021.02.079. [DOI] [PubMed] [Google Scholar]
- 37.Hubert A., Seitz A., Pereyra V.M., Bekeredjian R., Sechtem U., Ong P. Coronary artery spasm: the interplay between endothelial dysfunction and vascular smooth muscle cell hyperreactivity. Eur Cardiol. 2020;15 doi: 10.15420/ecr.2019.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sara J.D.S., Corban M.T., Prasad M., et al. Prevalence of myocardial bridging associated with coronary endothelial dysfunction in patients with chest pain and non-obstructive coronary artery disease. EuroIntervention. 2020;15(14):1262–1268. doi: 10.4244/EIJ-D-18-00920. [DOI] [PubMed] [Google Scholar]
- 39.Corban M.T., Hung O.Y., Eshtehardi P., et al. Myocardial bridging: contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J Am Coll Cardiol. 2014;63(22):2346–2355. doi: 10.1016/j.jacc.2014.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciampricotti R., el Gamal M. Vasospastic coronary occlusion associated with a myocardial bridge. Cathet Cardiovasc Diagn. 1988;14(2):118–120. doi: 10.1002/ccd.1810140213. [DOI] [PubMed] [Google Scholar]
- 41.Rinaldi R., Colucci M., Torre I., et al. Predicting the response to acetylcholine in ischemia or infarction with non-obstructive coronary arteries: the ABCD score. Atherosclerosis. 2024;391 doi: 10.1016/j.atherosclerosis.2024.117503. [DOI] [PubMed] [Google Scholar]
- 42.Diamond G.A., Forrester J.S. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300(24):1350–1358. doi: 10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 43.Dey S., Flather M.D., Devlin G., et al. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart. 2009;95(1):20–26. doi: 10.1136/hrt.2007.138537. [DOI] [PubMed] [Google Scholar]
- 44.Dean J., Cruz S.D., Mehta P.K., Merz C.N.B. Coronary microvascular dysfunction: sex-specific risk, diagnosis, and therapy. Nat Rev Cardiol. 2015;12(7):406–414. doi: 10.1038/nrcardio.2015.72. [DOI] [PubMed] [Google Scholar]
- 45.Reis S.E., Holubkov R., Conrad Smith A.J., et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141(5):735–741. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 46.Beltrame J.F., Crea F., Kaski J.C., et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38(33):2565–2568. doi: 10.1093/eurheartj/ehv351. [DOI] [PubMed] [Google Scholar]
- 47.Happach V.C., Delk G.T., Ganti L. Myocardial bridging, the hidden risk factor for ischemia. Mil Med. 2022;187(9-10):e1230–e1232. doi: 10.1093/milmed/usab042. [DOI] [PubMed] [Google Scholar]
- 48.Sternheim D., Power D.A., Samtani R., Kini A., Fuster V., Sharma S. Myocardial bridging: diagnosis, functional assessment, and management: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78(22):2196–2212. doi: 10.1016/j.jacc.2021.09.859. [DOI] [PubMed] [Google Scholar]
- 49.Murtaza G., Mukherjee D., Gharacholou S.M., et al. An updated review on myocardial bridging. Cardiovasc Revasc Med. 2020;21(9):1169–1179. doi: 10.1016/j.carrev.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 50.Wessel T.R., Arant C.B., McGorray S.P., et al. Coronary microvascular reactivity is only partially predicted by atherosclerosis risk factors or coronary artery disease in women evaluated for suspected ischemia: results from the NHLBI Women’s Ischemia Syndrome Evaluation (WISE) Clin Cardiol. 2007;30(2):69–74. doi: 10.1002/clc.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bajaj N.S., Osborne M.T., Gupta A., et al. Coronary microvascular dysfunction and cardiovascular risk in obese patients. J Am Coll Cardiol. 2018;72(7):707–717. doi: 10.1016/j.jacc.2018.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arnett D.K., Blumenthal R.S., Albert M.A., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. Circulation. 2019;140(11):e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasupathy S., Air T., Dreyer R.P., Tavella R., Beltrame J.F. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131(10):861–870. doi: 10.1161/CIRCULATIONAHA.114.011201. [DOI] [PubMed] [Google Scholar]
