Abstract
Purpose
Asthma is a clinical syndrome with various underlying pathomechanisms and clinical phenotypes. Genetic, ethnic, and geographic factors may influence the differences in clinical presentation, severity, and prognosis. We compared the characteristics of asthma based on the geographical background by analyzing representative cohorts from the United States, Europe, South America, and Asia using the Severe Asthma Research Program (SARP), Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes (U-BIOPRED), Program for Control of Asthma in Bahia (ProAR), and Cohort for Reality and Evolution of Adult Asthma in Korea (COREA), respectively.
Methods
The clinical characteristics and medications for the SARP (n = 669), U-BIOPRED (n = 509), ProAR (n = 996), and COREA (n = 3,748) were analyzed. Subgroup analysis was performed for severe asthma.
Results
The mean age was highest and lowest in the COREA and SARP, respectively. The asthma onset age was lowest in the ProAR. The mean body mass index was highest and lowest in the SARP and COREA, respectively. Baseline pulmonary function was lowest and highest in the U-BIOPRED and COREA, respectively. The number of patients with acute exacerbation in the previous year was highest in U-BIOPRED. The mean blood eosinophil count was highest in COREA. The total immunoglobulin E was highest in the ProAR. The frequency of atopy was highest in the SARP. The principal component analysis plot revealed differences among all cohorts.
Conclusions
The cohorts from 4 different continents exhibited different clinical and physiological characteristics, probably resulting from the interplay between genetic susceptibility and geographical factors.
Keywords: Asthma, cohort studies, phenotype, ethnicity, environment, geography
INTRODUCTION
Asthma is a complex heterogeneous airway disease that is influenced by several genetic and environmental factors.1,2 The clinical characteristics of asthma differ depending on ethnicity and geography.3 The severity of the disease, responsiveness to medication, and prognosis vary according to the geographical and ethnic backgrounds in patients with asthma. For example, a study that compared the responsiveness to inhaled corticosteroids (ICSs) in patients with similar severity of asthma determined that patients of African descent exhibited a lower response to drugs than those of European descent, which may contribute to the elevated frequency of asthma-related morbidity and mortality.4,5 Moreover, the improvements in pulmonary function may differ due to ethnicity-specific drug-drug interactions.6 Environmental exposures related to geographical differences are probably more likely to affect these parameters compared to genetic differences. These regional differences also include access to health care, educational level, health-care systems, allergen exposure, air pollution (including indoor smoking, biomass fuel burning, and vehicular emissions), and diet.2,7 Some studies reported that the latitude-dependent difference in the amount of sunlight exposure may affect the development of asthma by altering the immune system via the availability of vitamin D levels.8 Moreover, access to medical care can lead to variations in disease outcomes, including acute exacerbation.9 Therefore, a comparative analysis of the cohorts representing different countries and regions is essential, to understand the differences in asthma based on ethnic and geographical characteristics.
Cohorts for asthma have been established in most countries to study the natural course and pathophysiology of asthma. The National Heart Lung and Blood Institute launched the Severe Asthma Research Program (SARP) to identify and characterize severe asthma in the United States.10 Patient enrollment is based on the definition of severe asthma provided by the European Respiratory Society and American Thoracic Society (ERS/ATS) guidelines for patients aged above 6 years.11,12
The Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes (U-BIOPRED), an European Union consortium of 20 academic institutions, 11 pharmaceutical companies, 4 small-medium enterprises, and 6 patient organizations, was established in 2009. The cohort contains patients with severe asthma, mild/moderate asthma, and healthy controls.13 The Program for Control of Asthma in Bahia (ProAR) was established in 2003 in the city of Salvador, Brazil, with individual follow-ups ranging from 6 months (minimal) to 10 years. The ProAR is the chief secondary public care outpatient reference center specializing in asthma in Salvador and receives patients with uncontrolled asthma, usually those requiring emergency visits and hospitalizations.14 The Cohort for Reality and Evolution of Adult Asthma in Korea (COREA) is a prospective, observational, and multi-centered cohort of Korean patients with asthma that was established in 2005. The broad population of patients with adult asthma in Korea was recruited by allergists or pulmonologists from 25 referral centers.15,16,17 Patients with asthma who met Global Initiative for Asthma (GINA) guidelines were enrolled.1 These asthma cohorts, i.e., the SARP, U-BIOPRED, ProAR, and COREA, are representative of the United States of America, Europe, South America, and Korea, respectively. An overview of the four cohorts is presented in Table 1.
Table 1. Comparison of clinical characteristics among the SARP, U-BIOPRED, ProAR, and COREA cohorts.
