Abstract
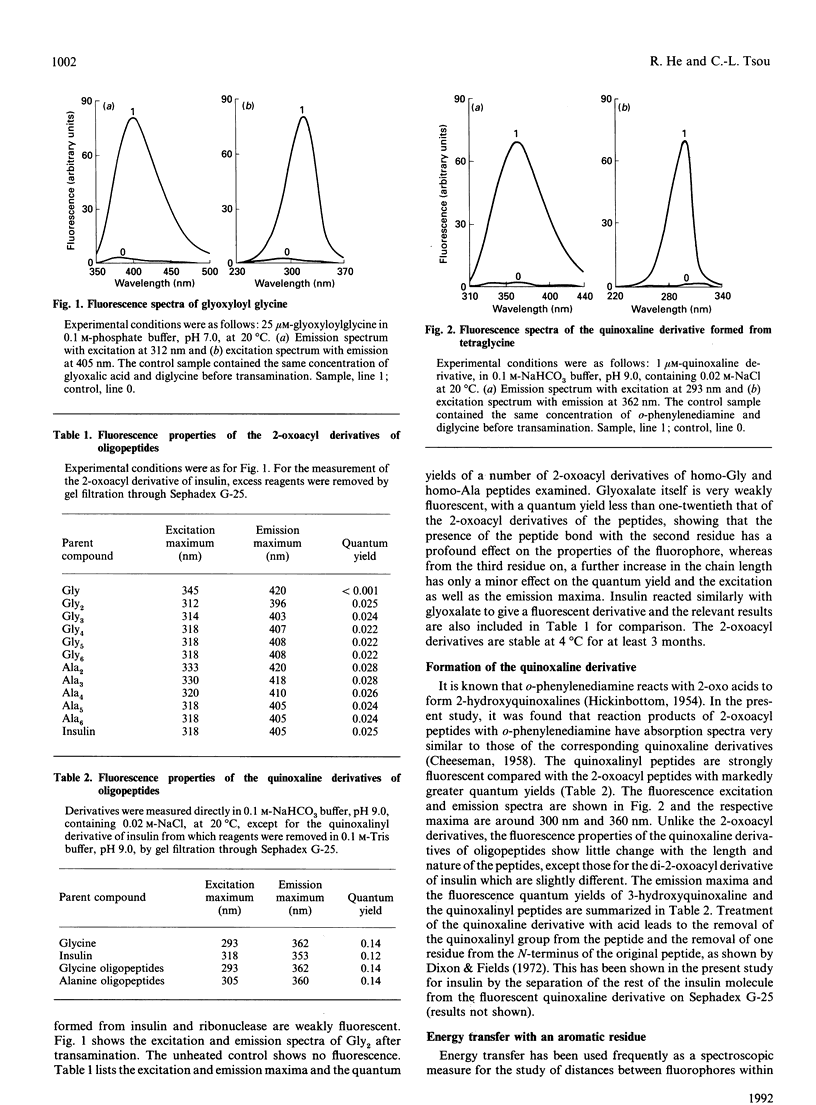
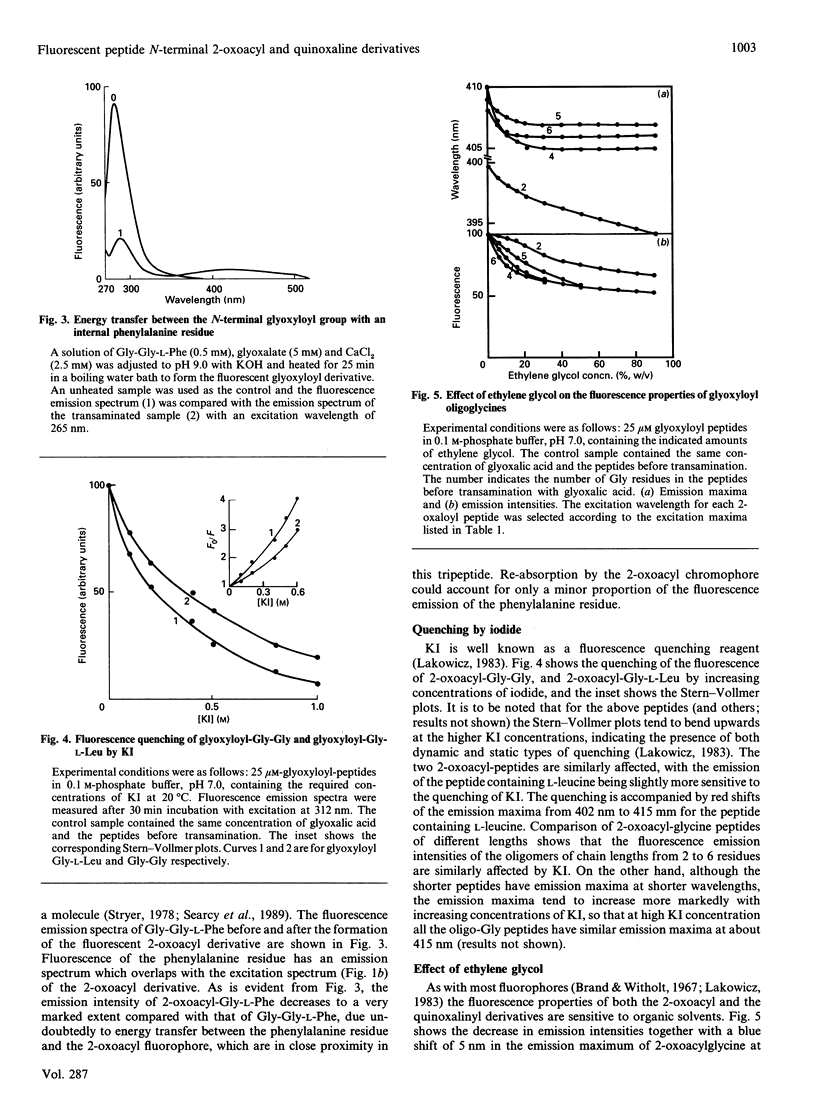
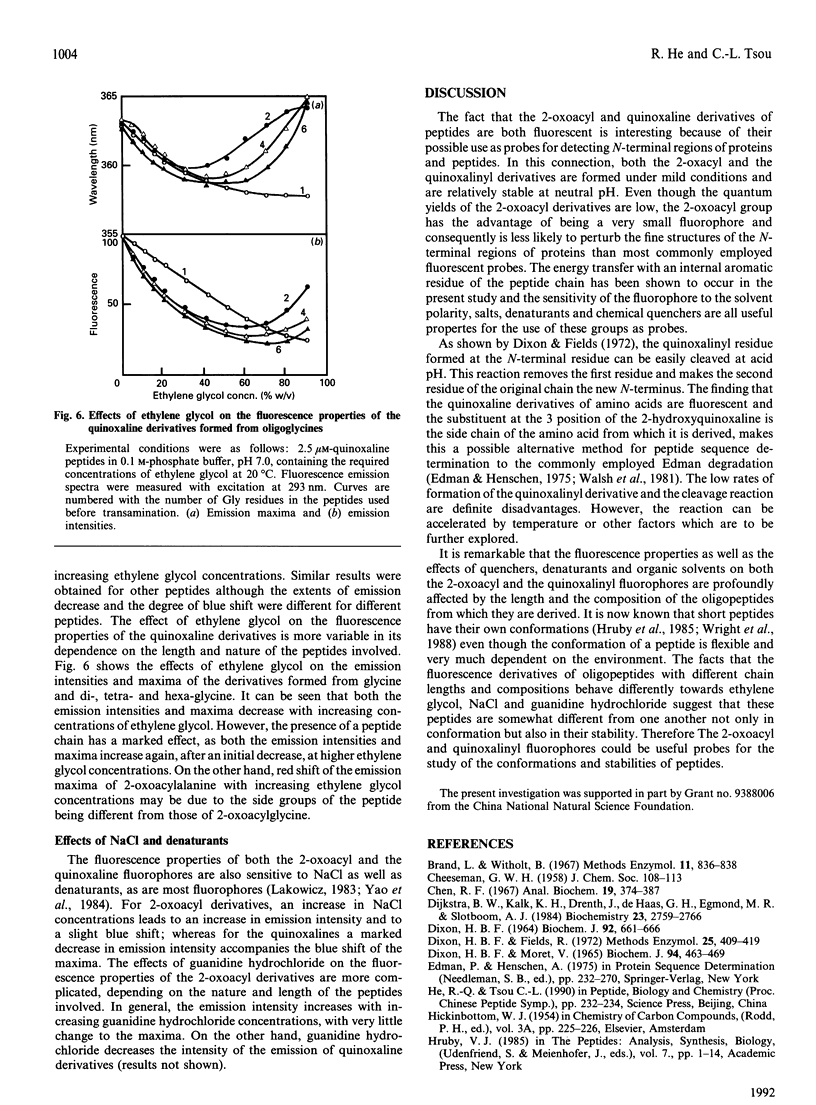
A peptide reacts with glyoxalic acid resulting in transamination of the N-terminal residue to form a 2-oxoacyl group. This further reacts with o-phenylenediamine, leading to a quinoxaline derivative of the original N-terminal amino acid, which is cleavable in mild acid [Dixon & Fields (1972) Methods Enzymol. 25, 409-419]. The 2-oxoacyl peptides are weakly fluorescent with emission maxima around 410 nm and excitation maxima at about 320 nm, depending on the nature and length of the peptide. Formation of the quinoxaline derivative results in a marked increase of fluorescence, with emission maximum of 363 nm when excited at 303 nm. The fluorescence properties of these derivatives change with the nature and length of the peptides and are affected by the presence of organic solvents, NaCl and denaturants. It is suggested that such fluorescent derivatives could be used as probes for the study of the conformation of the N-terminal region of peptides and proteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen R. F. Some characteristics of the fluorescence of quinine. Anal Biochem. 