Abstract
The biosynthesis of many bacterial siderophores employs a member of a family of ligases that have been defined as NRPS-independent siderophore (NIS) synthetases. These NIS synthetases use a molecule of ATP to produce an amide linkage between a carboxylate and an amine. Commonly used carboxylate substrates include citrate or α-ketoglutarate, or derivatives thereof, while the amines are often hydroxamate derivatives of lysine or ornithine, or their decarboxylated forms cadaverine and putrescine. Enzymes that employ three substrates to catalyze a reaction may proceed through alternate mechanisms. Some enzymes use sequential mechanisms in which all three substrates bind prior to any chemical steps. In such mechanisms, substrates can bind in a random, ordered, or mixed fashion. Alternately, other enzymes employ a ping-pong mechanism in which a chemical step occurs prior to the binding of all three substrates. Here we describe an enzyme assay that will distinguish among these different mechanisms for the NIS synthetase, using IucA, an enzyme involved in the production of aerobactin, as the model system.
Keywords: Siderophore biosynthesis, NRPS-independent siderophores, Aerobactin, NIS Syntheses, Mechanistic Enzymology
1. Introduction
When faced with a low-iron environment, many microorganisms produce the biosynthetic and uptake machinery for siderophores, small molecule iron chelators, that are secreted into the environment to acquire iron (Barry and Challis, 2009; Hider and Kong, 2010; Lamb, 2015). Siderophores are often classified chemically into different families on the basis of the biosynthetic pathway that is used for their production and for the chemical functional groups that are involved in the coordination of iron. A common mechanism for production of peptide siderophores employs the nonribosomal peptide synthetases (NRPSs), a family of modular, assembly line proteins that convert amino acids into small peptide chains. Independent of the ribosomes, NRPS products often contain non-proteinogenic amino acids and chemical modifications that are incorporated into the final product (Miethke and Marahiel, 2007). Many NRPS siderophores additionally contain aryl caps composed of salicylic acid (2-hydroxybenzoic acid) or 2,3-dihydroxybenzoic acid (Quadri, 2000; Kudo et al., 2019) as well as oxazoline and thiazoline heterocycles that are formed by the cyclodehydration of serine, threonine, and cysteine residues.
In contrast to the NRPS derived siderophores, a second family of compounds have been termed NRPS-independent siderophores (NISs) (Challis, 2005; Kadi et al., 2007; Oves-Costales et al., 2009). NISs generally contain one or more amine compounds that are converted to a hydroxamate through the activity of a hydroxylase and an acyl transferase. Often, the pathway also contains a decarboxylase resulting in a three-protein cascade of that converts lysine or ornithine to cadaverine or putrescine, respectively, followed by the N-hydroxylation and N-acylation to produce the final hydroxamate. The hydroxamates are then installed on a carboxylate substrate, often citrate, α-ketoglutarate, or succinate. These combined hydroxamate, hydroxyls, and carboxylate groups on different NISs facilitate iron binding. Common NISs include desferrioxamine (Ronan et al., 2018; Yang et al., 2022; Yang et al., 2023), petrobactin (Nusca et al., 2012), staphyloferrin (Tang et al., 2020), and aerobactin (Carbonetti and Williams, 1984; Bailey et al., 2016; Bailey et al., 2018).
Also shared within the NIS biosynthetic gene cluster is an NIS synthetase, a ligase that uses ATP to catalyze the formation of an amide bond between an amine substrate bearing the hydroxamate and the carboxylate substrate (Challis, 2005; Kadi et al., 2007; Oves-Costales et al., 2009). These enzymes catalyze a two-step reaction involving the formation of an acyl-adenylate intermediate and inorganic pyrophosphate (PPi). The amine then attacks the adenylate to displace AMP and form the amide linkage. The NIS synthetase enzymes therefore consume three substrates, ATP, the carboxylate, and the amine, and produce three products, AMP, PPi, and the final amide product. Aerobactin, an NIS that is produced by many Gram-negative bacteria (Carbonetti and Williams, 1984; Ford et al., 1986), including hypervirulent strains of Klebsiella pneumoniae (Nassif and Sansonetti, 1986), is a well-characterized NIS biosynthetic pathway that contains two NIS synthetases, IucA and IucC. Aerobactin is produced from primary metabolites lysine and citrate through the actions of the hydroxylase IucD, the acetyltransferase IucB, and the two NIS synthetases (Figure 1). Combined, the four-step pathway results in the production of aerobactin that is secreted from the cell. The ability to produce aerobactin is a critical virulence factor for hypervirulent strains of K. pneumoniae (Russo et al., 2014; Russo et al., 2015; Russo and Gulick, 2019).
Figure 1.