| Variables | SARP | U-BIOPRED | ProAR | COREA | |
|---|---|---|---|---|---|
| Region | U.S. | Europe | South America (Brazil) | Korea | |
| Enrolled patients | Asthma | Asthma | Adult Asthma | Adult asthma | |
| Asthma severity | All | All | All | All | |
| Presence of control group | Present | Present | Present | Absent | |
| Patient age | ≥ 18 yr | ≥ 18 yr | ≥ 18 yr | ≥ 18 yr | |
| Participating organizations | 10 medical centers | 40 organizations | 1 medical centers | 25 medical centers | |
| Smokers | Excluded (current or ≥ 5 PYs) | Included | Excluded if ≥ 10 PYs at screening, but not when the information was provided at the study visit | Included | |
| Summary of inclusion and exclusion criteria | Inclusion criteria | Exclusion criteria | |||
| SARP | • According to ATS/ERS guidelines | • Pregnancy during the characterization phase | |||
| (Definition of severe asthma* for patients aged ≥ 6 yr) | • Current smokers | ||||
| • Smoking history > 5 PYs | |||||
| • Pulmonary disease associated with asthma-like symptoms (but not limited to): cystic fibrosis, COPD, vocal cord dysfunction, severe scoliosis or chest wall deformities, and congenital pulmonary disorders | |||||
| U-BIOPRED | • Four cohorts were recruited | • Pregnancy during the characterization phase | |||
| A) Severe non-smoking asthmatics (SAn): < 5 PY smoking history with severe asthma | • < 6 wk post-partum or < 6 wk cessation of breast feeding | ||||
| B) Smokers and ex-smokers with severe asthma (SAs/ex): current smokers or ex-smokers with a PY history > 5 yr | • Subjects had a recent history of incapacitating psychiatric disorders | ||||
| C) Mild/moderate non-smoking asthmatics (MMA): < 5 PY smoking history, controlled or partially controlled asthma symptoms with a dose of less than 500 μg of fluticasone propionate/day or equivalent | • History or current evidence of an upper or lower respiratory infection or symptoms (including common cold) within 2 wk of baseline assessments (assessments should be deferred) | ||||
| D) Healthy non-smoking controls (HC): < 5 PY smoking history, no history of asthma or wheezing, no other chronic respiratory disease, pre-BD-FEV1 ≥ 80% (HC NOT included in analysis, pediatrics also not included) | |||||
| ProAR | • Four cohorts were recruited | • Current smokers were excluded | |||
| 1. Severe Asthma non-smoking; Based on WHO 2009. Majority in the difficult to treat asthma category | |||||
| 2. Severe Asthma non-smoking non-reversible; Based on WHO 2009. Majority in the difficult to treat category. These subjects don’t meet reversibility criteria | |||||
| 3. Mild to moderate non-smoking asthmatics as defined by their physician | |||||
| 4. No Asthma controls. Environmentally matched cohort | |||||
| COREA | • One or more of the following asthma-related symptoms persisting for more than 3 mon: dyspnea, coughing, or wheezing | • Patients have other conditions that can cause death or impairment during the study period (e.g., cancer, HF, CAD, CVD, renal failure, DM with severe complications, and uncontrolled HTN) | |||
| • Airway hyper-responsiveness or evidence of reversibility (GINA guidelines) | • Patients have other conditions that may affect PFT and CT image analysis (e.g., lung resection, TB destroyed lung, and BE) | ||||
SARP, Severe Asthma Research Program; U-BIOPRED, Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes; ProAR, Program for Control of Asthma in Bahia; COREA, Cohort for Reality and Evolution of Adult Asthma in Korea; PY, pack-year; ATS, American Thoracic Society; ERS, European Respiratory Society; COPD, chronic obstructive pulmonary disease; BD, bronchodilator; FEV1, forced expiratory volume in 1 second; WHO, World Health Organization; HF, heart failure; CAD, coronary artery disease; CVD, cerebrovascular disease; DM, diabetes; HTN, hypertension; PET, pulmonary function test; CT, computed tomography; TB, tuberculosis; BE, bronchiectasis; ICS, inhaled corticosteroid; OCS, oral corticosteroid.
*Severe asthma, uncontrolled symptoms defined according to GINA guidelines (1) and/or frequent cases of exacerbation (more than 2 per year) despite high-dose ICS (≥ 1,000 μg fluticasone propionate/day or equivalent dose, or a doubling of the dose of maintenance ICS for at least three days or requiring hospitalization), with or without OCS, plus at least one other controller medication.
This study compared the clinical characteristics of the SARP, U-BIOPRED, ProAR, and COREA cohorts. We analyzed the characteristics of asthma, identified the clinical factors affecting its prognosis on the basis of ethnicity and geographical background, and speculated on the future direction of asthma research.
MATERIALS AND METHODS
Participants and measurements
All data were derived from collected raw data, and each cohort was analyzed within the scope available for disclosure and sharing.
The original SARP cohort includes participants aged 12 years or above (n = 726) diagnosed with asthma by physicians. In this study, we included only adults aged above 18 years (n = 669), 444 of whom were classified as white (66.37%), 175 as black or African-American (26.16%), 16 as Asian (2.39%), 17 as multi-ethnic (2.54%), 15 as other ethnicities (2.24%), and 2 as uncertain (0.30%). All participants had no or less than a 5 pack-year smoking history; current smokers were excluded. SARP includes 36 variables under the following categories: basic patient information, baseline pulmonary function, medical history, medication use, health care utilization (HCU), serum total immunoglobulin E (IgE) level, and blood eosinophil count.
U-BIOPRED distinguishes between pediatric and adult asthma; this analysis included all adult patients with asthma aged over 18 years (n = 509). In this group, smoking history was not limited, therefore, heavy smokers (more than 10 pack-year) were included. U-BIOPRED also includes biologic indicators, such as blood and sputum eosinophil levels.
The ProAR cohort mainly consists of untreated non-smoking patients with severe asthma. All patients were over 18 years of age (n = 996). ProAR includes baseline characteristics, medical history, and information on blood eosinophil levels.
All patients in the COREA, the largest of the 4 cohorts, were recruited by allergists or pulmonologists from 25 referral centers in the Republic of Korea. Participants are older than 18 years of age (n = 3,748). The cohort data include baseline characteristics, medical history, smoking history, and a variety of serum and sputum biological markers. These patients represent the broad population of Korean adults with asthma.
The present study was cross-sectional in design and compared the clinical characteristics of each cohort at baseline. This analysis was conducted using variables that were common to each cohort because of the differences in the information available for each cohort. All patients provided written informed consent at the time of cohort enrollment, and this study was approved by the Institutional Review Boards of Asan Medical Center (2019-1676), and the ethics boards of the individual clinical centers participating in SARP, U-BIOPRED, and ProAR.
Definition of variables
Medication
All 4 cohorts included information related to medication. However, the information collected differed slightly among the cohorts; thus, a direct comparison could not be made. Therefore, the approximate proportions of drug combinations used were assessed. We defined controller drugs on the basis of the GINA guidelines and classified ICS doses into low, medium, or high.18
HCU
HCU in the past year was defined as follows: 0 = none, 1 = more than 1 urgent hospital visit, 2 = emergency department (ED) visit, 3 = use of oral corticosteroids (OCS) for more than 3 consecutive days, 4 = hospitalization, and 5 = intensive care unit (ICU) visit.
