Significance
Repetitive DNA sequences in the genome can cause problems for our cells. To protect the genome, cells turn off these repetitive sequences by placing them in inactive heterochromatic areas and adding repressive markers. However, we don’t know exactly how this is achieved during early embryonic development. Here, we found that the repetitive sequences are densely labeled with an active marker H3K14ac. This marker to our surprise attracts a protein called Eggless/SetDB1 to foster a repressive environment, turning off these repetitive sequences in early fruit fly embryos. Experimentally disrupting this process unleashes many repetitive DNA sequences and harms organismal viability. Our finding uncovers a mode to turn off repetitive sequences in the development of early embryos.
Keywords: Drosophila, early embryo, heterochromatin, H3K14ac, Eggless/SetDB1
Abstract
Constitutive heterochromatin, a fundamental feature of eukaryotic nucleus essential for transposon silencing and genome stability, is rebuilt on various types of repetitive DNA in the zygotic genome during early embryogenesis. However, the molecular program underlying this process remains poorly understood. Here, we show that histone H3 lysine 14 acetylation (H3K14ac) is engaged in the reinstallation of constitutive heterochromatin in Drosophila early embryos. H3K14ac partially colocalizes with H3 lysine 9 trimethylation (H3K9me3) and its methyltransferase Eggless/SetDB1 around the mid-blastula transition. Concealing H3K14ac by either antibody injection or maternal knockdown of Gcn5 diminishes Eggless/SetDB1 nuclear foci and reduces the deposition of H3K9me3. Structural analysis reveals that Eggless/SetDB1 recognizes H3K14ac via its tandem Tudor domains, and disrupting the binding interface causes defects in Eggless/SetDB1 distribution and derepression of a subset of transposons. Therefore, H3K14ac, a histone modification normally associated with active transcription, is a crucial component of the early embryonic machinery that introduces constitutive heterochromatic features to the newly formed zygotic genome.
After fertilization, profound epigenetic resetting transforms the genome inherited from two fully differentiated gametes into a relatively naïve state, preparing a blank scroll for the painting of a new life cycle. Basic epigenetic landscapes are then laid out in subsequent embryogenesis following stereotyped developmental programs, including the stepwise package of various repetitive DNA elements into transcriptionally inert constitutive heterochromatin (1–4). In Drosophila melanogaster, approximately 20% of the genome, composed of large tandem repeats and dispersed transposable elements, initiates heterochromatinization when embryos slow down the cell cycles at the major embryonic transformation period called the mid-blastula transition (MBT) (5–7). This establishment of constitutive heterochromatin is orchestrated largely by maternal programs that are of significant complexity (8), with Eggless/SetDB1-catalyzed deposition of H3K9me3 being the major chromatin silencing effector (6, 7). Different repetitive DNA elements seem to take on distinct molecular pathways to arrive at a heterochromatic state. Tandem AAGAG repeat relies on specific recognition by the pioneer factor GAF to initiate H3K9me3 deposition and transcriptional silencing (9). Some transposable elements, however, utilize piRNA-directed mechanism to engage Piwi and the PICTS complex with the nascent transposon transcripts to recruit Eggless/SetDB1 and instruct local heterochromatin formation in the early embryos (10–14). Besides, the emergence of heterochromatic features on different repetitive DNA elements is heterochronic. A group of tandem repetitive elements such as the 359-bp and AAGAG repeats attain H3K9me3 decoration and HP1a accumulation at the MBT, preceding other repeats (8, 9). To date, our understanding of the molecular basis underlying the precise recruitment of Eggless/SetDB1 and hence the reestablishment of constitutive heterochromatin in the zygotic genome during early embryonic development is far from complete.
Unlike histone methylation that can decorate active or silent chromatins, acetylation of histone tails is thought to be invariably linked to transcriptional activation (15–17). H3K14 is acetylated mainly by histone acetyltransferase Gcn5, which serves as the catalytic subunit of four different transcriptional coactivator complexes that stimulate gene expression in Drosophila (18). Null Gcn5 alleles block the oocyte development and larva-to-adult metamorphosis (19). Transgenic flies carrying histone gene unites (His-GUs) in which H3K14 is substituted for alanine or arginine (H3K14A or H3K14R) in a histone deficiency mutant background manifest lethality at the embryonic or second-instar larval stage (20, 21). In late embryonic development, H3K14ac enriches over the gene body of a group of tissue-specific genes, defining a unique chromatin state essential for organogenesis (21). Intriguingly, in vitro peptide binding assay revealed that human SetDB1, a methyltransferase of H3K9me3, can interact with doubly modified H3 tails containing H3K14ac and H3K9me2/3 through its N-terminal tandem Tudor domains (TD). Crystal structure demonstrated that the interface between TD2 and TD3 of SetDB1 forms the binding pockets for these modifications (22). These observations suggest a functional connection between H3K14ac and the repressive H3K9me3 histone mark. However, the early embryonic dynamics of H3K14ac relative to H3K9me3, as well as the potential biological significance of H3K14ac in the establishment of constitutive heterochromatin, remain undetermined.
Here, we show that in Drosophila H3K14ac is transmitted from the female germline to the zygotic embryos. It can be recognized by the tandem TD of Eggless/SetDB1, facilitating the concentration of Eggless/SetDB1 and deposition of H3K9me3 on a subset of repetitive DNA elements around the time of MBT. Either erasing the H3K14ac modification or disrupting the recognition of H3K14ac by Eggless/SetDB1 leads to reduced deposition of H3K9me3 and derepression of a group of transposons, compromising the integrity of constitutive heterochromatin and organismal viability. Our study identifies the H3K14ac-mediated recruitment of Eggless/SetDB1 as an important module of the molecular machinery underneath the reinstallation of constitutive heterochromatin during early embryogenesis.
Results
H3K14ac Is Maternally Transmitted and Concentrates at the Apical Pole of Nuclei with H3K9me2/3 and Eggless/SetDB1 Around the Mid-Blastula Transition.
Drosophila early embryogenesis begins with rapid nuclear divisions, deferring the emergence of constitutive heterochromatic hallmarks in the zygotic genome until the cell cycles slow down at the MBT (7, 8). We set out to characterize the spatial distribution of H3K14ac in the syncytial and cellular blastoderm embryos in comparison to H3K9me2/3 (Fig. 1A). While H3K9me2 histone modification became detectable slightly earlier than H3K9me3, both repressive marks progressively accumulated in cycle 13 and cycle 14 embryos at the apical pole of nuclei where numerous repetitive DNA elements reside. H3K14ac was already abundant in nuclei of early syncytial blastoderm embryos (cycle 11 to 12), forming several nuclear puncta in addition to the ubiquitous nuclear distribution. In cycle 13 and cycle 14 embryos, H3K14ac became enriched at the apical regions of nuclei, colocalizing with the H3K9me2/3 modifications (Fig. 1A). We quantified the H3K14ac antibody staining signals within and without the H3K9me3-decorated compartments in cycle 14 embryos, and detected that the mean fluorescent intensity of H3K14ac was significantly higher in the H3K9me3-positive regions (Fig. 1B). Eggless/SetDB1 is the major histone methyltransferase in the early embryos to deposit H3K9me2/3 modifications (7). We collected embryos from a knock-in Drosophila line in which the Eggless/SetDB1 is tagged with GFP at its N terminus (GFP-SetDB1), and performed immunostaining to visualize H3K14ac. Similar to H3K9me3, GFP-SetDB1 accumulated at the apical poles of nuclei in cycle 14 embryos and significantly overlapped with H3K14ac (Fig. 1 A and C). The apical regions of nuclei are enriched with repetitive DNA elements, exhibiting higher DNA density and more compact nucleosome organization even before emergence of many heterochromatic features in Drosophila early embryos (23) (SI Appendix, Fig. S1 A–C). We performed 3D reconstruction to quantify the distribution of H3K14ac. Within the apical His2Av-compacted region which occupies 3.84% of total nuclear volume, the concentration of H3K14ac reflected by fluorescent intensity per cubic micrometer was approximately 8 times of that in the nonapical nuclear region (SI Appendix, Fig. S1 D–E). Both H3K14ac and H3K9ac are catalyzed by the acetyltransferase Gcn5 (18, 19, 24). However, unlike H3K14ac, H3K9ac was detected mainly on chromosome arms and manifested no enrichment at the apical nuclear poles at this stage (Fig. 1 D–E and SI Appendix, Fig. S1A).
Fig. 1.
Distribution of H3K14ac during Drosophila gametogenesis and early embryogenesis. (A) Immunofluorescence of fixed embryos at different developmental stages. The estimated embryonic cell cycles, determined using either internuclear distances for the syncytial blastoderm embryos or nuclear lengths for the cellular blastoderm embryos, are labeled at the top. The antibody staining of H3K14ac is shown in green, and H3K9me2/3 or SetDB1 in red. DAPI-stained DNA is shown in blue. The numbered images show enlarged views of the corresponding dashed line-labeled regions. Bars: 5 μm. (B) Quantification of nuclear H3K14ac fluorescent signals in H3K9me3 positive (H3K9me3 +) and H3K9me3 negative (H3K9me3−) regions in cycle 14 embryos. Unpaired t-test, ****P < 0.0001. Error bars represent the SD. (C) Quantification of nuclear H3K14ac signals in SetDB1 positive (SetDB1+) and negative (SetDB1−) regions in cycle 14 embryos. Unpaired t-test, ****P < 0.0001. Error bars represent the SD. (D) Immunofluorescence of fixed cycle 14 embryos with H3K9ac. The antibody staining of H3K9ac is shown in green, and DAPI-stained DNA is shown in red. Bars: 5 μm. (E) Quantification of nuclear H3K9ac fluorescent signals at the DAPI-intense apical regions and the rest nonapical regions in cycle 14 embryos. Unpaired t-test, ****P < 0.0001. Error bars represent the SD. (F) Immunofluorescence of Drosophila testis with H3K14ac (green) and H3K9me3 (red) antibodies. DNA was labeled with DAPI (blue). A cartoon of Drosophila testis is shown on the left. The dashed line-labeled regions are zoomed-in in (G). Bar: 20 μm. (G) H3K14ac and H3K9me3 fluorescent signals at the apical tip of testis and in individualized sperms. (H) Immunofluorescence of Drosophila ovaries and early embryos with H3K14ac (green) and H3K9me3 (red) antibodies. DNA was stained with DAPI (blue). The enlarged views of the dashed line-labeled regions are shown on the right. Metaphase chromosomes of paternal or maternal origin in cycle 1 embryos are labeled with ♂ or ♀ respectively. Bars: 15 μm.