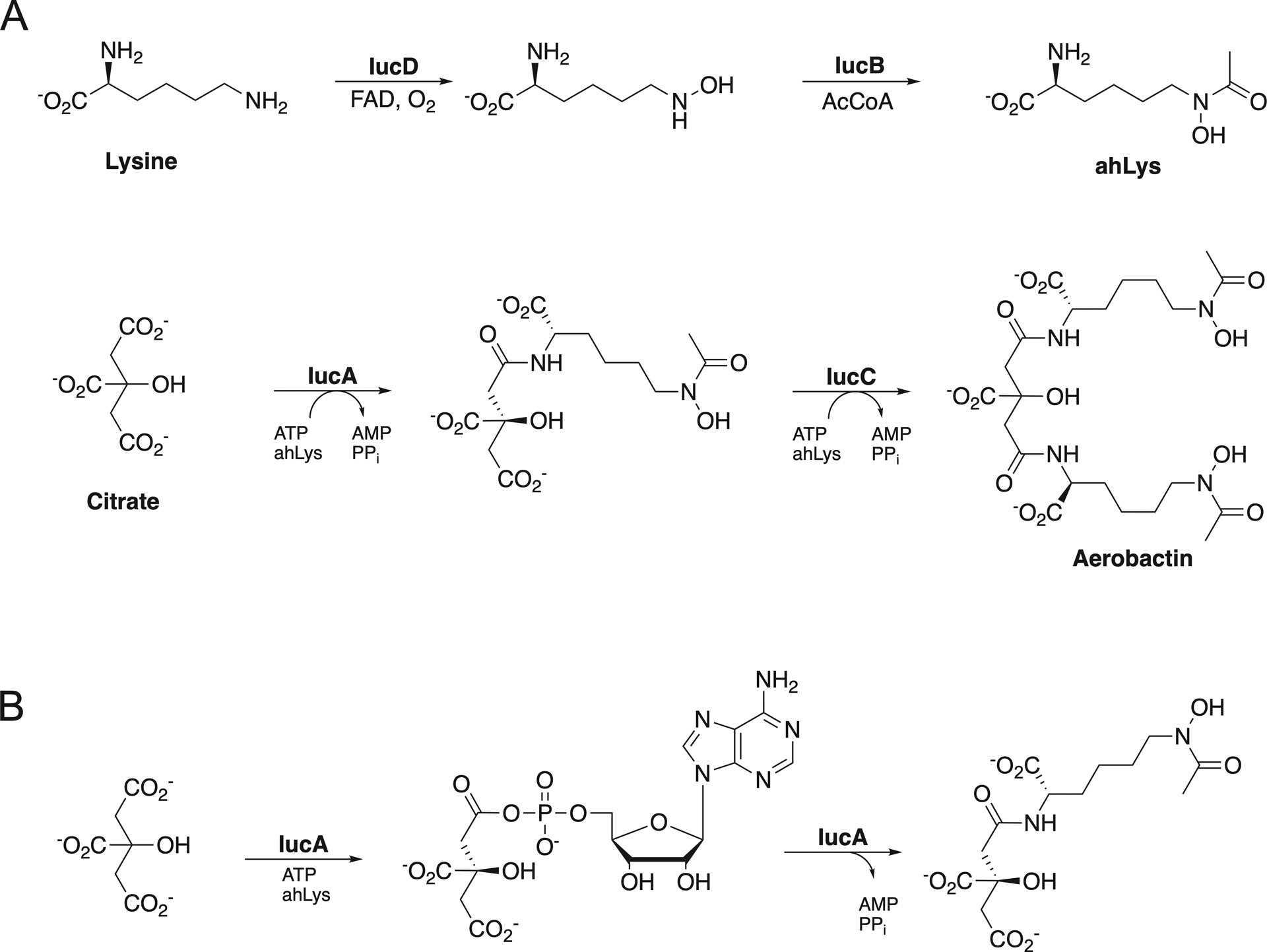
Aerobactin Biosynthesis. A. Biosynthesis of aerobactin from lysine and citrate. The hydroxylase IucD and acetyl transferase IucB produce N6-acetyl-N6-hydroxylysine (ahLys), which is then installed in two reactions catalyzed by the NIS synthetases IucA and IucC. B. The NIS synthetase IucA catalyzes formation of citryl-ahLys through a two-step ATP-dependent reaction.
Enzymes that use three substrates can employ different mechanisms that can broadly be divided into two general classes. Enzymes with sequential mechanisms bind all three substrates prior to any chemical steps. The binding order for the substrates may be random, ordered, or partially ordered, with one substrate binding in a fixed position (first, second, last) and the order of binding of the remaining two being unimportant. In contrast, some three-substrate enzymes employ a ping-pong mechanism in which one or two substrates fail to bind prior to the first chemical step. Some ligases, such as the adenylate-forming family to which the NRPS adenylation domains belong, use a bi-uni-uni-bi reaction mechanism (Gulick, 2009). For example, AMP-forming acyl-CoA synthetases catalyze the formation of acetyl-CoA by first binding the carboxylate and ATP, which are converted to an acyl-AMP intermediate and PPi. Following release of PPi, CoA binds and the enzyme produces acetyl- or propionyl-CoA and AMP, which are both released from the enzyme (Horswill and Escalante-Semerena, 2002; Reger et al., 2007; Wu et al., 2008).
Here, we present a kinetic approach to distinguish among the different catalytic mechanisms of the three substrate NIS synthetase enzymes. The approach involves initial velocity measurements at multiple substrate concentrations, which are combined to distinguish among the different approaches. Methods are described for protein purification, kinetic measurements using an assay that couples AMP formation to NADH oxidation through three coupling enzymes (Wu and Hill, 1993), and the approach for plotting the initial rates to determine the mechanism of substrate binding. This technique has been used with the NIS synthetase IucA to identify an ordered binding mechanism, with ATP binding first, followed by citrate, and ahLys (Mydy et al., 2020). The use of this technique to characterize other NIS synthetases should confirm a consistent ordered mechanism among different members of this family.
2. Protein production and purification
For enzyme assays, proteins should be purified to homogeneity, allowing accurate determination of concentration and ensuring consistent results can be obtained across different experiments. While the general strategy adopted will be specific to a particular protein, an overall strategy that has proven successful for numerous enzymes, including multiple NIS synthetases, employs a pET plasmid that allows for the production of a protein harboring a 6 × histidine purification tag and a protease cleavage site that allows for the removal of the tag. In our laboratory, a TEV protease site has been incorporated into the pET15 plasmid. After lysis of the cell paste, the lysate is clarified via high-speed ultracentrifugation, passed through a immobilized metal ion affinity chromatography (IMAC) column charged with Ni2+, and eluted with increasing concentrations of imidazole. The eluted protein is dialyzed to remove imidazole, and simultaneously treated with TEV protease to cleave the tag. The cleaved, partially purified protein is then passed over the IMAC column a second time, removing contaminants, uncleaved enzyme, cleaved His-tag, and the TEV protease. Finally, the protein is polished through a size exclusion chromatography step to remove aggregates. The protein is dialyzed into final storage buffer and frozen at −80°C in small aliquots until needed for kinetic experiments.
2.1. Equipment
New Brunswick Innova 44R shaker/incubators
BioRad NGC Quest Chromatograph Systems chromatography system
2.2. Buffers, Strains, and Reagents
E. coli BL21(DE3) cell line containing pET15TEV-iucA expression plasmid
LB Media, autoclaved (10 g tryptone, 10 g NaCl, 5 g yeast extract, per L)
Lysis Buffer (50 mM HEPES, pH 7.5 at 4°C, 250 mM NaCl, 20 mM imidazole, 0.2 mM triscarboxyethylphosphine (TCEP), and 10 % glycerol)
Elution Buffer (50 mM HEPES, pH 7.5 at 4°C, 250 mM NaCl, 300 mM imidazole, 0.2 mM TCEP, and 10 % glycerol)
Storage buffer (50 mM HEPES, pH 7.5 at 4°C, 150 mM NaCl, 0.2 mM TCEP)
Isopropyl-β-D-thiogalactoside (IPTG), 100 mM stock in water, filter sterilized
5 mL HisTrap HP chromatography IMAC column, Cytiva Life Sciences
Superdex-200 16/600 Size Exclusion Chromatography Column, Cytiva Life Sciences
TEV protease, isolated as described (Tropea et al., 2009; Raran-Kurussi et al., 2017)
2.3. Procedure
Protein expression
-
1
Grow an overnight starter culture of expression cells in 5 ml sterile LB media containing 100 μg/ml ampicillin in a sterile 15 ml culture tube at 37° C in a shaker.