Acute exacerbation
Moderate to severe exacerbation was defined as one of the following categories in this study: 1) requirement of high-dose OCS for at least 3 days, 2) doubling of the maintenance OCS dose for at least 3 days, and 3) hospitalization. Information regarding the total number of exacerbation events was included. We analyzed the exacerbation based on the above-mentioned HCU information for the SARP cohort. We applied the same criteria for all cohorts for the comparison with the COREA cohort.
Severe asthma
Although the characteristics and registration criteria of all cohorts were different, fortunately all cohorts were classified according to their severity. Therefore, we could compare and analyze only patients with severe asthma.
Statistical analysis
All statistical analyses were conducted using R software (R Project for Statistical Computing, Vienna, Austria; www.r-project.org). The results were presented as the mean ± standard deviation for continuous variables and percentage for categorical variables. Baseline characteristics were compared using t-tests for continuous variables and χ2 and Fisher’s exact tests for categorical variables. The analysis of variance was also used to compare the 3 or 4 groups.
The principal component analysis (PCA) was conducted to determine whether the cohorts represented different groups. Based on the cross-sectional analysis results of the cohorts, we selected the variables that showed differences. The following all possible variables were selected from each cohort for PCA: 1) age, 2) sex, 3) body mass index (BMI), 4) age of asthma onset, 5) asthma duration, 6) pre-bronchodilator forced expiratory volume in 1 second (FEV1) (%), 7) pre-bronchodilator forced vital capacity (FVC) (%), 8) pre-bronchodilator FEV1/FVC ratio, 9) total IgE, and 10) acute exacerbation (previous 1 year).
RESULTS
General characteristic comparisons among all 4 cohorts
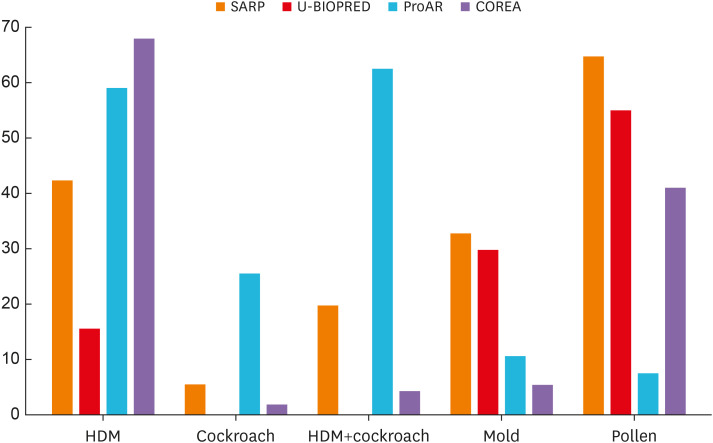
The clinical characteristics of participants differed amongst each cohort (SARP, U-BIOPRED, ProAR, and COREA) (Table 2). The mean patient age was lowest in the SARP group, followed by the ProAR, U-BIOPRED and COREA groups. The proportion of women with asthma was more than 50% in all groups and was highest in the ProAR group at approximately 80%. The BMI was highest in the SARP group, followed by U-BIOPRED and ProAR. The BMI of patients in the COREA was significantly lower than those in the other three groups. Heavy smokers (ex-smokers with a history of more than 5 pack-years or current smokers) were excluded from the SARP cohort. Thus, although it was difficult to make a comparison, the proportion of patients with a history of smoking was greater in the COREA group. The proportion of patients with atopy was higher in the SARP. Interestingly, allergen distribution significantly differed among groups (Fig. 1).
Table 2. Comparison of baseline characteristics among the cohorts in this study.
| Characteristics | SARP | U-BIOPRED | ProAR | COREA | P value | |
|---|---|---|---|---|---|---|
| Total patients | 669 | 509 | 996 | 3,748 | ||
| Age (≥ 18 yr) | 38.58 ± 12.68 (n = 669) | 50.15 ± 14.34 (n = 509) | 45.04 ± 15.15 (n = 996) | 50.79 ± 15.75 (n = 3,748) | < 0.001 | |
| Females | 457/669 (68.31) | 305/509 (59.92) | 794/996 (79.72) | 2,138/3,748 (57.04) | < 0.001 | |
| BMI (kg/m2) | 29.74 ± 7.88 (n = 669) | 28.62 ± 6.14 (n = 509) | 28.14 ± 5.68 (n = 995) | 24.12 ± 3.49 (n = 3,599) | < 0.001 | |
| Smoking history | < 0.001 | |||||
| Never | 561/669 (83.86) | 340/509 (66.80) | 766/993 (77.14) | 1,881/3,362 (55.95) | ||
| Light (< 10 PYs) | 108/669 (16.14) | 81/509 (15.91) | 152/993 (15.31) | 390/3,362 (11.60) | ||
| Heavy (current or ≥ 10 PYs) | NA | 88/509 (17.29) | 75/993 (7.55) | 1,091/3,362 (32.45) | ||
| Family history* | 332/669 (49.62) | 225/509 (44.20) | 285/996 (28.60) | 1,608/1,228 (58.22) | < 0.001 | |
| Allergic rhinitis | NA | 248/509 (48.91) | 563/904 (62.28) | 1,342/1,901 (70.59) | < 0.001 | |
| Atopy | 561/669 (83.86) | 349/509 (68.84) | 600/905 (66.3) | 950/1,857 (51.16) | < 0.001 | |
| HDM | 239/493 (48.48) | 70/449 (15.59) | 535/905 (59.1) | 647/950 (68.10) | < 0.001 | |
| CR | 32/493 (6.49) | NA | 233/905 (25.7) | 20/950 (2.11) | < 0.001 | |
| HDM and CR | 112/493 (22.72) | NA | 567/905 (62.6) | 42/950 (4.42) | < 0.001 | |
| Mold | 185/493 (37.53) | 121/403 (30.02) | 98/905 (10.8) | 57/950 (6.00) | < 0.001 | |
| Pollen | 364/493 (73.83) | 281/509 (55.21) | 70/905 (7.7) | 393/950 (41.37) | < 0.001 | |