Since H3K14ac was already detectable in cycle 11 embryos, we traced its presence during gametogenesis as well as in preblastoderm embryos. Drosophila testis is a blind-ended coiled tube, with the germline stem cells at the apical tip and matured sperms in the distal regions (Fig. 1F). While both H3K14ac and H3K9me3 were detected in cells at the testis tip, these two histone modifications showed little overlap. However, neither of these modifications was maintained in the individualized sperms (Fig. 1G). Drosophila ovary is composed of a bundle of ovarioles in which the stem cells are located at the tip of the germarium and the developing egg chambers are arranged in a linear manner (Fig. 1H). The oocyte localizes to the posterior side of each egg chamber, with its chromatin harboring an extremely diversified repertoire of histone modifications (25). We detected strong H3K14ac and H3K9me3 signals in oocytes at different developmental stages. The H3K9me3 was concentrated in the middle of the germinal vesicle, whereas H3K14ac appeared more expanded and amorphous in its distribution (Fig. 1H, previtellogenic stage 8). Therefore, both H3K14ac and H3K9me3 were absent in mature sperms but present in oocytes. After fertilization, the chromosomes of maternal and paternal origins are still kept separate in mitosis of the first cell cycle. Compared to H3K9me3, which was removed from all the zygotic genome, the H3K14ac signal was detected on half of the metaphase chromosomes that were likely inherited from the maternal pronucleus. Subsequently, the H3K14ac decorated the zygotic nuclei throughout the preblastoderm stage (Fig. 1H). These results indicate that H3K14ac is maternally transmitted, persists throughout the early embryogenesis, and partially colocalizes with hallmarks of constitutive heterochromatin during the MBT time frame.
Masking or Erasing the H3K14ac Histone Code Disrupts Eggless/SetDB1 Nuclear Foci Formation in Syncytial Blastoderm Embryos.
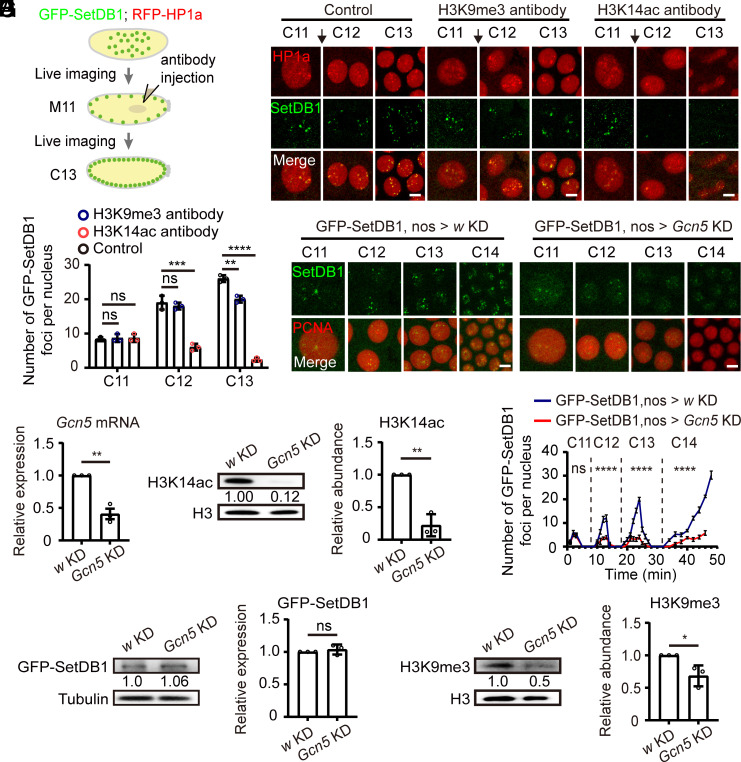
The colocalization of H3K14ac with Eggless/SetDB1 and H3K9me3 during the period of heterochromatin formation prompted us to investigate its functional significance. To this end, we injected H3K14ac antibody into embryos at the mitosis of cycle 11 to mask the modification, and monitored the localization of the endogenously tagged GFP-SetDB1 as well as RFP-HP1a expressed from a transgene (Fig. 2A). H3K9me3 antibody was used for comparison because H3K9me3 at this stage has not been established (8). Injection of H3K9me3 antibody showed little effect on the nuclear foci formation of GFP-SetDB1 and RFP-HP1a until the embryos developed to the interphase of cycle 13, during which the presence of H3K9me3 antibody mildly reduced the number of GFP-SetDB1 as well as RFP-HP1a foci (Fig. 2 B and C, and Movies S1 and S2). However, injection of H3K14ac antibody gave rise to a stronger phenotype. The nuclear foci of GFP-SetDB1 and RFP-HP1a were significantly disrupted in the interphases of both cycle 12 and cycle 13 embryos, and the chromosome segregations in mitosis 12 were unsuccessful (Fig. 2 B and C and Movie S3).
Fig. 2.
Perturbation of H3K14ac impacts SetDB1 localization during early embryonic development. (A) Schematics of live imaging experiments with antibody-injected embryos. (B) Time-lapse images of nuclei in embryos expressing RFP-HP1a (red) and GFP-SetDB1 (green). H3K9me3 or H3K14ac antibody diluted in PBS was injected during mitosis 11 (black arrows). PBS was served as a control. Bars: 5 μm. (C) Quantification of the numbers of nuclear GFP-SetDB1 foci in the embryos before and after injection. Unpaired t-test, ns: no significance, **P < 0.01, ***P < 0.001, ****P < 0.0001. Error bars represent the SD. (D) RT-qPCR analysis of Gcn5 mRNA abundance in 0 to 2 h embryos from GFP-SetDB1, nos-Gal4 > w (w KD) or GFP-SetDB1, nos-Gal4 > Gcn5 (Gcn5 KD) parents. Unpaired t-test, **P < 0.01. The error bar represents the SD. (E) Western blot analysis of w KD and Gcn5 KD 0 to 2 h embryos with H3 and H3K14ac antibodies. The H3K14ac modification is downregulated in Gcn5 KD embryos. Unpaired t-test, **P < 0.01. The error bar represents the SD. (F) Time-lapse images of nuclei in embryos harboring GFP-SetDB1 (green) and mCherry-PCNA (red) after w or Gcn5 knockdown. Bars: 5 μm. (G) Line charts represent the numbers of nuclear GFP-SetDB1 foci in w KD and Gcn5 KD embryos from embryonic cycle 11 to cycle 14. Two-way ANOVA, ns: no significance, ****P < 0.0001. Error bars represent the SD. (H) Western blot analysis of w KD and Gcn5 KD 0 to 2 h embryos with tubulin and GFP antibodies. The protein level of the endogenously tagged GFP-SetDB1 shows no statistical difference. Unpaired t-test, ns: no significance. The error bar represents the SD. (I) Western blot analysis of w KD and Gcn5 KD 0 to 2 h embryos with H3 and H3K9me3 antibodies. The H3K9me3 modification is downregulated in Gcn5 KD embryos. Unpaired t-test, *P < 0.05. The error bar represents the SD.
To further ascertain the contribution of H3K14ac to the nuclear foci formation of Eggless/SetDB1, we knocked down the expression of the major H3K14ac acetyltransferase Gcn5 in the ovaries and early embryos using nos-Gal4 (Fig. 2D) (25). The nos-Gal4-driven Gcn5 knockdown (KD) decreased the level of H3K14ac modification in both ovaries and early embryos (SI Appendix, Fig. S2A and Fig. 2E). H3K9ac modification was also decreased although to a smaller extent after Gcn5 KD, whereas other histone acetylations such as H4K5ac and H4K8ac were not affected (SI Appendix, Fig. S2 A and B). In Gcn5 KD embryos, the accumulation of GFP-SetDB1 on nuclear foci in interphases of cycle 12 to cycle 14 was significantly diminished (Fig. 2 F and G and Movies S4 and S5). The protein level of GFP-SetDB1 was unaffected by the maternal KD of Gcn5 (Fig. 2H), however, the deposition of H3K9me3 was reduced, likely due to the compromised nuclear foci formation of Eggless/SetDB1 (Fig. 2I). Taking into consideration that H3K9ac was not enriched at the apical nuclear pole (Fig. 1D), we concluded that the observed reduction of GFP-SetDB1 foci and H3K9me3 in Gcn5 KD embryos was primarily attributed to the decreased level of H3K14ac modification.
Erasing the H3K14ac Compromises Transposon Silencing and Heterochromatin Integrity.