- 54.Ridker P.M., Buring J.E., Rifai N., Cook N.R. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 55.Sinha A., Dutta U., Demir O.M., et al. Rethinking false positive exercise electrocardiographic stress tests by assessing coronary microvascular function. J Am Coll Cardiol. 2024;83(2):291–299. doi: 10.1016/j.jacc.2023.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodriguez Lozano P.F., Rrapo Kaso E., Bourque J.M., et al. Cardiovascular imaging for ischemic heart disease in women: time for a paradigm shift. JACC Cardiovasc Imaging. 2022;15(8):1488–1501. doi: 10.1016/j.jcmg.2022.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thai J.N., Abidov A., Jie T., Krupinski E.A., Kuo P.H. Nuclear myocardial perfusion imaging versus stress echocardiography in the preoperative evaluation of patients for kidney transplantation. J Nucl Med Technol. 2015;43(3):201–205. doi: 10.2967/jnmt.115.159400. [DOI] [PubMed] [Google Scholar]
- 58.Parwani P., Mohammad A., Liberman Y., Litmanovich D.E. Approach to imaging ischemia in women. J Thorac Imaging. 2023;38(4):204–211. doi: 10.1097/RTI.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 59.Kim M.N., Kim S.A., Kim Y.H., et al. Head to head comparison of stress echocardiography with exercise electrocardiography for the detection of coronary artery stenosis in women. J Cardiovasc Ultrasound. 2016;24(2):135–143. doi: 10.4250/jcu.2016.24.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haase R., Schlattmann P., Gueret P., et al. Diagnosis of obstructive coronary artery disease using computed tomography angiography in patients with stable chest pain depending on clinical probability and in clinically important subgroups: meta-analysis of individual patient data. BMJ. 2019;365:l1945. doi: 10.1136/bmj.l1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sicari R., Nihoyannopoulos P., Evangelista A., et al. Stress echocardiography expert consensus statement—executive summary: European Association of Echocardiography (EAE) (a registered branch of the ESC) Eur Heart J. 2009;30(3):278–289. doi: 10.1093/eurheartj/ehn492. [DOI] [PubMed] [Google Scholar]
- 62.Michelsen M.M., Pena A., Mygind N.D., et al. Coronary microvascular dysfunction and myocardial contractile reserve in women with angina and no obstructive coronary artery disease. Echocardiography. 2018;35(2):196–203. doi: 10.1111/echo.13767. [DOI] [PubMed] [Google Scholar]
- 63.Patterson R.E., Churchwell K.B., Eisner R.L. Diagnosis of coronary artery disease in women: roles of three dimensional imaging with magnetic resonance or positron emission tomography. Am J Card Imaging. 1996;10(1):78–88. [PubMed] [Google Scholar]
- 64.Schwaiger M. Myocardial perfusion imaging with PET. J Nucl Med. 1994;35(4):693–698. [PubMed] [Google Scholar]
- 65.Bateman T.M., Heller G.V., McGhie A.I., et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol. 2006;13(1):24–33. doi: 10.1016/j.nuclcard.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Nakazato R., Heo R., Leipsic J., Min J.K. CFR and FFR assessment with PET and CTA: strengths and limitations. Curr Cardiol Rep. 2014;16(5):484. doi: 10.1007/s11886-014-0484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dorbala S., Di Carli M.F. Cardiac PET perfusion: prognosis, risk stratification, and clinical management. Semin Nucl Med. 2014;44(5):344–357. doi: 10.1053/j.semnuclmed.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monroy-Gonzalez A.G., Tio R.A., de Groot J.C., et al. Long-term prognostic value of quantitative myocardial perfusion in patients with chest pain and normal coronary arteries. J Nucl Cardiol. 2019;26(6):1844–1852. doi: 10.1007/s12350-018-1448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pagidipati N.J., Hemal K., Coles A., et al. Sex differences in functional and CT angiography testing in patients with suspected coronary artery disease. J Am Coll Cardiol. 2016;67(22):2607–2616. doi: 10.1016/j.jacc.2016.03.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thomson L.E.J., Wei J., Agarwal M., et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8(4) doi: 10.1161/CIRCIMAGING.114.002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.SCOT-HEART Investigators, Newby D.E., Adamson P.D., et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924–933. doi: 10.1056/NEJMoa1805971. [DOI] [PubMed] [Google Scholar]