| Asthma onset age | 15.56 ± 14.48 (n = 669) | 25.43 ± 18.49 (n = 494) | 14.14 ± 15.36 (n = 982) | 45.74 ± 16.80 (n = 2,762) | < 0.001 | |
| Duration of asthma (yr) | 23.01 ± 14.06 (n = 669) | 24.63 ± 16.03 (n = 494) | 31.02 ± 15.98 (n = 982) | 5.19 ± 7.64 (n = 2,762) | < 0.001 | |
| Pre-bronchodilator FEV1 (%) | 73.61 ± 22.26 (n = 669) | 70.08 ± 22.24 (n = 502) | 71.44 ± 18.51 (n = 984) | 80.02 ± 20.76 (n = 3,378) | < 0.001 | |
| Pre-bronchodilator FVC (%) | 85.42 ± 19.43 (n = 669) | 90.26 ± 20.24 (n = 502) | 81.29 ± 14.74 (n = 984) | 88.07 ± 17.68 (n = 3,378) | < 0.001 | |
| Pre-bronchodilator FEV1/FVC | 0.7 ± 0.12 (n = 669) | 0.81 ± 0.17 (n = 500) | 0.72 ± 0.13 (n = 984) | 0.72 ± 0.12 (n = 3,378) | < 0.001 | |
| Post-bronchodilator FEV1 (%) | 86.46 ± 19.87 (n = 669) | 79.56 ± 22.21 (n = 502) | 77.64 ± 17.53 (n = 980) | 82.05 ± 21.82 (n = 2,001) | < 0.001 | |
| Post-bronchodilator FVC (%) | 95.26 ± 16.55 (n = 669) | 97.80 ± 19.01 (n = 502) | 85.3 ± 13.77 (n = 980) | 87.81 ± 17.14 (n = 2,001) | < 0.001 | |
| FEV1 change† (%) | 16.27 ± 16.69 (n = 669) | 16.15 ± 16.69 (n = 493) | 10.25 ± 12.52 (n = 980) | 7.10 ± 13.92 (n = 1,959) | < 0.001 | |
| Blood eosinophil, count/μL | 270.0 ± 290.0 (n = 669) | 306.95 ± 395.81 (n = 494) | 311.56 ± 267.81 (n = 989) | 356.39 ± 404.74 (n = 3,009) | < 0.001 | |
| Total IgE (IU/mL) | 287.72 ± 569.60 (n = 669) | 317.46 ± 624.78 (n = 496) | 493.02 ± 656.46 (n = 980) | 398.31 ± 654.66 (n = 1,438) | < 0.001 | |
| Sputum eosinophil (%) | NA | 11.61 ± 18.15 (n = 159) | NA | 11.92 ± 17.03 (n = 729) | < 0.001 | |
| Sputum neutrophil (%) | NA | 51.59 ± 26.79 (n = 159) | NA | 27.61 ± 30.00 (n = 709) | 0.396 | |
| Exacerbation‡ (≥ 1) | 360/669 (53.81) | 365/509 (71.70) | 547/996 (54.92) | 663/3,378 (19.62) | < 0.001 | |
Data presented as the mean ± standard error or n/N (%).
SARP, Severe Asthma Research Program; U-BIOPRED, Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes; ProAR, Program for Control of Asthma in Bahia; COREA, Cohort for Reality and Evolution of Adult Asthma in Korea; BMI, body mass index; PYs, pack-years; NA, not applicable; HDM, house dust mite; CR, cockroach; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IgE, immunoglobulin E.
*Family history: asthma family history (including parents and siblings).
†FEV1 change (%): [{(Post-bronchodilator FEV1) − (Pre-bronchodilator FEV1)}/Pre-bronchodilator FEV1]*100.
‡Exacerbation: history of acute exacerbation over the past year.
Fig. 1. Allergen sensitization in the SARP, U-BIOPRED, ProAR, and COREA cohorts. Allergen distribution significantly differed among the four groups. Pollen and HDMs were the most common allergens in the SARP and COREA, respectively.
SARP, Severe Asthma Research Program; U-BIOPRED, Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes; ProAR, Program for Control of Asthma in Bahia; COREA, Cohort for Reality and Evolution of Adult Asthma in Korea; HDM, house dust mite.
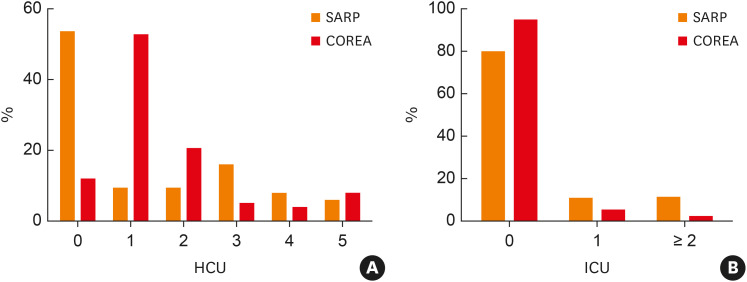
Pollen was the most common allergen in the SARP, while ProAR showed high sensitization rates to cockroaches and house dust mites (HDMs), similar to COREA, which also showed the highest sensitization to HDMs. The age of asthma onset was notably low in the ProAR and SARP. The onset of asthma occurred during adolescence and middle age in the U-BIOPRED and COREA groups, respectively. The mean baseline pulmonary function (pre-bronchodilator FEV1%) was lowest in the U-BIOPRED group, followed by ProAR, SARP, and COREA. COREA exhibited high blood eosinophil counts, which were lowest in SARP at less than 300 counts/μL. Moreover, the total IgE levels were significantly higher in ProAR, followed by COREA, U-BIOPRED, and SARP. Sputum indicators could be compared only between U-BIOPRED and COREA: the sputum neutrophil count was higher in U-BIOPRED patients. The history of acute exacerbation over the past year was most common in U-BIOPRED, similar for the SARP and ProAR, and fewest in the COREA. The difference in HCU was largest between SARP and COREA, and HCU over the past year also significantly differed between these 2 groups. The SARP group included a high proportion of patients with no HCU history, while the COREA group included a high proportion of patients with more than one urgent hospital or ED visit over the last 12 months (Fig. 2A). However, the proportion of patients with a history of ICU care over the past year was slightly higher in the SARP group (Fig. 2B).