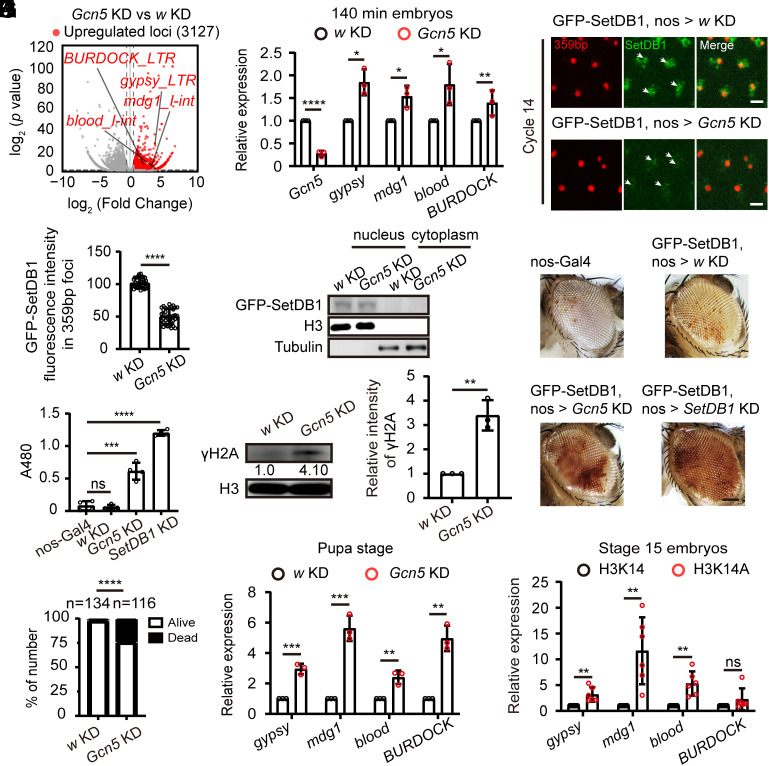
The disruption of Eggless/SetDB1 localization and reduction of H3K9me3 after maternal Gcn5 KD indicated errors in the establishment of heterochromatic silencing. We collected control and Gcn5 KD embryos at cycle 14 or embryonic stage five, and performed RNA-sequencing (RNA-seq) analysis to examine the expression of repetitive DNA elements. A total of 3,127 repeat loci exhibited an upregulated expression level in Gcn5 KD embryos (Fold Change ≥ 1.5, P < 0.05), among which 1,067 loci were satellite elements and transposons belonging to 122 different classes (Fig. 3A and Dataset S1). For validation, we selected several affected transposons, including gypsy, blood, BURDOCK, and mdg1, and confirmed their increased transcription by RT-qPCR in embryos with Gcn5 KD (Fig. 3B).
Fig. 3.
nos-Gal4-driven Gcn5 knockdown compromises constitutive heterochromatin. (A) Volcano plot of repeats expressions in w KD control versus Gcn5 KD embryos (stage 5, 140 min). Upregulated repeat loci after Gcn5 KD (Fold Change ≥ 1.5, P < 0.05) are plotted in red. (B) RT-qPCR validation of upregulation of 4 representative transposons (gypsy, mdg1, blood, and BURDOCK) in Gcn5 KD embryos. Unpaired t-test, *P < 0.05, **P < 0.01, ****P < 0.0001. Error bars represent the SD. (C) Video frames from live imaging experiments showing reduced recruitment of GFP-SetDB1 (green) to the 359-bp satellite sequences in Gcn5 KD embryos. The 359-bp loci were visualized by its TALE-light probe (red). The white arrows indicate the 359-bp regions. Bars: 5 μm. (D) Quantification of GFP-SetDB1 fluorescent signals within 359-bp regions in w KD and Gcn5 KD embryos. Unpaired t-test, ****P < 0.0001. Error bars represent the SD. (E) Western blot analysis of GFP-SetDB1 in the nuclear and cytoplasmic fractions of stage 5 w KD or Gcn5 KD embryos. Histone H3 and tubulin were used as nuclear and cytoplasmic markers, respectively. (F) Eye pictures from female wm4h (inversion on chromosome X) PEV reporter flies with nos-Gal4-driven knockdown of w, Gcn5, or SetDB1 respectively. Bar: 50 μm. (G) Pigment assay of wm4h reporter flies after knockdown of the indicated genes. Pigment was extracted and the OD at 480 nm was measured. Unpaired t-test, ns: no significance, ***P < 0.001, ****P < 0.0001. Error bars represent the SD. (H) Quantification of successful eclosions in w KD control versus Gcn5 KD flies. Chi-square test, ****P < 0.0001. (I) RT-qPCR analysis of transposons expression in nos-Gal4-driven w KD or Gcn5 KD flies at pupal stage. Unpaired t-test, **P < 0.01, ***P < 0.001. Error bars represent the SD. (J) Western blot analysis of pupae from nos-Gal4-driven w KD or Gcn5 KD flies with histone H3 and γH2A antibodies. The quantification shows increased γH2A signal in pupae from Gcn5 KD files. Unpaired t-test, **P < 0.01. The error bar represents the SD. (K) RT-qPCR analysis of transposons expression in stage 15 embryos carrying the H3K14 or H3K14A rescue His-GUs. Unpaired t-test, ns: no significance, **P < 0.01. Error bars represent the SD.
We further enlisted different reporting systems to evaluate the integrity of transcriptional silencing and heterochromatin on various repetitive sequences at different developmental stages. The abnormal activation of gypsy can be detected early during oogenesis by the gypsy-LacZ reporter (26). Following Gcn5 KD, we observed moderate X-gal staining signals, slightly weaker than that detected in the piwi KD ovaries (SI Appendix, Fig. S3A). The abnormally produced transcriptional products of repetitive DNA elements in the Gcn5 KD ovaries might be transmitted to the embryos. We collected unfertilized eggs and stage 5 embryos from w or Gcn5 KD parents and assessed the expression levels of gypsy, mdg1, blood, and BURDOCK by RT-qPCR. The level of gypsy was increased slightly in Gcn5 KD unfertilized eggs relative to that of w KD control, suggesting that some of the gypsy transcripts generated in the ovaries were loaded into mature eggs. However, in Gcn5 KD stage 5 embryos, the expression levels of gypsy, mdg1, blood, and BURDOCK were all significantly upregulated comparing to that in either w KD embryos or Gcn5 KD unfertilized eggs (SI Appendix, Fig. S3B). We therefore concluded that the aberrantly activated transposons detected in stage 5 Gcn5 KD embryos were largely due to abnormal heterochromatin establishment during embryonic development, with small contribution of maternal transmission.
Previous TALE-light imaging of tandem satellite repeats revealed that the 359-bp repetitive element recruits Eggless/SetDB1 and starts heterochromatinization during the late syncytial blastoderm stage (7, 8). Consistently, significant enrichment of GFP-SetDB1 within the 359-bp repeats region was detected in the interphase of cycle 14 control embryos, however, in embryos with Gcn5 KD, the accumulation of GFP-SetDB1 at the 359-bp loci was markedly abrogated (Fig. 3 C and D, and Movies S6 and S7). This was not due to compromised nuclear localization of GFP-SetDB1, as the nuclear-cytoplasmic fractionation assay showed that the GFP-SetDB1 remained nuclear and its protein level was not altered in the Gcn5 KD embryos (Fig. 3E).
The position-effect variegation (PEV) strain wm4h, which carries an inversion on the X chromosome that positions the white gene close to pericentromeric heterochromatin, is a strongly variegating reporter used to assess heterochromatin integrity (27). Compromised heterochromatic silencing leads to derepression of white that causes eye pigmentation. Maternal KD of Eggless/SetDB1 driven by nos-Gal4 resulted in increased pigment level compared to the control group. A similar but milder increase of pigmentation was also observed in flies with maternal KD of Gcn5 (Fig. 3 F and G), suggesting that proper Gcn5 activity in oocytes and early embryos is required for ensuring the fidelity of heterochromatin formation.
The compromised heterochromatin integrity after Gcn5 KD was associated with decreased viability. Approximately 25% of the progeny from the maternal Gcn5 KD flies died at the pupal stage (Fig. 3H). We collected these pupae and assessed transposon activation and genome stability. In the offspring pupae from the nos-Gal4-driven Gcn5 KD parents, elevated transcription of gypsy, blood, BURDOCK, and mdg1, alongside increased DNA damage indicated by γH2A abundance was observed (Fig. 3 I and J). Mutation of H3K14 to alanine (H3K14A) can abolish the acetylation modification and is embryonic lethal (20, 21). We collected homozygous H3K14A and H3K14 embryos at stage 15 and measured the transcription of gypsy, blood, BURDOCK, and mdg1. The embryos carrying the H3K14A rescue His-GUs in a histone deficiency background manifested higher transposon activity than that with the wild type H3K14 rescue His-GUs (Fig. 3K).
Together, these results suggest that the presence of H3K14ac is required for the establishment of transposon silencing and the fidelity of heterochromatin formation during early embryonic development.
Gcn5 KD Results in Decreased Eggless/SetDB1 and H3K9me3 on a Fraction of Transposons and Satellites.