-
2
Inoculate 1 L of sterile LB media containing 100 μg/ml ampicillin in a 2 L baffled shaker flask. Shake cells at 37°C for 3–5 h, monitor growth at OD600.
-
3
At an OD600 of 0.6 – 0.8, chill cells on ice for 10 min, cool incubator to 16 °C. Prior to returning cells to the incubator, induce protein expression with 500 μM sterile IPTG. Continue protein expression overnight.
-
4
After 16–18 hr of growth and expression, harvest cells by centrifugation at 6000 × g for 15 min. The cell paste can be frozen and stored at −80 °C until use, or directly continued into purification.
Purification
-
5
Cell paste is resuspended in a lysis buffer at a concentration of ~5–10 ml buffer per 1 g paste. Cells are lysed by sonication at 4 °C at 50% amplitude, using 20 cycles of 30 s on, 45 s off. The cell lysate is clarified by ultracentrifugation at 185×103 g for 45 min. The supernatant is then filtered over a 0.45 μm polysulfone membrane to prepare for the chromatographic purification.
-
6
Clarified lysate is passed over a pre-equilibrated 5 mL HisTrap HP column. Upon loading the full lysate, the column is washed with lysis buffer until absorbance returns to baseline, approximately 10 column volumes.
-
7
The column is then washed with lysis buffer containing 50 mM imidazole. If an FPLC system is used in which the A line contains lysis buffer and the B line contains the elution buffer, this wash step can be performed using 90 % A and 10 % B, which combines to 50 mM imidazole. The wash will result in the elution of several weakly bound contaminating proteins and should be monitored for return of the absorbance to baseline.
-
8
Elute the protein with 100 % B, resulting in the elution of the bound tagged protein.
-
9
Fractions containing the eluted protein should be identified through SDS-PAGE, the chromatographic trace, or UV absorbance. The fractions should be combined, concentrated to ~5–10 ml, and dialyzed against Lysis Buffer at 4 °C to remove imidazole. The protein is then dialyzed for 2–4 hr against 500 mL of buffer. At this stage, the dialysis bag is carefully opened, and TEV protease added at a ratio of approximately 1:100 mg:mg with target protein. The dialysis bag is then be transferred to a fresh 500 ml of buffer for dialysis overnight.
-
10
The following morning, harvest the protein from the dialysis bag, clarify the sample via centrifugation in a 4 °C microcentrifuge or filter through 0.45 μm polysulfone membrane, and load the protein onto a 5 mL HisTrap HP column that has been equilibrated with lysis buffer. Untagged protein will pass through the column to be collected. The column can be washed and eluted with 10 % and 100 % buffer B; fractions from these steps can be monitored by SDS-PAGE to assess cleavage efficiency.
-
11
Fractions containing the untagged protein can be combined and concentrated.
-
12
The protein should be passed over a size exclusion chromatography column, such as Superdex-S200 16/600. Protein should be monitored for homogeneity and elution as a single peak. Comparison with standards of known molecular weight and oligomeric status can inform understanding of the oligomeric state of the enzyme. The column should be preequilibrated with final storage buffer. Eluted protein can be concentrated to desired final concentration and frozen in small aliquots by directly pipetting into liquid nitrogen, and storage at −80 °C.
2.4. Notes
The expression system should be tailored to the protein target and may involve alternate expression plasmids or host strains. Similarly, the strategy for cell growth and protein induction may not be uniform for all NIS synthetases. The strategy described here has worked for many bacterial enzymes involved in siderophore biosynthesis that have been studied in our lab.
Purification and storage buffers will be protein-specific but should be designed to maintain protein solubility. The inclusion of additives such as reducing agents (dithiothreitol (DTT) and TCEP) or glycerol can be tested empirically for improvement in protein yields and stability.
2. Purification can be done manually in the absence of a FPLC system, using gravity flow of lysate and protein samples over the column. Flow-through and elution fractions should be analyzed via SDS-PAGE to identify protein.
3. NADH coupled adenylation assay
To rapidly characterize enzyme turnover in a generalizable format that can be employed for all NIS synthetases, an AMP-detection is assay is used that combines three enzymes, myokinase, pyruvate kinase, and lactate dehydrogenase, to couple the production of a single molecule of AMP to the conversion of two molecules of NADH to NAD+. The disappearance of NADH is monitored spectrophotometrically because of its absorbance at 340 nm. The coupled reaction scheme involves the reaction of the AMP product with ATP to form two molecules of ADP that is catalyzed by myokinase. The ADP is a substrate for pyruvate kinase, which uses a molecule of phosphoenolpyruvate to form pyruvate and ATP. Finally, the pyruvate molecule is a substrate for lactate dehydrogenase that couples reduction of pyruvate to lactate with oxidation of NADH, to form NAD+. The reaction is monitored in real-time and is readily adaptable to a 96-well plate reader with UV absorbance capabilities. The reaction is monitored for linear initial rate readings, that can be readily converted via an extinction coefficient of ε=6220 M−1 cm−1 to initial rate in units of μM/min.
3.1. Equipment
Biotek Synergy 4 Plate reader
96-well flat bottom UV transparent microplates (Caplugs)
3.2. Reagents
Common reagents
1 U/L Pyruvate Kinase, Sigma-Aldrich
1 U/L Lactate Dehydrogenase, Sigma-Aldrich
10 U/L Myokinase, Sigma-Aldrich
300 mM Phosphoenolpyruvate, Sigma-Aldrich
10 mM NADH, Sigma-Aldrich
1 M MOPS, pH 7.5
1 M MgCl2
25 mM ATP
Reagents specific to the NIS Synthetase under investigation
50 mM Citrate
50 mM ahLys (Bailey et al., 2018)
Purified NIS synthetase from step 2, diluted to 10 × concentration, here 10 μM.
3.3. Procedure
Substrate concentrations are then combined such that the varied substrate is provided in a dilution series and combined with the two remaining substrates that are held at a constant ratio in relation to their KM values. It is therefore necessary to determine preliminary apparent KM values through conventional kinetic approaches. An appropriate volume of master mix is produced that will be combined with enzyme and substrates in the multiwell-plate. The following protocol employs six substrate concentrations for the varied substrate, for which double reciprocal plots are generated at five concentrations of the fixed substrates. As each data point is measured in triplicate, for each line that is generated, it is necessary to generate a master mix for 18 reactions. For each reaction, 80 μl of master mix will be provided to initiate the reaction, combining to 1.44 ml of master mix. To ensure adequate material, 1.5 ml of master mix is created for each line that is generated.