Fig. 2. Comparison of hospital care behavior over the past year: (A) Distribution of patients based on the frequency of HCU. The SARP group included a high proportion of patients with no HCU history. The COREA group included a high proportion of patients with > 1 urgent hospital or emergency department visit over the last 12 months; (B) Distribution of patients based on the frequency of ICU admission. The proportion of patients with a history of ICU care was slightly higher in the SARP group.
HCU, hospital care unit; SARP, Severe Asthma Research Program; COREA, Cohort for Reality and Evolution of Adult Asthma in Korea; ICU, intensive care unit.
PCA plot
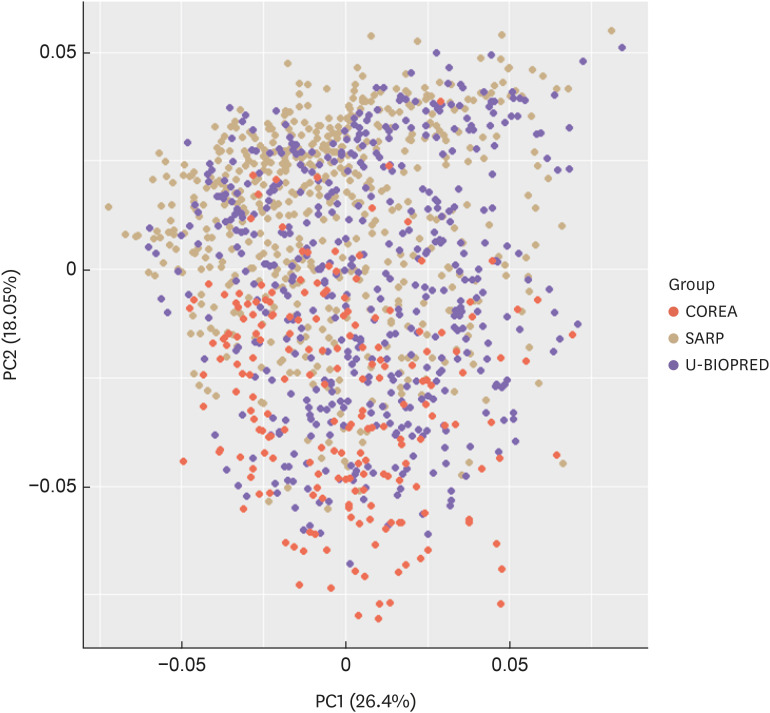
The PCA plot was generated using variables from 3 cohorts, since ProAR was excluded due to the restrictions on data disclosure. The selected variable combinations created new variables, referred to as components. The contribution of each variable to components 1 and 2 is shown in Supplementary Fig. S1; values closer to zero indicate lower contributions, while larger absolute values suggest higher contributions. The PCA plot confirmed that the COREA cohort differed from the other 2 cohorts (SARP and U-BIOPRED) (Fig. 3).
Fig. 3. Principal component analysis plot for the SARP, COREA, and U-BIOPRED cohorts. The plot shows that the COREA differed from the other 2 cohorts, i.e., SARP and U-BIOPRED.
SARP, Severe Asthma Research Program; COREA, Cohort for Reality and Evolution of Adult Asthma in Korea; U-BIOPRED, Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes.
Comparisons of patients with severe asthma
We repeated the analysis to exclude the influence of asthma severity by analyzing patients with severe asthma. The SARP, U-BIOPRED, ProAR, and COREA cohorts included 274, 421, 544, and 245 patients with severe asthma, respectively, which accounted for approximately 40.96%, 82.71%, 54.61%, and 6.53% of the total population of each cohort, respectively (Table 3). Similar results were observed on comparison with the previous analysis. The mean age was lowest in the SARP group; however, the intergroup differences decreased as the mean age increased across all groups. The proportion of women with asthma was higher in all groups: it was highest in the ProAR group at more than 80%. The mean BMI increased further for all groups, except the COREA. The mean BMI was highest in the SARP, followed by the U-BIOPRED and ProAR groups. Patients from the COREA group also had significantly lower BMI compared to the other 3 groups. The proportion of smokers was even higher in COREA cohort. The proportion of patients with a history of atopy was considerably higher, and that with a family history of atopy increased in the COREA group, unlike the other 3 cohorts. The age of asthma onset was very low in the ProAR group. The onset of asthma occurred during adolescence in the SARP and U-BIOPRED, and middle age in the COREA group. We confirmed the duration of asthma in patients with severe asthma in the COREA group was twice as long as the average of all cohorts. The overall mean baseline FEV1% decreased, and its average value was approximately 65%. The mean baseline pulmonary function (pre-bronchodilator FEV1%) was lowest in the SARP group, followed by ProAR, COREA, and U-BIOPRED; however, it showed a different pattern from the previous results. The average blood eosinophil count was highest in the COREA group. The total IgE level remained highest in ProAR, while similar values were observed in the remaining 3 groups. Sputum indicators showed a similar pattern as those obtained in the previous analysis. Notably, the rates of acute exacerbation significantly increased in all cohorts: more than 80% of patients in the SARP and U-BIOPRED experienced acute exacerbations over the past year.