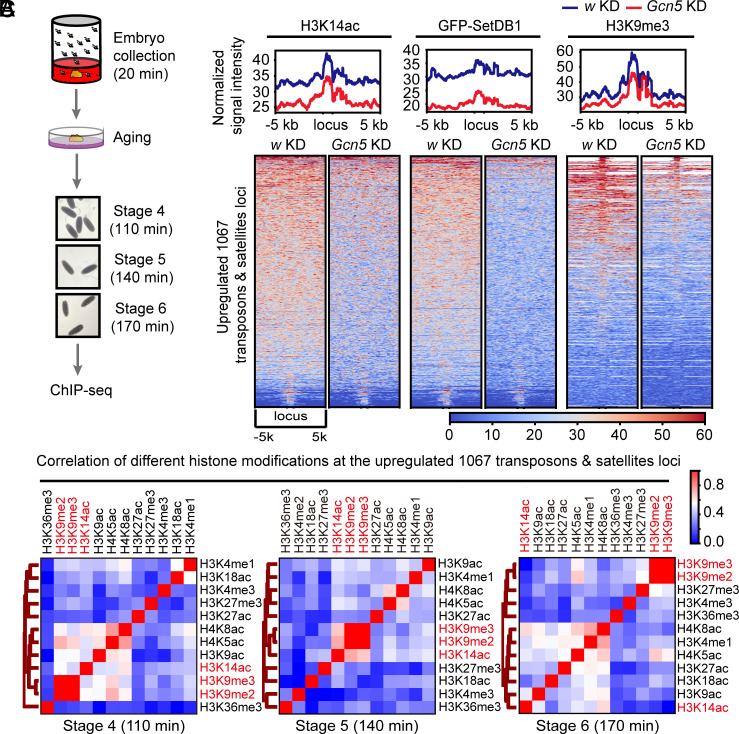
The establishment of heterochromatic silencing on different portions of the repetitive genome involves diverse molecular programs (8). RNA-seq analysis revealed that 1,067 transposons and satellite loci manifested elevated transcription upon maternal KD of Gcn5 (Fig. 3A), suggesting that these repetitive elements are regulated by Gcn5-dependent heterochromatin formation. To elucidate the developmental distribution of H3K14ac as well as H3K9me3 in these genomic regions, we conducted chromatin immunoprecipitation sequencing (ChIP-seq) using hand-staged embryos (Fig. 4A), and compared the results with published ChIP-seq datasets of other histone modifications generated with Drosophila early embryos (28).
Fig. 4.
nos-Gal4-driven Gcn5 KD results in decreased SetDB1 and H3K9me3 on a fraction of transposons and satellites loci. (A) Schematics of embryo collection and preparation for ChIP-seq analysis. (B) Heatmaps demonstrating the correlation coefficient among different histone modifications at different embryonic stages on the 1,067 upregulated transposons and satellites elements identified in the RNA-seq analysis of Gcn5 KD and w KD embryos. (C) At the top, averaged normalized ChIP-seq signals of H3K14ac, GFP-SetDB1, and H3K9me3 around the 1,067 upregulated repeats loci in Gcn5 KD and w KD embryos. At the bottom, heatmaps of H3K14ac, GFP-SetDB1, and H3K9me3 ChIP-seq signals around the 1,067 upregulated transposons and satellites loci in Gcn5 KD and w KD embryos.
At syncytial blastoderm stage (stage 4) when the process of heterochromatinization just initiates, the amounts of H3K9me2 and H3K9me3 modifications are minimum. Several histone modifications manifested moderate correlations with each other at the 1,067 transposons and satellites loci. As embryos developed to stage 5, the critical time window for constitutive heterochromatin formation, the correlation among H3K14ac and H3K9me2/3 stood out. In stage 6 embryos, H3K14ac was no longer clustered with H3K9 methylation modifications (Fig. 4B). This dynamic co-occupancy between H3K14ac and H3K9me2/3 at different developmental stages was reminiscent of antibody staining patterns of these modifications in Drosophila early embryos (Fig. 1A).
To directly investigate the impact of H3K14ac on the genomic distribution of Eggless/SetDB1 and H3K9me3, we collected stage 5 embryos from flies with maternal KD of Gcn5 and performed ChIP-seq analysis. While the baseline of the normalized ChIP-seq signals generated with w KD or Gcn5 KD embryos remained comparable (SI Appendix, Fig. S4A), Gcn5 KD resulted in a dramatic decline of H3K14ac genome-wide as well as at the 1067 repetitive loci that exhibited significant upregulation of expression in Gcn5 KD embryos. Remarkably, GFP-SetDB1 and H3K9me3 were concomitantly downregulated at these genomic regions (Fig. 4C). On the contrary, at the transposons and satellite loci that manifested decreased expression upon maternal KD of Gcn5, GFP-SetDB1 signal was unaffected and H3K9me3 signal even showed a slight increase (SI Appendix, Fig. S4B).
Together, these results suggest that the Gcn5-catalyzed H3K14ac histone mark is instrumental for the recruitment of Eggless/SetDB1, deposition of H3K9me3, and hence the establishment of heterochromatic silencing on a fraction of repetitive elements during Drosophila early embryogenesis.
Specific Recognition of H3K14ac by the Tandem TD of Eggless/SetDB1.
We next wanted to unravel the molecular basis underlying this contribution of H3K14ac to the establishment of constitutive heterochromatin in Drosophila early embryos. It has been reported that human SetDB1 can recognize the H3K14ac and H3K9me2/3 doubly modified H3 tails in vitro via its N-terminal triple Tudor domains (3TD) (22). To investigate whether Drosophila Eggless/SetDB1 could bind H3K14ac, we first conducted an analysis of sequence similarity of the 3TD among Drosophila, human, and mouse (SI Appendix, Fig. S5A). The sequence alignment revealed that, while the 3TD of human SetDB1 (hSETDB1) exhibited a 99% sequence identity with mouse SetDB1 (mSETDB1), it only displayed a 33% sequence identity with that of Drosophila Eggless/SetDB1 (DmSetDB1). Nonetheless, what stood out was the high degree of conservation of amino acid residues responsible for mediating the interaction with H3K14ac within the 3TD across Drosophila, mouse, and human (SI Appendix, Fig. S5A). Therefore, we hypothesized that Drosophila Eggless/SetDB1 has the potential to recognize the H3K14ac histone mark through its TD.
To verify this potential interaction, we determined the crystal structures of the 3TD of Drosophila Eggless/SetDB1 in both its apo-state and in complex with an H3 peptide containing H3K9me2 and H3K14ac modifications (H3K9me2K14ac). The 3TD of Drosophila Eggless/SetDB1, similar to its human counterpart, consists of three classical TD (TD1, TD2, and TD3) that adopted an antiparallel β-barrel-like structure (Fig. 5A). Superposition of the structures of Drosophila 3TD in its apo-state and in the H3K9me2K14ac peptide-bound complex showed that the two structures overlapped very well with an RMSD value of 1.52 Å over all the Cα-atoms of the 3TD. However, in the Drosophila 3TD-H3K9me2K14ac complex, only the S10-P16 fragment of the H3K9me2K14ac peptide was observed in the electron density map, while the residues T3-K9 in the peptide were disordered and invisible (Fig. 5 A and B). Further structural analysis showed that the H3K14ac peptide was positioned at the interface between the TD2 and TD3 (Fig. 5B). Since the 3TD of human SetDB1 interacts with H3K9me2/3 and H3K14ac doubly modified peptide (22), we compared the structures of the Drosophila 3TD-H3K14ac and the human 3TD-H3K9me2/3K14ac complexes. As expected, the residues involved in H3K14ac recognition exhibited a high degree of sequence and structural similarity (Fig. 5C). However, several aromatic residues involved in the H3K9me2/3 recognition by the human 3TD were not conserved in Drosophila 3TD. In human 3TD, the TD2 recognizes the H3K9me3 mark with an aromatic cage formed by residues Y268, W275, Y277, F297, and Y301 (Fig. 5D), and the TD3 forms an aromatic cage via the residues W358, W363, and F379 to accommodate the H3K9me2 modification with an additional hydrogen bond provided by residue D382 (Fig. 5E). Mutation of any of these aromatic residues in human 3TD abolished or reduced the binding affinity to the H3K9me1/2/3K14ac peptide (22). Structural comparison revealed that W275 in human TD2 was substituted with A557 in Drosophila TD2, and W358 and W363 in human TD3 were replaced by S640 and L645 in Drosophila TD3, respectively (Fig. 5 D and E). The absence of these aromatic residues in Drosophila 3TD disrupted the formation of complete aromatic cages, likely abrogating the recognition of H3K9 methylations.
Fig. 5.
Drosophila SetDB1 recognizes H3K14ac modification via its interface between TD2 and TD3. (A) Crystal structure of the DmSetDB1 3TD bound to the H3K9me2K14ac peptide. Residues T3-K9 of the peptide were disordered and invisible. On the left, the 3TD of DmSetDB1 is shown in the cartoon representation. On the right, electrostatic surface representation of the 3TD of DmSetDB1 bound to the H3K9me2K14ac peptide. The H3K9me2K14ac peptide is shown as a stick model and colored magenta. (B) 2mFo-DFc map for the H3K9me2K14ac peptide, which is contoured at 1σ by PyMOL. (C) Comparison of the H3K14ac residue recognition between the 3TDs of DmSetDB1 and hSETDB1 (PDB: 6BHI). (D and E) Comparisons of the aromatic cages in TD2 and TD3 between DmSetDB1 and hSETDB1 (PDB: 6BHI), respectively. The different residue is marked using a red dashed circle. The black dashed lines represent the hydrogen bonds formed between protein residues and the peptide. (F and G) Detailed interactions of the 3TD of DmSetDB1 bound to the H3K9me2K14ac peptide. Hydrogen bonds formed between protein and peptide are marked as black dashed lines. (H) SPR analysis of wild type (WT) TD and the F578A mutant binding to the H3K14ac peptides. The mean equilibrium dissociation constants (Kd) were determined from 3 independent experiments. (I) The table on the left lists the sequences of synthesized histone H3 and H4 peptides with different acetylation modifications. SPR analyses of DmSetDB1 3TD binding to H3K14ac, H3K9ac, H4K5ac, and H4K8ac peptide are shown on the right. The mean equilibrium dissociation constants (Kd) were determined from 3 independent experiments.