For IucA, apparent KM values were determined to be 50 μM for ATP, 540 μM for citrate, and 790 μM for ahLys. A representative recipe is provided (Table 1) to generate the master mix and perform the reaction for varying ATP and the lowest concentrations of citrate and ahLys. Four more master mixes will be generated to be used to produce the first series of plots for varied citrate and constant values of ATP and ahLys at a constant ratio relative to their apparent KM value.
Create the master mix solution that will be combined with the varied substrate. This recipe produces a master mix for a final reaction concentration 54 μM citrate and 79 μM ahLys, roughly 0.1 × apparent KM value.
Thaw enzyme rapidly then store on ice. Dilute enzyme stock at 10 × working concentration, here 10 μM.
Create varied substrate dilution series at six 10 × concentrations that bracket the apparent KM value. For ATP, with KM value of 50 μM, create ATP stock concentrations at 250, 375, 500, 800, and 2000 μM.
Arrange three columns of the 96 well plate to perform one series of replicates for the double reciprocal plot. The first three wells in the top row receive 10 μl of enzyme added to one side of the well, and 10 μl of water to the other. These three wells will serve as a negative control, or blank. To the remainder of the wells in the column add 10 μl of enzyme and 10 μl of the increasing concentrations of ATP, such that 10 μl of the 25 μM ATP is added to row 2, 10 μl of 37.5 μM ATP is added to row 3, and so on. Be careful not to allow enzyme and substrate to mix so that the reaction is initiated by addition of 80 μl of the master mix. In a separate column add 250 μl of master mix to be added later for triplicate wells of reaction. Incubate plate in plate reader for 5 minutes to allow reaction set up to achieve 37 °C.
To each well, as rapidly as possible, add 80 μl of master mix with a multichannel pipettor, allowing the substrate, enzyme, and master mix components to mix.
Initiate the reaction and monitor the reaction for 10 min. When the identity of the blank wells are provided, the plate reader provides corrected values for the ΔOD/min. These values can be converted into an initial rate that can be used for generation of the double reciprocal plot.
Table 1.
Master Mix recipe
| Component | Stock Concentration | Volume Stock | Master Mix Final Concentrationa |
|---|---|---|---|
| Pyruvate Kinase | 1000 U/ml | 18.8 μl | 12.5 U/ml |
| Myokinase | 10000 U/ml | 1.9 μl | 12.5 U/ml |
| Lactate Dehydrogenase | 1000 U/ml | 18.8 μl | 12.5 U/ml |
| Phosphoenolpyruvate | 300 mM | 18.8 μl | 3.75 mM |
| NADH | 10 mM | 150 μl | 1000 μM |
| MOPS | 1 M | 375 μl | 250 mM |
| MgCl2 | 1 M | 28.1 μl | 18.74 mM |
| Citrate (0.1 × KM) | 25 mM | 4.1 μl | 67.5 μM |
| ahLys (0.1 × KM) | 25 mM | 5.9 μl | 98.75 μM |
| Water | 878.6 μl | ||
| Total Volume | 1500 μl |
Final concentration in master mix is 1.25 × concentration in the final reaction.
3.4. Notes
While modern kinetic analysis uses least squares fitting for the determination of kinetic constants, the approach here with double reciprocal, Lineweaver Burk plots, allows for facile graphical representation of the data that is indicative of different mechanisms, as described in the next section.
Initial values for apparent KM values should be determined via standard techniques by saturating two of the three substrates and varying the third. Initial velocity rates are plotted against the concentration of the varied substrate. These values can be used for defining the fixed concentrations of non-varied substrates at a constant ratio relative to their KM value.
For reactions lacking a nucleophile like ahLys, a surrogate nucleophile like 50–150 mM hydroxylamine could be also used.
For reactions requiring a specific buffer, MOPS could be replaced with a buffer like 100–250 mM HEPES pH 7.5.
4. Kinetic analysis to distinguish among potential mechanisms
The initial rate plots generated in section 3 are then used to identify the kinetic mechanisms, which are distinguished by the linearity and non-linearity of the different plots, as well as the position of the intercepts of different plots. These features have been described in detail for three-substrate reaction mechanisms (Segel, 1975; Rudolph and Fromm, 1979). Because of the errors in measurements, particularly at low substrate concentration(s), and minor deviations from linearity, it can be difficult to fully distinguish among potential mechanisms. Nonetheless, careful analysis of the plots and potentially additional biochemical and biophysical methods can be employed to distinguish among the possible catalytic mechanisms.
4.1. Possible mechanisms
Three-substrate reaction mechanisms can be divided broadly into ordered and ping-pong mechanisms (Table 2). With each, there are five mechanisms that relate to the nature of substrate binding within each mechanism. The five ordered mechanisms encompass the first five mechanisms and describe reactions in which 1. substrate binding is completely random, 2. substrates are required to bind in an ordered fashion, and 3–5. partially ordered reactions in which the second, third, and first substrate binds in a requisite position while the remaining two substrates can bind randomly.
Table 2.
Graphical analysis of kinetic data for ter-reactant enzymesa
| Initial Rate Plotsb | Slope and Intercept Replotsc | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mechanism | 1/A | 1/B | 1/C | ASlope | AInt | BSlope | BInt | CSlope | CInt |
| 1. Random | I | I | I | N | N | N | N | N | N |
| 2. Ordered | I | I | I | N | N | N | L | N | N |
| 3. Partially Random (AC Random) | I | I | I | N | N | N0 | L | N | N |
| 4. Partially Random (AB Random) | I | I | 0 | N0 | N | N0 | N | N | * |
| 5. Partially Random (BC Random) | I | I | I | N0 | N | N | L | N | L |
| 6. Hexa Uni Ping Pong | P | P | P | L | L | L | L | L | L |
| 7. Ordered Bi Uni Uni Bi Ping Pong | I | I | P | L | L | L | L | L | N |
| 8. Ordered Uni Uni Bi Bi Ping Pong | P | I | I | L | N | L | L | L | L |
| 9. Random Bi Uni Uni Bi Ping Pong | I | I | P | L | L | L | L | L | N |
| 10. Random Uni Uni Bi Bi Ping Pong | P | I | I | L | N | L | L | L | L |
Reprinted with permission from Mydy, L. S., Bailey, D. C., Patel, K. D., Rice, M. R. and Gulick, A. M. (2020). “The Siderophore Synthetase IucA of the Aerobactin Biosynthetic Pathway Uses an Ordered Mechanism.” Biochemistry 59: 2143–2153. Copyright 2020 American Chemical Society.