Table 3. Comparison of baseline characteristics among the cohorts for patients with a severe asthma.
| Characteristics | SARP | U-BIOPRED | ProAR | COREA | P value | |
|---|---|---|---|---|---|---|
| Total patients | 274 | 421 | 544 | 245 | ||
| Age (≥ 18 yr) | 43.65 ± 12.48 (n = 274) | 51.92 ± 13.44 (n = 421) | 51.90 ± 0.60 | 54.37 ± 14.71 (n = 245) | < 0.001 | |
| Females | 179/274 (65.33) | 261/421 (62.00) | 444/544 (81.6) | 129/245 (52.65) | < 0.001 | |
| BMI (kg/m2) | 31.02 ± 7.71 (n = 274) | 29.23 ± 6.28 (n = 421) | 29.00 ± 4.66 (n = 544) | 24.08 ± 3.62 (n = 242) | < 0.001 | |
| Smoking history | < 0.001 | |||||
| Never | 224/274 (81.75) | 264/421 (62.71) | NA | 104/243 (42.80) | ||
| Light (< 10 PYs) | 50/274 (18.25) | 62/421 (14.73) | NA | 36/243 (14.81) | ||
| Heavy (current or ≥ 10 PYs) | 0 (0) | 95/421 (22.57) | NA | 103/243 (42.39) | ||
| Pack-years | NA | 16.43 ± 17.38 (n = 157) | 12.00 ± 17.87 (n = 149) | 21.60 ± 21.65 (n = 139) | < 0.001 | |
| Family history* | 127/274 (46.35) | 72/170 (42.35) | NA | 58/84 (69.05) | 0.044 | |
| Allergic rhinitis | NA | 208/378 (55.03) | 299/491 (60.9) | 40/67 (59.70) | 0.564 | |
| Atopy | 207/274 (75.55) | 273/419 (65.2) | 313/491 (63.8) | 103/141 (73.05) | 0.003 | |
| Asthma onset age | 17.10 ± 15.86 (n = 274) | 26.54 ± 18.65 (n = 411) | 15.50 ± 16.00 (n = 543) | 47.00 ± 17.43 (n = 133) | < 0.001 | |
| Duration of asthma (yr) | 26.55 ± 14.52 (n = 274) | 24.65 ± 16.46 (n = 421) | NA | 10.65 ± 13.12 (n = 243) | < 0.001 | |
| Pre-bronchodilator FEV1 (%) | 60.36 ± 21.16 (n = 274) | 67.45 ± 21.32 (n = 418) | 63.50 ± 18.54 (n = 537) | 65.50 ± 22.52 (n = 234) | < 0.001 | |
| Pre-bronchodilator FVC (%) | 75.41 ± 19.13 (n = 274) | 87.88 ± 19.27 (n = 418) | 78.8 ± 16.22 (n = 537) | 80.12 ± 18.93 (n = 234) | < 0.001 | |
| Pre-bronchodilator FEV1/FVC | 0.64 ± 0.13 (n = 274) | 0.63 ± 0.14 (n = 418) | 0.65 ± 0.02 (n = 537) | 0.63 ± 0.14 (n = 234) | < 0.001 | |
| Post-bronchodilator FEV1 (%) | 75.20 ± 20.07 (n = 274) | NA | NA | 64.39 ± 21.34 (n = 182) | < 0.001 | |
| Post-bronchodilator FVC (%) | 88.93 ± 17.42 (n = 274) | NA | NA | 81.06 ± 18.04 (n = 182) | < 0.001 | |
| FEV1 change† (%) | 14.83 ± 12.95 (n = 274) | NA | NA | 2.90 ± 10.24 (n = 180) | < 0.001 | |
| Blood eosinophil (count/μL) | 300.0 ± 400.0 (n = 232) | 322.87 ± 430.19 (n = 406) | 323.55 ± 289.08 (n = 540) | 399.11 ± 497.94 (n = 186) | < 0.001 | |
| Total IgE (IU/mL) | 312.53 ± 692.70 (n = 195) | 321.57 ± 634.79 (n = 406) | 533.38 ± 687.99 (n = 533) | 320.97 ± 440.30 (n = 88) | 0.972 | |
| Sputum eosinophil (%) | NA | 12.09 ± 18.29 (n = 181) | NA | 15.49 ± 18.93 (n = 40) | 0.292 | |
| Sputum neutrophil (%) | NA | 53.72 ± 25.62 (n = 181) | NA | 27.14 ± 27.00 (n = 37) | < 0.001 | |
| Exacerbation (≥ 1) | 222/274 (81.02) | 342/421 (81.24) | 332/544 (61.03) | 104/245 (42.45) | < 0.001 | |
Data presented as the mean ± standard error or n/N (%).
SARP, Severe Asthma Research Program; U-BIOPRED, Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes; ProAR, Program for Control of Asthma in Bahia; COREA, Cohort for Reality and Evolution of Adult Asthma in Korea; BMI, body mass index; PYs, pack-years; NA, not applicable; HDM, house dust mite; CR, cockroach; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IgE, immunoglobulin E.
*Family history: asthma family history (including parents and siblings).
†FEV1 change (%): [{(Post-bronchodilator FEV1) − (Pre-bronchodilator FEV1)}/Pre-bronchodilator FEV1]*100.
‡Exacerbation: history of acute exacerbation over the past year.