To investigate whether the interaction between Drosophila 3TD and H3K14ac is specific, we analyzed the binding interface between Drosophila 3TD and the H3K14ac peptide. In the complex structure, the K14ac mark was stabilized through hydrophobic interactions with the side chains of F578, T584, F613, I671, and Y672 (Fig. 5F). The main chains of G582 and Y672 also contributed to the recognition of the K14ac mark by forming two hydrogen bonds with the acetyl group of K14ac (Fig. 5F). In addition to the acetyl group, the main chain of K14ac formed a hydrogen bond with the side chain of S675 (Fig. 5F). For other residues of the peptide, the residue T11 was stabilized by forming a hydrogen bond with G582 of TD2, while G12 and G13 in the peptide made hydrogen bonding interactions with the side chains of E669 and R677 of TD3, respectively. Furthermore, the main chain carbonyl oxygen of P16 formed a hydrogen bond with the side chain of E639 of TD3 (Fig. 5G). To directly measure the binding affinity between Drosophila 3TD and H3K14ac peptide, we employed surface plasmon resonance (SPR) analysis. The equilibrium dissociation constants (Kd) of wild type 3TD toward H3K14ac peptide was 338.7 μM. When F578, a key residue interacting with the H3K14 acetyl group, was mutated to alanine (F578A), the Drosophila 3TD could no longer bind to the H3K14ac peptide (Fig. 5H).
To further determine whether the recognition of acetylated lysine by Drosophila 3TD is specific to H3K14, we synthesized other acetylated histone peptides including H3K9ac, H4K5ac, and H4K8ac, and performed SPR analysis to evaluate their binding affinity to Drosophila 3TD (Fig. 5I). The Kd values of Drosophila 3TD toward H3K14ac, H3K9ac, H4K5ac, and H4K8ac peptides were 338.7 μM, 822.6 μM, 925.1 μM, and 653.8 μM, respectively, indicating that Drosophila 3TD exhibits the highest affinity for the H3K14ac peptide. The 3TD-H3K14ac structure showed that the sequence spanning from T11 to P16 in the H3 peptide is essential for the interactions of Drosophila and human 3TDs (22) (Fig. 5G). Mutating G12 and G13 of H3K14ac peptide to alanine or any bigger residue would diminish or prevent the binding of human 3TD due to the narrow groove between TD2 and TD3 (22). Sequence analysis revealed that the G12 and G13 of H3K14ac peptide are substituted with alanine and arginine, respectively, in the corresponding positions of the H3K9ac peptide, explaining the reduced binding of Drosophila 3TD to H3K9ac peptide (SI Appendix, Fig. S5B). Similarly, the T11 and G12 of the H3K14ac peptide are replaced by glycine and arginine, respectively, in the corresponding positions of the H4K5ac peptide. Structural model showed that the substitution of T11 with glycine disrupts the interaction between T11 and G582 of TD2. Additionally, when G12 is substituted with arginine, the arginine side chain clashes with the residues of TD3 (SI Appendix, Fig. S5C). In the H4K8ac peptide, the T11 of the H3K14ac peptide is substituted with lysine in the corresponding position. Structural analysis indicated that this substitution also disrupts the hydrogen bonding interaction between T11 and G582 of TD2, introducing a steric clash between the lysine side chain and the loop in TD2 (SI Appendix, Fig. S5D). Thus, these structural modeling also support that Drosophila 3TD bears a preference for binding to H3K14ac over other three acetylation marks containing peptides.
In summation, our comprehensive structural and binding analyses shed light on the fact that the TD of Drosophila Eggless/SetDB1 can recognize the H3K14ac histone mark in a specific manner, without the need for H3K9 methylations, although the in vitro binding affinity is relatively modest.
Disruption of the Interaction Between Eggless/SetDB1 and H3K14ac Reduces Eggless/SetDB1 Nuclear Foci Formation in the Early Embryo.
To investigate the in vivo function of the recognition of H3K14ac by Eggless/SetDB1, we injected Drosophila embryos with a recently developed binder competitive inhibitor (R,R)-59 of the tandem TD of SetDB1 (29), and then performed live imaging of the distributions of the endogenously tagged GFP-SetDB1 as well as the His2Av-mRFP expressed from a transgene (SI Appendix, Fig. S6A). Injection of (R,R)-59 weakened the accumulation of GFP-SetDB1 into nuclear foci and caused cell cycle arrest in Drosophila syncytial embryos (Movies S8 and S9), although we could not rule out the possibility of off-target effects of the injected small molecular inhibitor.
To precisely disrupt the interaction between Eggless/SetDB1 and H3K14ac, we generated knock-in mutations via CRISPR-Cas9 to recode F578 to alanine (F578A) in both wild type flies and that carry the endogenously GFP-tagged Eggless/SetDB1 alleles (SI Appendix, Fig. S6B). The homozygous F578A mutants were infertile and semilethal, as the ratio of heterozygotes to homozygotes in the progeny of heterozygous parents significantly deviated from the expected 2:1 seen in the wild type (SI Appendix, Fig. S6C). Moreover, the SetDB1F578A seemed to have a dominant negative effect, because even the heterozygous F578A mutants showed increased developmental defects and decreased viability.
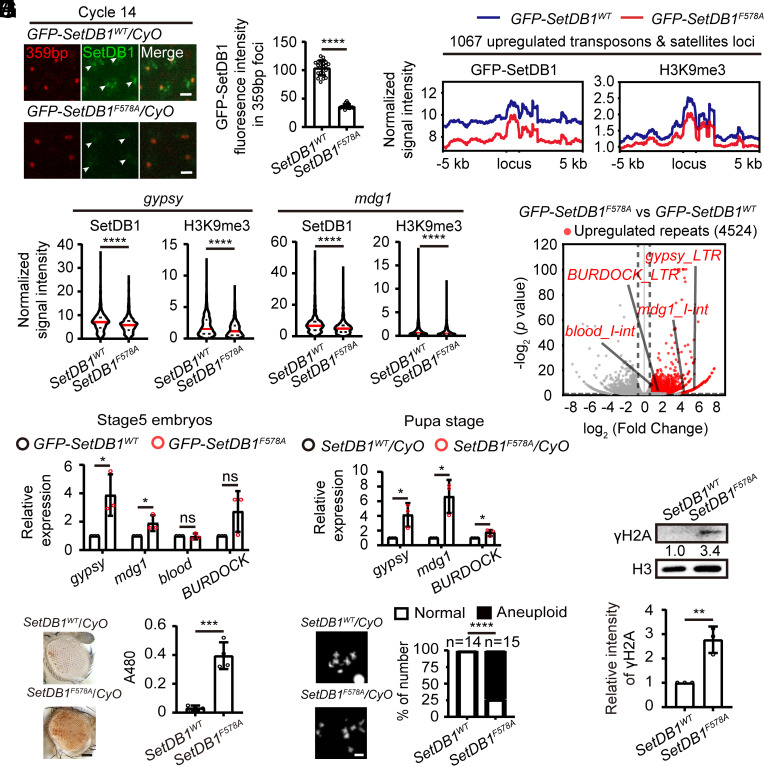
To investigate the impact of F578A mutation on the distribution of GFP-SetDB1, we collected embryos from heterozygous parents and performed live imaging analysis (Fig. 6A). Compared to wild type GFP-SetDB1 that was recruited to several nuclear foci including the 359-bp repeats region in cycle 14 embryos, GFP-SetDB1F578A appeared to be more diffusive, with decreased fluorescent intensity in the nuclei as well as within the 359-bp repeat loci (Fig. 6 A and B and Movies S10 and S11). To test the potential dominant negative effect of the F578A mutation, we generated GFP-SetDB1WT/SetDB1WT and GFP-SetDB1WT/SetDB1F578A flies and collected their embryos for live imaging (SI Appendix, Fig. S6D and Movies S12 and S13). GFP-SetDB1 was abundantly recruited to the 359-bp repeat loci in the wild type background. However, in the presence of SetDB1F578A, the recruitment of wild type GFP-SetDB1 to the 359-bp repeat loci was reduced (SI Appendix, Fig. S6 E and F), confirming a dominant negative effect of SetDB1F578A on the localization of wild type SetDB1. Western blot analysis revealed that the protein level of GFP-SetDB1F578A remained comparable to that of wild type (SI Appendix, Fig. S6 G and H), suggesting that the mutation did not affect protein stability. Of note, we observed a slight decrease of H3K9me3 in embryos collected from GFP-SetDB1F578A heterozygous parents (SI Appendix, Fig. S6I). These changes were reminiscent of that observed in the embryos with Gcn5 KD (Fig. 2 H and I), indicating that disruption of Eggless/SetDB1 localization compromises the deposition of H3K9me3.
Fig. 6.
F578A knock-in of SetDB1 compromises transposon silencing and heterochromatin integrity. (A) Video frames from live imaging analysis of embryos from GFP-SetDB1WT/CyO or GFP-SetDB1F578A/CyO flies. GFP-SetDB1 is shown in green, and the 359-bp loci labeled with TALE-light is shown in red. White arrows indicate the 359-bp repeats regions. Bars: 5 μm. (B) Quantification of the mean GFP fluorescent intensity within the 359-bp repeats loci in cycle 14 embryos. Unpaired t-test, ****P < 0.0001. Error bars represent the SD. (C) Averaged ChIP-seq signals of GFP-SetDB1 and H3K9me3 around the aforementioned 1,067 upregulated transposons and satellites loci in embryos collected from GFP-SetDB1WT/CyO or GFP-SetDB1F578A/CyO flies. (D) Violin plots of the ChIP-seq signals of GFP-SetDB1 or H3K9me3 at gypsy and mdg1 genomic loci. Mann–Whitney test, ****P < 0.0001. (E) Volcano plot of repeats expressions in embryos (stage 5, 140 min) collected from GFP-SetDB1WT/CyO or GFP-SetDB1F578A/CyO flies. Upregulated repeat elements in the SetDB1F578A experimental group (Fold Change ≥ 1.5, p < 0.05) are plotted in red. (F) RT-qPCR analysis of the 4 Gcn5 KD-sensitive transposons (gypsy, mdg1, blood, and BURDOCK) in stage5 embryos collected from GFP-SetDB1WT/CyO or GFP-SetDB1F578A/CyO flies. Unpaired t-test, ns: no significance, *P < 0.05. Error bars represent the SD. (G) On the left side, eye pictures from the female wm4h PEV reporter flies with the indicated genotypes. Bar: 50 μm. On the right side, pigment assay of the control flies or that carrying the SetDB1F578A knock-in mutation. Unpaired t-test, ***P < 0.001. Error bars represent the SD. (H) Chromosome spreads of 3rd instar neuroblasts from control or the SetDB1F578A knock-in mutant. Chi-square test, ****P < 0.0001. (I) RT-qPCR analysis of the indicated transposons at pupal stage. Unpaired t-test, *P < 0.05. Error bars represent the SD. (J) Western blot analysis of pupae from the indicated flies with H3 and γH2A antibodies. The quantification shows increased γH2A signal in the SetDB1F578A knock-in mutant. Unpaired t-test, **P < 0.01. The error bar represents the SD.