I, lines intersect to the left of the 1/v axis. 0, lines intersect on the 1/v axis. P, parallel lines
N, non-linear. L, linear. N0, non-linear, passes through the origin.
all intercepts are the same.
The five ping-pong reaction mechanisms are defined by the number of substrates that bind and are released between the different chemical steps. In the Hexa Uni mechanism (mechanism 6), there is a chemical step that occurs between the binding of each substrate and the release of a product. In the Bi Uni Uni Bi (mechanism 7) and Uni Uni Bi Bi (mechanism 8) mechanisms, two or one substrate, respectively, bind prior to the first chemical step. Mechanisms 9 and 10 are similar, although involve a random binding of substrates. We note that often, although not always, ping pong reaction mechanisms can involve covalent acyl-enzyme intermediates. Although none is expected for NIS synthetases, the possibility of bound but not covalent intermediates, as seen with the acyl-CoA synthetase adenylate intermediates (Gulick, 2009), raises the possibility of a ping-pong enzyme mechanism.
4.2. Initial double reciprocal plots
The initial rate plots obtained with different substrate concentrations are now replotted to distinguish among the ten mechanisms. Three sets of plots for IucA were created, using varied citrate at fixed concentrations of ATP and ahLys, varied ATP at fixed concentrations of citrate and ahLys, and varied ahLys at fixed concentrations of ATP and citrate. These plots (Figure 2) clearly show a series of intercepting lines, which clearly rules out any of the ping pong mechanisms. That none of the points of intersection lies on the Y-axis, allows us to also rule out mechanism 4.
Figure 2.
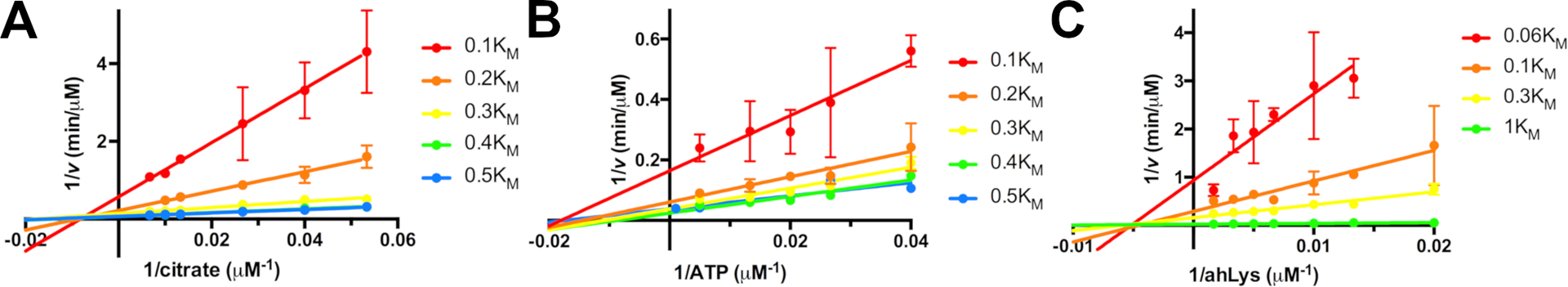
Initial Rate plots for IucA, varying concentrations of one substrate at fixed concentrations of the remaining two substrates at a constant ratio relative to their respective KM values. A. Citrate concentration was varied at multiple concentrations of ATP and ahLys. B. ATP concentration was varied at multiple concentrations of citrate and ahLys. C. ahLys concentration was varied at multiple concentrations of ATP and citrate. Double reciprocal plots were created illustrating intersecting patterns for all three substrates. Reprinted with permission from Mydy, L. S., D. C. Bailey, K. D. Patel, M. R. Rice and A. M. Gulick (2020). “The Siderophore Synthetase IucA of the Aerobactin Biosynthetic Pathway Uses an Ordered Mechanism.” Biochemistry 59: 2143–2153. Copyright 2020 American Chemical Society.
4.3. Slope and intercept replots
To distinguish among the remaining mechanisms, the slopes and intercepts of the lines fit in the initial rate plots are replotted against the reciprocal of the concentration of one of the fixed substrates (Figure 3). Analyzing these plots in relation to the table from above provides insight into the final reaction mechanism. All of the replots show better fits to non-linear than linear. However, the citrate intercept replot (in Figure 3D) approximates a straight line and may indeed represent a straight line. We interpreted this in our earlier work (Mydy et al., 2020) as supporting an ordered mechanism in which citrate binds second (Mechanism 2). We ultimately concluded that the best fit to all of the data, including additional kinetic studies using saturating amounts of the two fixed substrates, the impact of substrate binding on stabilizing the protein as indicated by a melting temperature analysis, and the crystal structure of IucA bound to ATP, that the catalytic mechanism is ordered with ATP binding preceding citrate, and finally ahLys.
Figure 3.