- 72.Hoffmann U., Ferencik M., Udelson J.E., et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) Circulation. 2017;135(24):2320–2332. doi: 10.1161/CIRCULATIONAHA.116.024360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rovera C., Moretti C., Bisanti F., De Zan G., Guglielmo M. Myocardial bridging: review on the role of coronary computed tomography angiography. J Clin Med. 2023;12(18):5949. doi: 10.3390/jcm12185949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wei J., Mehta P.K., Johnson B.D., et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv. 2012;5(6):646–653. doi: 10.1016/j.jcin.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pepine C.J., Ferdinand K.C., Shaw L.J., et al. Emergence of nonobstructive coronary artery disease: a woman’s problem and need for change in definition on angiography. J Am Coll Cardiol. 2015;66(17):1918–1933. doi: 10.1016/j.jacc.2015.08.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.AlBadri A., Bairey Merz C.N., Johnson B.D., et al. Impact of abnormal coronary reactivity on long-term clinical outcomes in women. J Am Coll Cardiol. 2019;73(6):684–693. doi: 10.1016/j.jacc.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Everaars H., de Waard G.A., Driessen R.S., et al. Doppler flow velocity and thermodilution to assess coronary flow reserve: a head-to-head comparison with [15O]H2O PET. JACC Cardiovasc Interv. 2018;11(20):2044–2054. doi: 10.1016/j.jcin.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 78.Fu B., Wei X., Lin Y., Chen J., Yu D. Pathophysiologic basis and diagnostic approaches for ischemia with non-obstructive coronary arteries: a literature review. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.731059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hansen B., Holtzman J.N., Juszczynski C., et al. Ischemia with no obstructive arteries (INOCA): a review of the prevalence, diagnosis and management. Curr Probl Cardiol. 2023;48(1) doi: 10.1016/j.cpcardiol.2022.101420. [DOI] [PubMed] [Google Scholar]
- 80.Takahashi T., Gupta A., Samuels B.A., Wei J. Invasive coronary assessment in myocardial ischemia with no obstructive coronary arteries. Curr Atheroscler Rep. 2023;25(10):729–740. doi: 10.1007/s11883-023-01144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hwang D., Park S.H., Koo B.K. Ischemia with nonobstructive coronary artery disease: concept, assessment, and management. JACC Asia. 2023;3(2):169–184. doi: 10.1016/j.jacasi.2023.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Santucci A., Jacoangeli F., Cavallini S., d’Ammando M., de Angelis F., Cavallini C. The myocardial bridge: incidence, diagnosis, and prognosis of a pathology of uncertain clinical significance. Eur Heart J Suppl. 2022;24(Suppl I):I61–I67. doi: 10.1093/eurheartjsupp/suac075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smilowitz N.R., Prasad M., Widmer R.J., et al. Comprehensive management of ANOCA, part 2-program development, treatment, and research initiatives: JACC state-of-the-art review. J Am Coll Cardiol. 2023;82(12):1264–1279. doi: 10.1016/j.jacc.2023.06.044. [DOI] [PubMed] [Google Scholar]
- 84.Ong P., Athanasiadis A., Sechtem U. Pharmacotherapy for coronary microvascular dysfunction. Eur Heart J Cardiovasc Pharmacother. 2015;1(1):65–71. doi: 10.1093/ehjcvp/pvu020. [DOI] [PubMed] [Google Scholar]
- 85.Ford T.J., Berry C. How to diagnose and manage angina without obstructive coronary artery disease: lessons from the British Heart Foundation CorMicA trial. Interv Cardiol. 2019;14(2):76–82. doi: 10.15420/icr.2019.04.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Turgeon R.D., Pearson G.J., Graham M.M. Pharmacologic treatment of patients with myocardial ischemia with no obstructive coronary artery disease. Am J Cardiol. 2018;121(7):888–895. doi: 10.1016/j.amjcard.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 87.Haverich A., Boyle E.C. Atherosclerosis Pathogenesis and Microvascular Dysfunction. Springer; 2019. Risk factors and prevention in light of atherosclerosis being a microvascular disease; pp. 75–95. [DOI] [Google Scholar]
- 88.Handberg E.M., Merz C.N.B., Cooper-Dehoff R.M., et al. Rationale and design of the Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD (WARRIOR) trial. Am Heart J. 2021;237:90–103. doi: 10.1016/j.ahj.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 89.Montone R.A., Niccoli G., Lanza G.A., Crea F. Personalized treatment of myocardial infarction and non-obstructive coronary arteries: an unmet need in a high-risk population. Eur Heart J. 2018;39(35):3335. doi: 10.1093/eurheartj/ehy305. [DOI] [PubMed] [Google Scholar]