Heterozygous Eggless/SetDB1F578A Knock-in Mutation Compromises Heterochromatin Integrity.
We mapped the genomic distribution of GFP-SetDB1F578A in comparison to GFP-SetDB1WT as well as the corresponding H3K9me3 histone modification in stage 5 embryos collected from heterozygous parental flies using ChIP-seq. The overall signals of GFP-SetDB1 and H3K9me3 after normalization were decreased in embryos carrying the F578A mutation (SI Appendix, Fig. S7A). The GFP-SetDB1 and H3K9me3 signals at the 1,067 transposons and satellite elements loci that were responsive to the KD of Gcn5 were also markedly declined in the GFP-SetDB1F578A mutant embryos (Fig. 6C and SI Appendix, S7B). At the gypsy and mdg1 loci, the amounts of GFP-SetDB1 and H3K9me3 signals were significantly lower in embryos carrying GFP-SetDB1F578A compared to that carrying the GFP-SetDB1WT (Fig. 6D). We further performed RNA-seq to analyze the aberrant transcription of repetitive DNA elements (Dataset S2). 74 out of the 122 classes of repetitive elements that were identified in the RNA-seq experiments with Gcn5 KD embryos manifested consistent transcriptional derepression in the GFP-SetDB1F578A embryos (Figs. 3 A and 6E and Dataset S3). We selected several transposons and validated their expression by RT-qPCR (Fig. 6F). Similar to the observation in Gcn5 KD embryos, the upregulated expression of transposons detected in stage 5 embryos carrying the SetDB1F578A mutation was mainly due to defects in heterochromatin establishment, rather than maternal transmission of abnormal transposons activities from the oocytes (SI Appendix, Figs. S7C and S3B).
The reduced deposition of H3K9me3 and transcriptional derepression of transposons suggested that the integrity of constitutive heterochromatin was compromised in the offspring of SetDB1F578A heterozygous parents. Consistently, we crossed the SetDB1F578A allele to the PEV reporter and observed that even heterozygous mutation of SetDB1F578A resulted in increased eye pigmentation (Fig. 6G). Defects in constitutive heterochromatin often accompany genomic instability. In the neuroblasts of third instar larvae, the aneuploidy rate was significantly higher in SetDB1F578A heterozygous mutants compared to wild type (Fig. 6H). At pupal stage, many transposons such as gypsy, mdg1, and BURDOCK showed increased activity in SetDB1F578A heterozygous mutants, and the DNA damage-associated γH2A abundance was upregulated concomitantly (Fig. 6 I and J).
These results indicate that, similar to Gcn5 KD which erases the H3K14ac histone mark, disrupting the interaction of Eggless/SetDB1 with the H3K14ac via substitution of F578 with alanine also causes defects in the genomic distributions of Eggless/SetDB1 and H3K9me3, compromising constitutive heterochromatin integrity and organismal viability.
Discussion
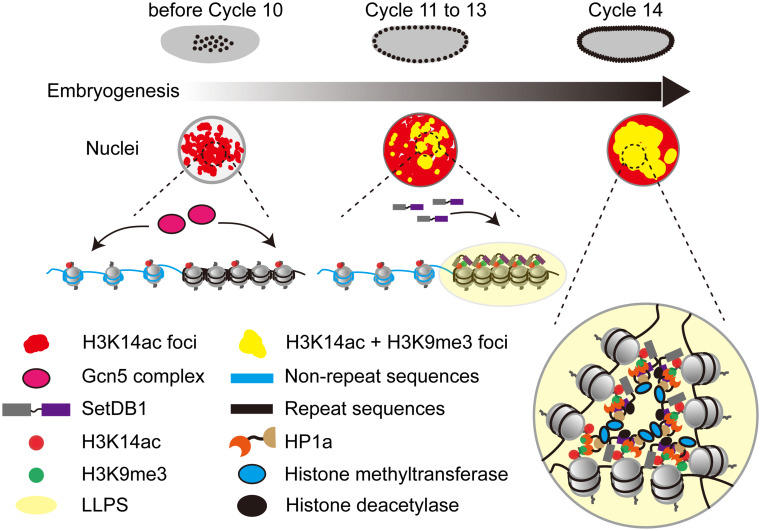
The restoration of epigenetic constraints in zygotic genome is central to early embryonic developmental programming. Constitutive heterochromatin, characterized by enrichment of H3K9me2/3 and HP1a, needs to be reestablished with precision to suppress repetitive DNA elements and maintain genomic stability. While the mechanisms that introduce the heterochromatic features to different repetitive sequences are complex (8), a key step in this process is the targeted recruitment of the H3K9 methyltransferase Eggless/SetDB1 (7). Our study reports a mode of recruitment of Eggless/SetDB1, in which an active histone modification H3K14ac directly attracts Eggless/SetDB1 via its 3TD. Structural analyses show that Eggless/SetDB1 can specifically recognize the H3K14ac histone mark independent of the H3K9 modification status, indicating that H3K14ac nucleosomes concentrate Eggless/SetDB1 to initiate heterochromatin formation during early embryogenesis. Consistently, either removing H3K14ac histone mark or disrupting the recognition of H3K14ac by Eggless/SetDB1 causes defects in both H3K9me3 deposition and transcriptional silencing of many repetitive elements. Therefore, we propose that H3K14ac is an essential component of the molecular machinery that reinstalls the constitutive heterochromatic features during early embryonic development (Fig. 7).
Fig. 7.
A proposed model illustrating how SetDB1 condensates on the H3K14ac-clustered repetitive sequences to participate in constitutive heterochromatin establishment.
Eggless/SetDB1 is evolutionarily conserved, with SUMO interacting motifs (SIM) and triple Tudor domains (3TD) at the N terminus, and a bifurcated SET domain harboring the H3K9 methyltransferase activity at the C terminus (30, 31). Previous studies suggest that the SIM can mediate the targeted recruitment of Eggless/SetDB1 by recognition of SUMOylated chromatin proteins. For example, in mouse embryonic stem cells, the KRAB zinc finger family proteins (KRAB-zfps) recognize different retrotransposon elements meanwhile interact with KAP1, an intramolecular E3 ligase that can self-SUMOylate its lysine residues. These SUMOylated lysines of KAP1 are in turn recognized by the SIM of Eggless/SetDB1, mediating its selective enrichment on retrotransposons (30). In Drosophila ovaries, the recruitment of Eggless/SetDB1 to transposable elements also seems to be dependent on the recognition of SUMOylated substrates by the SIM. Relying on its interacting small RNAs (piRNAs), the Argonaute family protein Piwi is selectively engaged with nascent transcripts from the corresponding transposons. Piwi forms a complex with Asterix/Gtsf1, Panoramix/silencio, Nxf2, Nxt1, Ctp, and the SUMO E3 ligase Su(var)2 to 10, which induces local SUMOylation on yet-to-be-identified substrates to recruit Eggless/SetDB1 (10–13). It is noteworthy that previous functional analyses have revealed that the SUMOylation-dependent mechanism only instructs heterochromatin on a fraction of repetitive elements (9, 14). The H3K14ac-mediated recruitment of Eggless/SetDB1 reported here is apparently another important mechanism to regulate repetitive elements in the early embryonic development, as disrupting the interaction between 3TD and H3K14ac causes a significant reduction of Eggless/SetDB1 binding and the H3K9me3 level on many genomic loci. The potential synergistic interactions of these two recruitment mechanisms during the reinstallation of constitutive heterochromatin in the early embryos warrant future elaboration.
As a histone modification normally associated with active transcription, the genomic distribution of H3K14ac is rather broad, manifesting dynamic co-occupancy with H3K9me2/3 only on a fraction of repetitive elements at specific developmental stage in Drosophila early embryos. Around the time window of the MBT (late stage 4 and stage 5) when cell cycles slow down and constitutive heterochromatin makes its first appearance, H3K14ac becomes increasingly concentrated at the apical poles of nuclei, forming bivalent genomic regions with H3K9me2/3. In stage 6 embryos after the establishment of constitutive heterochromatin, H3K14ac is no longer correlating with H3K9me2/3. How the distribution of H3K14ac is dynamically controlled remains unclear. The maintenance of H3K14ac during Drosophila early embryonic development is mainly mediated by Gcn5. Yet, at least four different Gcn5 complexes exist in Drosophila: the Spt-Ada-Gcn5 Acetyltransferase (SAGA), Ada2a-containing (ATAC), Ada2/Gcn5/Ada3 transcription activator (ADA), and Chiffon Histone Acetyltransferase (CHAT) complexes (18). While it is not clear which complex controls the H3K14ac dynamics in the early embryo, we think the CHAT complex is worthy of extra attention (32), because Chiffon also encodes a subunit for the Dbf4-dependent kinase (DDK) complex, which regulates cell cycle length and late replication of repetitive sequences by counteracting Rif1 activity (33). It is noteworthy that in addition to H3K14ac, Gcn5 can also catalyze H3K9ac (19). However, unlike H3K14ac, H3K9ac is predominantly deposited to chromosome arms rather than the apical nuclear regions. Future studies should address the spatial distribution of the CHAT complex in the early embryos and test the possibility whether it has a preference to acetylate H3K14 over H3K9. The deacetylase responsible for removing H3K14ac from heterochromatic regions in later development remains unknown, but one potential candidate is Rpd3, which can counteract genomic silencing both in Drosophila and yeast (34), and interact with Eggless/SetDB1 as well as nucleosome remodeler Mi-2 (12). Additionally, the in vitro binding affinity between 3TD of Eggless/SetDB1 and H3K14ac is modest, and we cannot rule out the possible involvement of other histone acetylations in the recruitment of Eggless/SetDB1 during the reinstallation of constitutive heterochromatin in Drosophila early embryos.