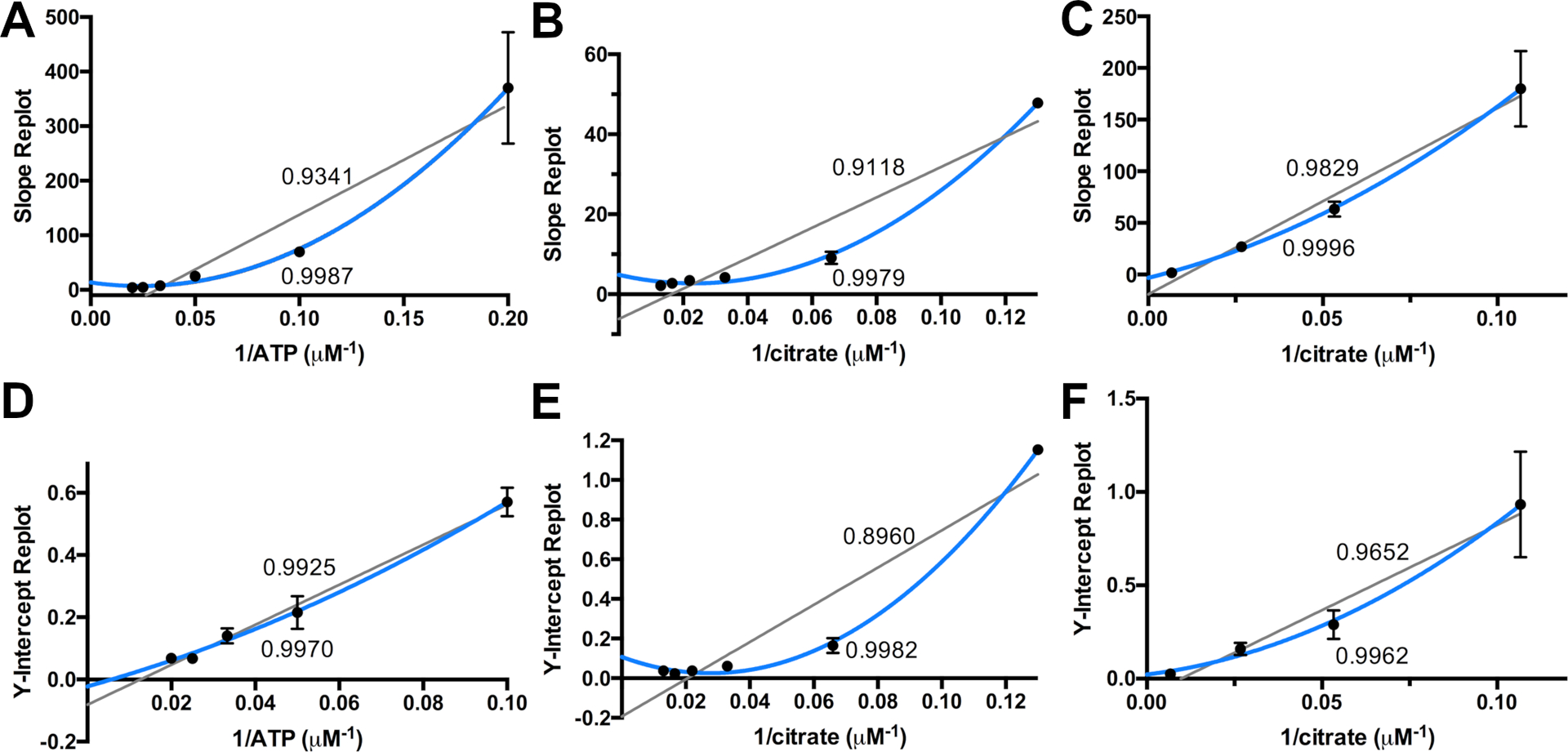
Slope and Intercept re-plots for IucA. The calculated slope (A-C) and intercepts (D-F) from Figure 2 are plotted against the concentration of one of the fixed, non-varied substrates. Fits of linear and non-linear plots are provided, along with the R2 value of the fit. The data from Figure 2A, varying citrate, are replotted in panels A. slope and D. intercept against the reciprocal concentration of ATP. The data from Figure 2B, varying ATP, are replotted in panels B. slope and E. intercept against the reciprocal concentration of citrate. The data from Figure 2A, varying ahLys, are replotted in panels C. slope and F. intercept against the reciprocal concentration of citrate. Reprinted with permission from Mydy, L. S., D. C. Bailey, K. D. Patel, M. R. Rice and A. M. Gulick (2020). “The Siderophore Synthetase IucA of the Aerobactin Biosynthetic Pathway Uses an Ordered Mechanism.” Biochemistry 59: 2143–2153. Copyright 2020 American Chemical Society.
4.4. Notes
We note that some studies of ter-reactant enzymes include additional potential mechanisms that include equilibrium or steady-state binding of certain substrates, or Theorell-Chance approaches that are adopted by enzymes in which the reaction and product release occur very quickly upon biding the third substrate (Viola and Cleland, 1982). For simplicity, we have reduced the number of possible mechanisms to the ten shown here.
It should be noted that errors in curves and minor deviations from linear can in some instances be difficult to distinguish. Many of the replots of Figure 3, for example, fit reasonably well to both linear and non-linear curves. Ultimately, investigators should consider multiple techniques and look for consistent observations that match all known features of the enzyme, structure, and chemical mechanism.
5. Alternate approaches
Alternate methods are available to analyze ter-reactant enzyme mechanisms, some of which were also performed and supported our data with the NIS synthetase IucA (Mydy et al., 2020). One approach is to saturate the enzyme with two, nonvarying substrates at > 100 × apparent KM value (Rudolph and Fromm, 1979). This strategy uses similar tables that associate with different ordered and sequential methods. One limitation of this approach can result from solubility or availability of custom substrates that may prevent the full analysis or suitably high concentrations.
6. Summary and conclusions
Bacterial siderophores play important roles in the adaptation to iron limiting environments, including the sites of infections for many human pathogens. Because of the diverse nature of the siderophore structures, understanding their biosynthesis may enable the development of novel inhibitors that block siderophore production and block growth in iron limiting media (Lamb, 2015). Many siderophores are derived from the activity of the modular, assembly-line NRPS proteins, and the development of specific inhibitors of NRPS enzymes (Ferreras et al., 2005; Miethke et al., 2006; Neres et al., 2008; Neres et al., 2013; Shelton et al., 2022), associated peptide modification enzymes on the siderophore pathway (Drake and Gulick, 2011; Theriault et al., 2013; Wurst et al., 2014), and enzymes involved in the generation of NRPS building blocks (Payne et al., 2005; Vasan et al., 2010; Meneely et al., 2014) have all been described.
In contrast, mechanistic and inhibition studies of NIS synthetases have been comparatively under-explored. The development of inhibitors requires the elucidation of the mechanism and understanding of the substrate binding properties. The discovery of an ordered mechanism for IucA and the formation of a quarternary complex with ATP, citrate, and ahLys suggests that the active site that must accommodate all three substrates prior to any chemical steps. This observation should be repeated with additional NIS synthetases. It is notable that some NIS synthetases are iterative, catalyzing the polymerization and often cyclization of multiple copies a single molecule that contains a carboxylate on one end and an amine on the other. The dimerization, trimerization, and cyclization of N-hydroxy-N-succinylcadaverine or putrescine molecules result in a family of hydroxamate-containing siderophores avaroferrin, putrebactin, desferrioxamine, and others (Rütschlin et al., 2017; Ronan et al., 2018; Yang et al., 2023). The mechanistic investigation of extension and cyclization, while likely employing a similar mechanism as IucA, should be interrogated experimentally.