Medication use and symptom control
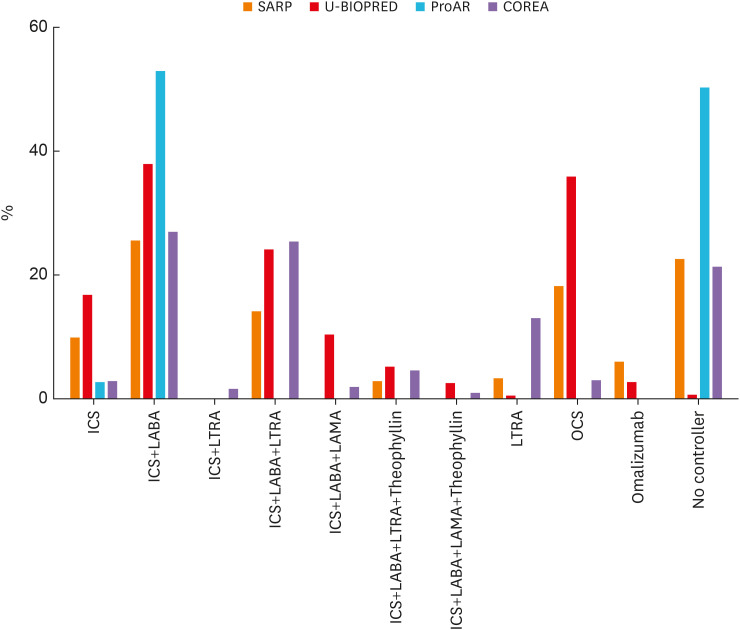
A large proportion of patients used two controllers in all 4 groups (Table 4). None of the patients in the ProAR used more than three controllers. Low-to-medium dose ICS was the most common medication used for asthma in the SARP and COREA groups, while high-dose ICS was the most common pharmacotherapy in the U-BIOPRED. Patients in the U-BIOPRED also showed a high rate of oral or systemic steroid administration. The use of medication was most frequent in the subset of patients using both ICS and long-acting beta agonists (LABA) in all groups. The proportion of patients who did not use a controller was highest in ProAR (50%), almost none in U-BIOPRED, and similar for SARP and COREA (approximately 21%). Most patients in the ProAR group used no controller or used only ICS+LABA (Table 4, Fig. 4).
Table 4. Comparison of medication use patterns.
| Variables | SARP | U-BIOPRED | ProAR | COREA | |
|---|---|---|---|---|---|
| Number of controllers | |||||
| 0 controller | 146/669 (21.82) | 2/508 (0.39) | 448/996 (50.00) | 285/1,778 (16.03) | |
| 1 controller | 79/669 (11.81) | 86/508 (16.93) | 23/996 (2.31) | 266/1,778 (14.96) | |
| 2 controllers | 190/669 (28.40) | 193/508 (37.99) | 525/996 (52.71) | 469/1,778 (26.38) | |
| 3 controllers | 152/669 (22.72) | 172/508 (33.86) | 0 | 469/1,778 (26.38) | |
| > 3 controllers | 102/669 (15.25) | 55/508 (9.48) | 0 | 289/1,778 (16.25) | |
| Steroid medication | |||||
| No steroids | 170/669 (25.41) | 5/508 (0.98) | 448/996 (50) | 4/1,302 (0.31) | |
| Low-medium-dose ICS | 208/669 (31.09) | 87/508 (17.13) | 974/1,302 (74.81) | ||
| High-dose ICS | 151/669 (22.57) | 416/508 (81.89) | 212/1,302 (16.28) | ||
| Oral or systemic steroids | 140/669 (20.93) | 195/508 (38.39) | 0/996 (0) | 112/1,302 (8.60) | |
| Medications | |||||
| ICS only | 9.57% | 16.54% | 2.31% | 2.66% | |
| ICS+LABA | 25.26% | 37.60% | 52.71% | 26.68% | |
| ICS+LTRA | NA | 0.00% | 0.00% | 1.40% | |
| ICS+LABA+LTRA | 13.75% | 23.82% | 0.00% | 25.13% | |
| ICS+LABA+LAMA | NA | 10.04% | 0.00% | 2.37% | |
| ICS+LABA+LTRA+Theophyllin | 2.54% | 4.92% | 0.00% | 4.29% | |
| ICS+LABA+LAMA+Theophyllin | NA | 2.16% | 0.00% | 0.73% | |
| LTRA only | 2.99% | 0.20% | 0.00% | 12.86% | |
| OCS | 17.94% | 35.63%* | 0.00% | 2.81% | |
| Omalizumab | 5.68% | 2.36%† | 0.00% | 0.00% | |
| No controllers | 22.27% | 0.39% | 50.00% | 21.06% | |
Data presented as n/N (%).
SARP, Severe Asthma Research Program; U-BIOPRED, Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes; ProAR, Program for Control of Asthma in Bahia; COREA, Cohort for Reality and Evolution of Adult Asthma in Korea; ICS, inhaled corticosteroid; LABA, long-acting β-agonist; LTRA, leukotriene-receptor antagonist; LAMA, long-acting muscarinic receptor antagonists; OCS, oral corticosteroid; NA, not applicable.
*More than once a month, †Immunotherapy.
Fig. 4. Medications used in the SARP, U-BIOPRED, ProAR, and COREA groups. ICS+LABA was the most commonly used controller in all cohorts. The rate of use of OCS was high in the U-BIOPRED cohort, and a large number of patients did not use controllers in the ProAR.
SARP, Severe Asthma Research Program; U-BIOPRED, Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes; ProAR, Program for Control of Asthma in Bahia; COREA, Cohort for Reality and Evolution of Adult Asthma in Korea; ICS, inhaled corticosteroid; LABA, long-acting beta agonists; OCS, oral corticosteroids; LTRA, leukotriene-receptor antagonist; LAMA, long-acting muscarinic receptor antagonists.
Symptomatic control of asthma was especially comparable between the patients from the SARP and COREA. The SARP group had a lower proportion of symptom-free patients. A high proportion of its patients had weekly symptoms (daytime or night symptoms) and activity limitation (Table 5).