The reinstallation of constitutive heterochromatin in the zygotic genome during early embryogenesis is precisely controlled in time and space. The timing of heterochromatin formation in Drosophila early embryos seems to be linked to the developmentally regulated slowing of the cell cycle (5). The fly embryos begin development with 13 rapid and metasynchronous nuclear divisions, and the frequent mitoses at this stage strips Eggless/SetDB1 as well as HP1a from chromatin and prevent their fruitful accumulation at the repetitive DNA loci (7, 8). At the MBT, Cdk1 activity is downregulated and interphase duration of cycle 14 is significantly prolonged, allowing for the progression of other developmental events including establishment of constitutive heterochromatin (5, 7). Consistently, arresting the early cell cycle by injection of dsRNA against mitotic cyclins permitted continued accumulation of Eggless/SetDB1 and HP1a into larger nuclear foci resembling that seen in later developmental stage, suggesting a timing function of interphase duration on heterochromatin formation (7). Therefore, even though H3K14ac and Eggless/SetDB1 are already present in the early embryos, the H3K14ac-mediated Eggless/SetDB1 recruitment is deferred until the cell cycle slows down at the MBT. On the other hand, our understanding of the spatial accuracy during heterochromatin formation, meaning which portion of the genome is selectively packaged into transcriptionally inert heterochromatic compartment, is only rudimentary. Considering that H3K14ac is widely distributed in the genome, it is not immediately clear how H3K14ac specifically concentrates Eggless/SetDB1 on a fraction of repetitive DNA elements to initiate heterochromatin formation. Phase separation mechanisms, driven by multivalent weak interactions, might underlie the H3K14ac-Eggless/SetDB1-dependent heterochromatic compartmentalization. Eggless/SetDB1 contains many low complexity sequences and is predicted to have the capacity to undergo phase separation. In addition, Eggless/SetDB1 carries several PXVXL motifs that can interact with HP1a, which has been reported to undergo liquid–liquid phase separation that drives the formation of heterochromatic domains in the early embryos (35). Many repetitive elements in the apical nuclear regions, such as the 359-bp repeats, exhibit high compaction in the early embryos before attaining heterochromatic repressive histone modifications (23). Accordingly, our quantification of H3K14ac in 3D reconstructed nuclei showed that the concentration of H3K14ac in the apical regions is about 8 times of that in the nonapical regions. We therefore speculate that higher local concentration of H3K14ac in the repeats-rich apical regions would recruit more Eggless/SetDB1. Once the phase separation threshold is exceeded, Eggless/SetDB1 and HP1a undergo liquid–liquid demixing, introducing H3K9me3 on adjacent nucleosomes. This process initiates nuclear compartmentalization, eventually leading to the establishment of constitutive heterochromatic regions (Fig. 7). This model may explain the observed dominant negative effect of SetDB1F578A on the localization of wild type SetDB1. SetDB1F578A loses the H3K14ac recognition activity but retains the ability to form condensates with wild type SetDB1, compromising its precise genomic recruitment. Experimental validation of Eggless/SetDB1’s phase separation capacity and in vitro reconstitution of the process of heterochromatin assembly on representative repetitive elements such as the 359-bp repeats are needed to formulate a more coherent view on the reinstallation of constitutive heterochromatin during early embryogenesis.
Materials and Methods
Methods and information for Drosophila culture, live imaging, immunofluorescence, western blot, ChIP-seq, RNA-seq, protein crystallization, surface plasmon resonance assays, and reagent are provided in SI Appendix, SI Materials and Methods and Dataset S6. For statistical analysis, data were analyzed using GraphPad Prism 9 software. Detailed descriptions of statistical analyses are in the figure legends. Data are expressed as mean ± SD.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Dataset S05 (DOCX)
Dataset S06 (DOCX)
Control embryo microinjected with PBS. Timelapses were captured every 1 minutes for a duration of circa 21 minutes from cycle 11 interphase to 13 interphase. Bars: 5 μm. The movie is related to Fig 2B.
The embryo microinjected with H3K9me3 antibody. Timelapses were captured every 1 minutes for a duration of circa 21 minutes from cycle 11 interphase to 13 interphase. Bars: 5 μm. The movie is related to Fig 2B.
The embryo microinjected with H3K14ac antibody. Timelapses were captured every 1 minutes for a duration of circa 20 minutes from cycle 11 interphase to 13 interphase. Bars: 5 μm. The movie is related to Fig 2B.
GFP-SetDB1 distribution in w KD embryo. Timelapses were captured every 1 minutes for a duration of circa 49 minutes from cycle 11 interphase to 14 interphase. Bars: 5 μm. The movie is related to Fig 2F.
GFP-SetDB1 distribution in Gcn5 KD embryo. Timelapses were captured every 1 minutes for a duration of circa 47 minutes from cycle 11 interphase to 14 interphase. Bars: 5 μm. The movie is related to Fig 2F.
GFP-SetDB1 accumulation at 359-bp loci in w KD embryo. Timelapses were captured every 2 minutes for a duration of circa 30 minutes in cycle 14 interphase. Bars: 5 μm. The movie is related to Fig 3C.
GFP-SetDB1 accumulation at 359-bp loci in Gcn5 KD embryo. Timelapses were captured every 2 minutes for a duration of circa 30 minutes in cycle 14 interphase. Bars: 5 μm. The movie is related to Fig 3C.
Control embryo microinjected with DMSO. Timelapses were captured every 1 minutes for a duration of circa 31 minutes form cycle 11 interphase to 13 interphase. Bars: 5 μm. The movie is related to SI Appendix, Fig S6A.
The embryo microinjected with (R,R)-59 inhibitor. Timelapses were captured every 1 minutes for a duration of circa 30 minutes form cycle 11 interphase to 13 interphase. Bars: 5 μm. The movie is related to SI Appendix, Fig S6A.
GFP-SetDB1WT accumulation at 359-bp loci. Timelapses were captured every 2 minutes for a duration of circa 38 minutes in cycle 14 interphase. Bars: 5 μm. The movie is related to Fig 6A.
GFP-SetDB1F578A accumulation at 359-bp loci. Timelapses were captured every 2 minutes for a duration of circa 38 minutes in cycle 14 interphase. Bars: 5 μm. The movie is related to Fig 6A.
GFP-SetDB1WT accumulation at 359-bp loci in embryo carrying SetDB1WT. Timelapses were captured every 2 minutes for a duration of circa 28 minutes in cycle 14 interphase. Bars: 5 μm. The movie is related to SI Appendix, Fig S6D.
GFP-SetDB1WT accumulation at 359-bp loci in embryo carrying SetDB1F578A. Timelapses were captured every 2 minutes for a duration of circa 28 minutes in cycle 14 interphase. Bars: 5 μm. The movie is related to SI Appendix, Fig S6D.
Acknowledgments
We gratefully acknowledge Drs. Patrick H. O’Farrell, Chun-Yi Cho, Shengyong Yang, Yikang Rong, Zhouhua Li, Yang Yu, Jianye Zang, Su Qin, Xuebiao Yao, the Developmental Studies Hybridoma Bank, the Bloomington Drosophila Stock Center, and TsingHua Fly Center for inspiring discussions or reagents. We would also like to acknowledge the assistance of Huan Liu in the crystallization and Fengling Li in the SPR analysis. This project has been supported by the National Natural Science Foundation of China (grants 32170821, 92153301, and 32370821 to K.Y. and 32101034 to F.C, 31770834 to K.L), National Key Research and Development Program of China (2021YFC2701200), Department of Science & Technology of Hunan Province (grants 2023RC1028, 2021JJ10054, and 2023SK2091 to K.Y and 2022JJ40762 to F.C).
Author contributions
K.L. and K.Y. designed research; R.T., M.Z., Y.C., Z.J., and X.F. performed research; G.G. and K.Y. contributed new reagents/analytic tools; R.T., M.Z., Y.C., J.Z., A.D., L.L., S.M., F.C., J.M., K.L., and K.Y. analyzed data; and R.T., K.L., and K.Y. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Ke Liu, Email: keliu2015@mail.ccnu.edu.cn.
Kai Yuan, Email: yuankai@csu.edu.cn.
Data, Materials, and Software Availability
All study data are included in the article and/or supporting information. Sequencing data in this study have been deposited in the Gene Expression Omnibus (GEO) under accession number GSE242239 (36). Coordinates and structure factors have been deposited in the Protein Data Bank under accession ID 7UW8 (37) and 7UVE (38).