The structural and mechanistic investigation additionally identifies a distinction between the NIS synthetases and NRPS adenylation domains, both of which activate a carboxylate for amide bond formation through an adenylation step. Many NRPS enzymes have been studied with isosteric acyl sulfamoyl adenylate inhibitors that mimic the acyl adenylate intermediate (Ferreras et al., 2005; Miethke et al., 2006; Somu et al., 2006). These high potency inhibitors were recently tested for DesD, the NIS synthetase involved in the biosynthesis of desferrioxamine (Yang et al., 2022). The inhibitor was surprising much less efficient, with an IC50 value that was ~1000 × greater than observed for the best NRPS inhibitors. Structural examination provided a reasonable explanation. NIS synthetases bind ATP with the β- and γ-phosphates buried deep within the protein core, making multiple interactions with several conserved arginine residues. The lack of binding energy provided in the adenylate mimic reduces the potency of the inhibitors. Indeed, addition of inorganic pyrophosphate to the inhibition experiment increased the potency of the acyl sulfamate inhibitor (Yang et al., 2022).
The investigation of catalytic mechanisms of enzymes remains an important determinant to the understanding of the protein function in the context of a broader biosynthetic pathway. Probing the function of individual enzymes informs our understanding of enzymes from uncharacterized biosynthetic pathways that may produce important natural products that have yet to be discovered.
Acknowledgment
Work in our lab is supported by NIH grant GM136235 (to AMG). The authors thank Daniel C. Bailey, Eric J. Drake, and Matthew R. Rice for early assistance and studies on IucA.
References
- Bailey DC, Alexander E, Rice MR, Drake EJ, Mydy LS, Aldrich CC and Gulick AM (2018). “Structural and functional delineation of aerobactin biosynthesis in hypervirulent Klebsiella pneumoniae.” J Biol Chem 293(20): 7841–7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DC, Drake EJ, Grant TD and Gulick AM (2016). “Structural and functional characterization of aerobactin synthetase IucA from a hypervirulent pathotype of Klebsiella pneumoniae.” Biochemistry 55(25): 3559–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry SM and Challis GL (2009). “Recent advances in siderophore biosynthesis.” Curr Opin Chem Biol 13(2): 205–215. [DOI] [PubMed] [Google Scholar]
- Carbonetti NH and Williams PH (1984). “A cluster of five genes specifying the aerobactin iron uptake system of plasmid ColV-K30.” Infect Immun 46(1): 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis GL (2005). “A widely distributed bacterial pathway for siderophore biosynthesis independent of nonribosomal peptide synthetases.” Chembiochem 6(4): 601–611. [DOI] [PubMed] [Google Scholar]
- Drake EJ and Gulick AM (2011). “Structural characterization and high-throughput screening of inhibitors of PvdQ, an NTN hydrolase involved in pyoverdine synthesis.” ACS Chem Biol 6(11): 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreras JA, Ryu JS, Di Lello F, Tan DS and Quadri LE (2005). “Small-molecule inhibition of siderophore biosynthesis in Mycobacterium tuberculosis and Yersinia pestis.” Nat Chem Bio 1(1): 29–32. [DOI] [PubMed] [Google Scholar]
- Ford S, Cooper RA and Williams PH (1986). “Biochemical genetics of aerobactin biosynthesis in Escherichia coli.” Fems Microbiology Letters 36(2–3): 281–285. [Google Scholar]
- Gulick AM (2009). “Conformational dynamics in the acyl-CoA synthetases, adenylation domains of non-ribosomal peptide synthetases, and firefly luciferase.” ACS Chem Biol 4: 811–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hider RC and Kong X (2010). “Chemistry and biology of siderophores.” Nat Prod Rep 27(5): 637–657. [DOI] [PubMed] [Google Scholar]
- Horswill AR and Escalante-Semerena JC (2002). “Characterization of the propionyl-CoA synthetase (PrpE) enzyme of Salmonella enterica: residue Lys592 is required for propionyl-AMP synthesis.” Biochemistry 41(7): 2379–2387. [DOI] [PubMed] [Google Scholar]
- Kadi N, Oves-Costales D, Barona-Gomez F and Challis GL (2007). “A new family of ATP-dependent oligomerization-macrocyclization biocatalysts.” Nat Chem Biol 3(10): 652–656. [DOI] [PubMed] [Google Scholar]
- Kudo F, Miyanaga A and Eguchi T (2019). “Structural basis of the nonribosomal codes for nonproteinogenic amino acid selective adenylation enzymes in the biosynthesis of natural products.” J Ind Microbiol Biotechnol 46(3–4): 515–536. [DOI] [PubMed] [Google Scholar]
- Lamb AL (2015). “Breaking a pathogen’s iron will: Inhibiting siderophore production as an antimicrobial strategy.” Biochim Biophys Acta 1854(8): 1054–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneely KM, Luo Q, Riley AP, Taylor B, Roy A, Stein RL, Prisinzano TE and Lamb AL (2014). “Expanding the results of a high throughput screen against an isochorismate-pyruvate lyase to enzymes of a similar scaffold or mechanism.” Bioorg Med Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miethke M, Bisseret P, Beckering CL, Vignard D, Eustache J and Marahiel MA (2006). “Inhibition of aryl acid adenylation domains involved in bacterial siderophore synthesis.” Febs J 273(2): 409–419. [DOI] [PubMed] [Google Scholar]
- Miethke M and Marahiel MA (2007). “Siderophore-based iron acquisition and pathogen control.” Microbiol Mol Biol Rev 71(3): 413–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mydy LS, Bailey DC, Patel KD, Rice MR and Gulick AM (2020). “The siderophore synthetase IucA of the aerobactin biosynthetic pathway uses an ordered mechanism.” Biochemistry 59(23): 2143–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif X and Sansonetti PJ (1986). “Correlation of the virulence of Klebsiella pneumoniae K1 and K2 with the presence of a plasmid encoding aerobactin.” Infect Immun 54(3): 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neres J, Engelhart CA, Drake EJ, Wilson DJ, Fu P, Boshoff HI, Barry CE 3rd, Gulick AM and Aldrich CC (2013). “Non-nucleoside inhibitors of BasE, an adenylating enzyme in the siderophore biosynthetic pathway of the opportunistic pathogen Acinetobacter baumannii.” J Med Chem 56(6): 2385–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neres J, Labello NP, Somu RV, Boshoff HI, Wilson DJ, Vannada J, Chen L, Barry CE 3rd, Bennett EM and