- 90.Lanza G.A., Maseri A. Coronary artery spasm. Curr Treat Options Cardiovasc Med. 2000;2(1):83–90. doi: 10.1007/s11936-000-0031-0. [DOI] [PubMed] [Google Scholar]
- 91.Takahashi J., Nihei T., Takagi Y., et al. Prognostic impact of chronic nitrate therapy in patients with vasospastic angina: multicentre registry study of the Japanese coronary spasm association. Eur Heart J. 2015;36(4):228–237. doi: 10.1093/eurheartj/ehu313. [DOI] [PubMed] [Google Scholar]
- 92.Schindler T.H., Dilsizian V. Coronary microvascular dysfunction: clinical considerations and noninvasive diagnosis. JACC Cardiovasc Imaging. 2020;13(1 Pt 1):140–155. doi: 10.1016/j.jcmg.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 93.Shah S.M., Quesada O., Henry T.D. The challenge of myocardial bridging. J Soc Cardiovasc Angiogr Interv. 2023;2(2) doi: 10.1016/j.jscai.2022.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mancini G.B., Henry G.C., Macaya C., et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) study. Circulation. 1996;94(3):258–265. doi: 10.1161/01.cir.94.3.258. [DOI] [PubMed] [Google Scholar]
- 95.Sinha A., Rahman H., Douiri A., et al. ChaMP-CMD: a phenotype-blinded, randomized controlled, cross-over trial. Circulation. 2024;149(1):36–47. doi: 10.1161/CIRCULATIONAHA.123.066680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jansen T.P.J., Konst R.E., de Vos A., et al. Efficacy of diltiazem to improve coronary vasomotor dysfunction in ANOCA: the EDIT-CMD randomized clinical trial. JACC Cardiovasc Imaging. 2022;15(8):1473–1484. doi: 10.1016/j.jcmg.2022.03.012. [DOI] [PubMed] [Google Scholar]
- 97.Tunc E., Eve A.A., Madak-Erdogan Z. Coronary microvascular dysfunction and estrogen receptor signaling. Trends Endocrinol Metab. 2020;31(3):228–238. doi: 10.1016/j.tem.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 98.Merz C.N.B., Olson M.B., McClure C., et al. A randomized controlled trial of low-dose hormone therapy on myocardial ischemia in postmenopausal women with no obstructive coronary artery disease: results from the National Institutes of Health/National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Am Heart J. 2010;159(6):987.e1–987.e7. doi: 10.1016/j.ahj.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heggie R., Briggs A., Stanley B., et al. Stratified medicine using invasive coronary function testing in angina: a cost-effectiveness analysis of the British Heart Foundation CorMicA trial. Int J Cardiol. 2021;337:44–51. doi: 10.1016/j.ijcard.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 100.Ahmad A., Corban M.T., Moriarty J.P., et al. Coronary reactivity assessment is associated with lower health care–associated costs in patients presenting with angina and nonobstructive coronary artery disease. Circ Cardiovasc Interv. 2023;16(7) doi: 10.1161/CIRCINTERVENTIONS.122.012387. [DOI] [PubMed] [Google Scholar]
- 101.Montone R.A., Gurgoglione F.L., Del Buono M.G., et al. Interplay between myocardial bridging and coronary spasm in patients with myocardial ischemia and non-obstructive coronary arteries: pathogenic and prognostic implications. J Am Heart Assoc. 2021;10(14) doi: 10.1161/JAHA.120.020535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ong P., Athanasiadis A., Borgulya G., et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation. 2014;129(17):1723–1730. doi: 10.1161/CIRCULATIONAHA.113.004096. [DOI] [PubMed] [Google Scholar]
- 103.Mehta P.K., Wei J., Shufelt C., Quesada O., Shaw L., Bairey Merz C.N. Gender-related differences in chest pain syndromes in the frontiers in CV medicine special issue: sex & gender in CV medicine. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.744788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shah SM, Tremmel JA, Henry TD, et al. Determining the cause of coronary vasomotor disorders in patients with ischemia and nonobstructive coronary arteries: design and rationale of the DISCOVER INOCA prospective, multicenter registry. J Soc Cardiovasc Angiogr Interv. 2024;3(6):102046. doi: 10.1016/j.jscai.2024.102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carberry J., Aubiniere-Robb L., Kamdar A., Lomholt-Welch H., Berry C. Reappraising ischemic heart disease in women. Rev Cardiovasc Med. 2023;24(4):118. doi: 10.31083/j.rcm2404118. [DOI] [PMC free article] [PubMed] [Google Scholar]