Supporting Information
References
- 1.Campos E. I., Stafford J. M., Reinberg D., Epigenetic inheritance: Histone bookmarks across generations. Trends Cell Biol. 24, 664–674 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allshire R. C., Madhani H. D., Ten principles of heterochromatin formation and function. Nat. Rev. Mol. Cell Biol. 19, 229–244 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J., Ali M., Zhou Q., Establishment and evolution of heterochromatin. Ann. N Y Acad. Sci. 1476, 59–77 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padeken J., Methot S. P., Gasser S. M., Establishment of H3K9-methylated heterochromatin and its functions in tissue differentiation and maintenance. Nat. Rev. Mol. Cell Biol. 23, 623–640 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan K., Seller C. A., Shermoen A. W., O’Farrell P. H., Timing the drosophila mid-blastula transition: A cell cycle-centered view. Trends Genet. 32, 496–507 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong R. L., Duronio R. J., Phasing in heterochromatin during development. Genes. Dev. 33, 379–381 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seller C. A., Cho C. Y., O’Farrell P. H., Rapid embryonic cell cycles defer the establishment of heterochromatin by Eggless/SetDB1 in Drosophila. Genes. Dev. 33, 403–417 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan K., O’Farrell P. H., TALE-light imaging reveals maternally guided, H3K9me2/3-independent emergence of functional heterochromatin in Drosophila embryos. Genes. Dev. 30, 579–593 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaskill M. M., et al. , Localization of the Drosophila pioneer factor GAF to subnuclear foci is driven by DNA binding and required to silence satellite repeat expression. Dev. Cell 58, 1610–1624 e1618 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batki J., et al. , The nascent RNA binding complex SFiNX licenses piRNA-guided heterochromatin formation. Nat. Struct. Mol. Biol. 26, 720–731 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao K., et al. , A Pandas complex adapted for piRNA-guided transcriptional silencing and heterochromatin formation. Nat. Cell Biol. 21, 1261–1272 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Mugat B., et al. , The Mi-2 nucleosome remodeler and the Rpd3 histone deacetylase are involved in piRNA-guided heterochromatin formation. Nat. Commun. 11, 2818 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ninova M., et al. , The SUMO Ligase Su(var)2–10 controls hetero- and euchromatic gene expression via establishing H3K9 trimethylation and negative feedback regulation. Mol. Cell 77, 571–585 e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabry M. H., et al. , Maternally inherited piRNAs direct transient heterochromatin formation at active transposons during early Drosophila embryogenesis. eLife 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenuwein T., Allis C. D., Translating the histone code. Science 293, 1074–1080 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Filion G. J., et al. , Systematic protein location mapping reveals five principal chromatin types in Drosophila cells. Cell 143, 212–224 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kharchenko P. V., et al. , Comprehensive analysis of the chromatin landscape in Drosophila melanogaster. Nature 471, 480–485 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres-Zelada E. F., Weake V. M., The Gcn5 complexes in Drosophila as a model for metazoa. Biochim. Biophys. Acta Gene. Regul. Mech. 1864, 194610 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Carre C., Szymczak D., Pidoux J., Antoniewski C., The histone H3 acetylase dGcn5 is a key player in Drosophila melanogaster metamorphosis. Mol. Cell Biol. 25, 8228–8238 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W., et al. , Probing the function of metazoan histones with a systematic library of H3 and H4 mutants. Dev. Cell 48, 406–419 e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regadas I., et al. , A unique histone 3 lysine 14 chromatin signature underlies tissue-specific gene regulation. Mol. Cell 81, 1766–1780 e1710 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Jurkowska R. Z., et al. , H3K14ac is linked to methylation of H3K9 by the triple Tudor domain of SETDB1. Nat. Commun. 8, 2057 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shermoen A. W., McCleland M. L., O’Farrell P. H., Developmental control of late replication and S phase length. Curr. Biol. 20, 2067–2077 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciabrelli F., et al. , CBP and Gcn5 drive zygotic genome activation independently of their catalytic activity. Sci. Adv. 9, eadf2687 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarro-Costa P., et al. , Early programming of the oocyte epigenome temporally controls late prophase I transcription and chromatin remodelling. Nat. Commun. 7, 12331 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarot E., Payen-Groschene G., Bucheton A., Pelisson A., Evidence for a piwi-dependent RNA silencing of the gypsy endogenous retrovirus by the Drosophila melanogaster flamenco gene. Genetics 166, 1313–1321 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elgin S. C., Reuter G., Position-effect variegation, heterochromatin formation, and gene silencing in Drosophila. Cold Spring Harb Perspect. Biol. 5, a017780 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X. Y., Harrison M. M., Villalta J. E., Kaplan T., Eisen M. B., Establishment of regions of genomic activity during the Drosophila maternal to zygotic transition. eLife 3, e03737 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Y., et al. , Structure-guided discovery of a potent and selective cell-active inhibitor of SETDB1 tudor domain. Angew. Chem. Int. Ed 60, 8760–8765 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Fukuda K., Shinkai Y., SETDB1-mediated silencing of retroelements. Viruses 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ninova M., et al. , Su(var)2–10 and the SUMO pathway link piRNA-guided target recognition to chromatin silencing. Mol. Cell 77, 556–570.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres-Zelada E. F., George S., Blum H. R., Weake V. M., Chiffon triggers global histone H3 acetylation and expression of developmental genes in Drosophila embryos. J. Cell Sci. 135, jcs259132 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seller C. A., O’Farrell P. H., Rif1 prolongs the embryonic S phase at the Drosophila mid-blastula transition. PLoS Biol. 16, e2005687 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Rubertis F., et al. , The histone deacetylase RPD3 counteracts genomic silencing in Drosophila and yeast. Nature 384, 589–591 (1996). [DOI] [PubMed] [Google Scholar]
- 35.Strom A. R., et al. , Phase separation drives heterochromatin domain formation. Nature 547, 241–245 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang R., Jiang Z., Yuan K., Establishment of constitutive heterochromatin in Drosophila early embryos involves H3K14ac-mediated recruitment of Eggless/SetDB1. Gene Expression Omnibus (GEO). https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE242239. Deposited 3 September 2023.
- 37.Zhou M., et al. , Drosophila melanogaster setdb1-tuor domain. RCSB PDB Protein Data Bank. 10.2210/pdb7UW8/pdb. Deposited 3 May 2022. [DOI]
- 38.Zhou M., et al. , Drosophila melanogaster setdb1-tuor domain with peptide H3K9me2K14ac. RCSB PDB Protein Data Bank. 10.2210/pdb7UVE/pdb. Deposited 1 May 2022. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Dataset S05 (DOCX)
Dataset S06 (DOCX)
Control embryo microinjected with PBS. Timelapses were captured every 1 minutes for a duration of circa 21 minutes from cycle 11 interphase to 13 interphase. Bars: 5 μm. The movie is related to Fig 2B.
The embryo microinjected with H3K9me3 antibody. Timelapses were captured every 1 minutes for a duration of circa 21 minutes from cycle 11 interphase to 13 interphase. Bars: 5 μm. The movie is related to Fig 2B.
The embryo microinjected with H3K14ac antibody. Timelapses were captured every 1 minutes for a duration of circa 20 minutes from cycle 11 interphase to 13 interphase. Bars: 5 μm. The movie is related to Fig 2B.
GFP-SetDB1 distribution in w KD embryo. Timelapses were captured every 1 minutes for a duration of circa 49 minutes from cycle 11 interphase to 14 interphase. Bars: 5 μm. The movie is related to Fig 2F.
GFP-SetDB1 distribution in Gcn5 KD embryo. Timelapses were captured every 1 minutes for a duration of circa 47 minutes from cycle 11 interphase to 14 interphase. Bars: 5 μm. The movie is related to Fig 2F.
GFP-SetDB1 accumulation at 359-bp loci in w KD embryo. Timelapses were captured every 2 minutes for a duration of circa 30 minutes in cycle 14 interphase. Bars: 5 μm. The movie is related to Fig 3C.
GFP-SetDB1 accumulation at 359-bp loci in Gcn5 KD embryo. Timelapses were captured every 2 minutes for a duration of circa 30 minutes in cycle 14 interphase. Bars: 5 μm. The movie is related to Fig 3C.
Control embryo microinjected with DMSO. Timelapses were captured every 1 minutes for a duration of circa 31 minutes form cycle 11 interphase to 13 interphase. Bars: 5 μm. The movie is related to SI Appendix, Fig S6A.
The embryo microinjected with (R,R)-59 inhibitor. Timelapses were captured every 1 minutes for a duration of circa 30 minutes form cycle 11 interphase to 13 interphase. Bars: 5 μm. The movie is related to SI Appendix, Fig S6A.
GFP-SetDB1WT accumulation at 359-bp loci. Timelapses were captured every 2 minutes for a duration of circa 38 minutes in cycle 14 interphase. Bars: 5 μm. The movie is related to Fig 6A.
GFP-SetDB1F578A accumulation at 359-bp loci. Timelapses were captured every 2 minutes for a duration of circa 38 minutes in cycle 14 interphase. Bars: 5 μm. The movie is related to Fig 6A.
GFP-SetDB1WT accumulation at 359-bp loci in embryo carrying SetDB1WT. Timelapses were captured every 2 minutes for a duration of circa 28 minutes in cycle 14 interphase. Bars: 5 μm. The movie is related to SI Appendix, Fig S6D.
GFP-SetDB1WT accumulation at 359-bp loci in embryo carrying SetDB1F578A. Timelapses were captured every 2 minutes for a duration of circa 28 minutes in cycle 14 interphase. Bars: 5 μm. The movie is related to SI Appendix, Fig S6D.
Data Availability Statement
All study data are included in the article and/or supporting information. Sequencing data in this study have been deposited in the Gene Expression Omnibus (GEO) under accession number GSE242239 (36). Coordinates and structure factors have been deposited in the Protein Data Bank under accession ID 7UW8 (37) and 7UVE (38).