Aldrich CC (2008). “Inhibition of siderophore biosynthesis in Mycobacterium tuberculosis with nucleoside bisubstrate analogues: structure-activity relationships of the nucleobase domain of 5’-O-[N-(salicyl)sulfamoyl]adenosine.” J Med Chem 51(17): 5349–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusca TD, Kim Y, Maltseva N, Lee JY, Eschenfeldt W, Stols L, Schofield MM, Scaglione JB, Dixon SD, Oves-Costales D, Challis GL, Hanna PC, Pfleger BF, Joachimiak A and Sherman DH (2012). “Functional and structural analysis of the siderophore synthetase AsbB through reconstitution of the petrobactin biosynthetic pathway from Bacillus anthracis.” J Biol Chem 287(19): 16058–16072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oves-Costales D, Kadi N and Challis GL (2009). “The long-overlooked enzymology of a nonribosomal peptide synthetase-independent pathway for virulence-conferring siderophore biosynthesis.” Chem Commun (Camb)(43): 6530–6541. [DOI] [PubMed] [Google Scholar]
- Payne RJ, Kerbarh O, Miguel RN, Abell AD and Abell C (2005). “Inhibition studies on salicylate synthase.” Org Biomol Chem 3(10): 1825–1827. [DOI] [PubMed] [Google Scholar]
- Quadri LE (2000). “Assembly of aryl-capped siderophores by modular peptide synthetases and polyketide synthases.” Mol Microbiol 37(1): 1–12. [DOI] [PubMed] [Google Scholar]
- Raran-Kurussi S, Cherry S, Zhang D and Waugh DS (2017). “Removal of affinity tags with TEV protease.” Methods Mol Biol 1586: 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger AS, Carney JM and Gulick AM (2007). “Biochemical and crystallographic analysis of substrate binding and conformational changes in acetyl-CoA synthetase.” Biochemistry 46(22): 6536–6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronan JL, Kadi N, McMahon SA, Naismith JH, Alkhalaf LM and Challis GL (2018). “Desferrioxamine biosynthesis: diverse hydroxamate assembly by substrate-tolerant acyl transferase DesC.” Philos Trans R Soc Lond B Biol Sci 373(1748). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph FB and Fromm HJ (1979). “Plotting methods for analyzing enzyme rate data.” Methods Enzymol 63: 138–159. [DOI] [PubMed] [Google Scholar]
- Russo TA and Gulick AM (2019). “Aerobactin synthesis proteins as antivirulence targets in hypervirulent Klebsiella pneumoniae.” ACS Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo TA, Olson R, MacDonald U, Beanan J and Davidson BA (2015). “Aerobactin, but not yersiniabactin, salmochelin and enterobactin, enables the growth/survival of hypervirulent (hypermucoviscous) Klebsiella pneumoniae ex vivo and in vivo.” Infect Immun 83: 3325–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo TA, Olson R, MacDonald U, Metzger D, Maltese LM, Drake EJ and Gulick AM (2014). “Aerobactin mediates virulence and accounts for the increased siderophore production under iron limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae.” Infect Immun 82: 2356–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rütschlin S, Gunesch S and Böttcher T (2017). “One Enzyme, Three Metabolites: Shewanella algae controls siderophore production via the cellular substrate pool.” Cell Chem Biol 24(5): 598–604 e510. [DOI] [PubMed] [Google Scholar]
- Segel IH (1975). Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems. New York, John Wiley & Sons. [Google Scholar]
- Shelton CL, Meneely KM, Ronnebaum TA, Chilton AS, Riley AP, Prisinzano TE and Lamb AL (2022). “Rational inhibitor design for Pseudomonas aeruginosa salicylate adenylation enzyme PchD.” J Biol Inorg Chem 27(6): 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somu RV, Boshoff H, Qiao C, Bennett EM, Barry CE 3rd and Aldrich CC (2006). “Rationally designed nucleoside antibiotics that inhibit siderophore biosynthesis of Mycobacterium tuberculosis.” J Med Chem 49(1): 31–34. [DOI] [PubMed] [Google Scholar]
- Tang J, Ju Y, Zhou J, Guo J, Gu Q, Xu J and Zhou H (2020). “Structural and Biochemical Characterization of SbnC as a Representative Type B Siderophore Synthetase.” ACS Chem Biol 15(10): 2731–2740. [DOI] [PubMed] [Google Scholar]
- Theriault JR, Wurst J, Jewett I, Verplank L, Perez JR, Gulick AM, Drake EJ, Palmer M, Moskowitz S, Dasgupta N, Brannon MK, Dandapani S, Munoz B and Schreiber S (2013). Identification of a small molecule inhibitor of Pseudomonas aeruginosa PvdQ acylase, an enzyme involved in siderophore pyoverdine synthesis. Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD), National Center for Biotchnology Information (US). [PubMed] [Google Scholar]
- Tropea JE, Cherry S and Waugh DS (2009). “Expression and purification of soluble His(6)-tagged TEV protease.” Methods Mol Biol 498: 297–307. [DOI] [PubMed] [Google Scholar]
- Vasan M, Neres J, Williams J, Wilson DJ, Teitelbaum AM, Remmel RP and Aldrich CC (2010). “Inhibitors of the salicylate synthase (MbtI) from Mycobacterium tuberculosis discovered by high-throughput screening.” ChemMedChem 5(12): 2079–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola RE and Cleland WW (1982). “Initial velocity analysis for terreactant mechanisms.” Methods Enzymol 87: 353–366. [DOI] [PubMed] [Google Scholar]
- Wu MX and Hill KA (1993). “A continuous spectrophotometric assay for the aminoacylation of transfer RNA by alanyl-transfer RNA synthetase.” Anal Biochem 211(2): 320–323. [DOI] [PubMed] [Google Scholar]
- Wu R, Cao J, Lu X, Reger AS, Gulick AM and Dunaway-Mariano D (2008). “Mechanism of 4-chlorobenzoate:coenzyme a ligase catalysis.” Biochemistry 47(31): 8026–8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurst JM, Drake EJ, Theriault JR, Jewett IT, VerPlank L, Perez JR, Dandapani S, Palmer M, Moskowitz SM, Schreiber SL, Munoz B and Gulick AM (2014). “Identification of inhibitors of PvdQ, an enzyme involved in the synthesis of the siderophore pyoverdine.” ACS Chem Biol 9(7): 1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Banas VS, Patel KD, Rivera GSM, Mydy LS, Gulick AM and Wencewicz TA (2022). “An acyl-adenylate mimic reveals the structural basis for substrate recognition by the iterative siderophore synthetase DesD.” J Biol Chem 298(8): 102166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Banas VS, Rivera GSM and Wencewicz TA (2023). “Siderophore synthetase DesD catalyzes N-to-C condensation in desferrioxamine biosynthesis.” ACS Chem Biol 18(6): 1266–1270. [DOI] [PubMed] [Google Scholar]