1967 May;19(2):374–387. doi: 10.1016/0003-2697(67)90174-1. [DOI] [PubMed] [Google Scholar]
- DIXON H. B., MORET V. REMOVAL OF THE N-TERMINAL RESIDUE OF A PROTEIN AFTER TRANSAMINATION. Biochem J. 1965 Feb;94:463–469. doi: 10.1042/bj0940463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra B. W., Kalk K. H., Drenth J., de Haas G. H., Egmond M. R., Slotboom A. J. Role of the N-terminus in the interaction of pancreatic phospholipase A2 with aggregated substrates. Properties and crystal structure of transaminated phospholipase A2. Biochemistry. 1984 Jun 5;23(12):2759–2766. doi: 10.1021/bi00307a035. [DOI] [PubMed] [Google Scholar]
- Dixon H. B. Transamination of peptides. Biochem J. 1964 Sep;92(3):661–666. doi: 10.1042/bj0920661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nozaki Y. The preparation of guanidine hydrochloride. Methods Enzymol. 1972;26:43–50. doi: 10.1016/s0076-6879(72)26005-0. [DOI] [PubMed] [Google Scholar]
- Searcy D. G., Montenay-Garestier T., Hélène C. Phenylalanine-to-tyrosine singlet energy transfer in the archaebacterial histone-like protein HTa. Biochemistry. 1989 Nov 14;28(23):9058–9065. doi: 10.1021/bi00449a015. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Walsh K. A., Ericsson L. H., Parmelee D. C., Titani K. Advances in protein sequencing. Annu Rev Biochem. 1981;50:261–284. doi: 10.1146/annurev.bi.50.070181.001401. [DOI] [PubMed] [Google Scholar]
- Wright P. E., Dyson H. J., Lerner R. A. Conformation of peptide fragments of proteins in aqueous solution: implications for initiation of protein folding. Biochemistry. 1988 Sep 20;27(19):7167–7175. doi: 10.1021/bi00419a001. [DOI] [PubMed] [Google Scholar]
- Yao Q. Z., Tian M., Tsou C. L. Comparison of the rates of inactivation and conformational changes of creatine kinase during urea denaturation. Biochemistry. 1984 Jun 5;23(12):2740–2744. doi: 10.1021/bi00307a032. [DOI] [PubMed] [Google Scholar]
- van Heyningen S., Tipton K. F., Dixon H. B. Transamination of the N-terminal residue of carboxypeptidase. Biochem J. 1968 Jul;108(3):508–509. doi: 10.1042/bj1080508. [DOI] [PMC free article] [PubMed] [Google Scholar]