Table 5. Comparison of symptom control between SARP and COREA.
| Symptom control | SARP | COREA | |
|---|---|---|---|
| Period | |||
| No symptoms | 17/669 (2.54) | 1,120/3,146 (35.60) | |
| Weekly symptoms | 586/669 (87.59) | 952/3,146 (30.26) | |
| Monthly symptoms | 66/669 (9.87) | 1,074/3,146 (34.14) | |
| Activity | |||
| No symptoms | 81/669 (12.11) | 1,651/3,748 (44.05) | |
| Symptoms with exercise only | 144/669 (21.52) | 1,427/3,748 (38.07) | |
| Symptoms with routine activity | 444/669 (66.37) | 670/3,748 (17.88) | |
Data presented as n/N (%).
SARP, Severe Asthma Research Program; COREA, Cohort for Reality and Evolution of Adult Asthma in Korea.
DISCUSSION
Asthma is a global health issue that affects people of all ages.1 Therefore, international cooperation is required to overcome this disease, which can be realized through the analysis of cohorts based on geographical and ethnic backgrounds. Recent attempts were made to analyze the characteristics of the ProAR and U-BIOPRED groups.19 Therefore, we compared and analyzed the SARP, U-BIOPRED, ProAR and COREA, which are representative asthma cohorts from the United States, Europe, South America, and Korea, respectively, for which no comparative analysis had been attempted previously.
Our analysis found that these cohorts exhibited distinct characteristics. First, the mean age of patients with asthma in COREA was found to be higher than that of patients in the SARP, U-BIOPRED, and ProAR. Moreover, the SARP, U-BIOPRED, and ProAR groups exhibited early-onset asthma, whereas the COREA group showed late-onset asthma. Asthma in the elderly can be divided into two phenotypes: long-standing asthma and late-onset asthma.20 Clinical evidence suggests that late-onset asthma is a heterogeneous condition resulting from complex interactions between the environment, microbiome, comorbidities, and genetics.20,21,22,23,24 Moreover, previous studies have shown that the prevalence of asthma increases with age among Asian individuals.25,26,27 This may be attributed to the differences in the pathogenesis of asthma or differences in the natural course of the disease.
The second notable result involved the relationship between asthma and obesity; obese asthma has been studied extensively as an asthma subtype.28,29 Patients in the SARP, U-BIOPRED, and ProAR cohorts exhibited a mean BMI of approximately 30 kg/m2, the global standard for obesity. In contrast, the mean BMI of the Korean patients was approximately 24 kg/m2, which means that the BMI was not high among the patients in this cohort.30 Tracking over 10 years in the Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study found that mean BMI increased from 28.3 kg/m2 (classified as the overweight range) in the original TENOR I study to 30.6 kg/m2 (classified as obese) in the TENOR II study.31 Few studies have focused on obese asthma in Asian populations, including Koreans. These results suggest that obese asthma is a subtype that requires greater attention, particularly among American and European patients.
We found that the proportion of women with asthma was > 50% in each cohort. This suggests that asthma among women is an important subtype, as other studies on the prevalence of severe asthma have reported rates ≥ 60% among women. It has been suggested that sex hormones such as estrogen and progesterone could be the cause of the unique clinical features of asthma in women.27,32,33,34
Pollen was the most common allergen in the SARP group, while allergies to HDM were common in the ProAR and COREA. Sensitization to mold antigen was found to be lower in the ProAR and COREA patient groups than in the SARP or U-BIOPRED cohorts. Very little research has been conducted on the ethnic differences in allergen sensitization, and it is not clear whether environmental effects or genetic factors are more important in allergen sensitization. The National Health and Nutrition Examination Survey, which provides rates of sensitization in the United States, reported that non-Hispanic black individuals were more likely to have had at least one positive skin test result compared to non-Hispanic white individuals.35
Asthma severity, history of exacerbation, and medication use also exhibited notable results. The proportion of patients with severe asthma was highest in the U-BIOPRED group, and lowest in the COREA group. The mean baseline lung function (FEV1%) was lowest in the U-BIOPRED group and highest in the COREA group. However, the mean baseline lung function (FEV1%) was lowest in the SARP group and highest in the U-BIOPRED group in patients with severe asthma. The proportion of patients with a history of asthma exacerbation over the past year was higher in U-BIOPRED compared to the other cohorts. Furthermore, the number of eligible patients in SARP and U-BIOPRED groups significantly increased by more than 80%, while comparing patients with severe asthma.
Moreover, the distributions of the type of controllers used for treatment differed among all cohorts. These results are thought to be related to the differences in education levels, medical expenses, insurance systems, and levels of hospital awareness.
This study has some limitations. First, the study conducted only a cross-sectional analysis. The study on the natural course of asthma with longitudinal data may provide more valuable results. Secondly, although this study compared and analyzed relatively representative large-scale cohorts in each country, the results may not represent all patients with asthma in that country. Thirdly, there were differences in the variables among the cohorts, and the patients were not enrolled in accordance with a single standardized protocol. Finally, a replication of the results may be difficult. It is necessary to investigate other representative asthma cohorts, such as TENOR (USA) and the clinical course and biomarkers in a cohort of severe chronic airway disease (BIOAIR, pan-European).
A wide variety of studies have investigated asthma in single cohorts; however, few studies have conducted a comparative analysis of large cohorts, especially representative nationwide cohorts. This study showed differences in the characteristics of simple cohorts that resulted from the criteria used for cohort registration. Moreover, it may not actually represent the characteristics of asthma in each country or ethnicity. However, the comparison of the differences between the representative cohorts of each country is meaningful because most studies derive results from these representative cohorts that can represent the status of asthma in each country. Our study demonstrated the differences in the characteristics of asthma based on ethnicity and geography. Therefore, it is essential to conduct a comparative analysis among the countries and regions to understand the differences in asthma based on these factors. Moreover, it may be necessary to establish an integrated global asthma cohort to understand the differences among geographical and ethnic backgrounds.
ACKNOWLEDGMENTS
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (2019M3E5D3073365). U-BIOPRED was funded by a public-private grant from the Innovative Medicines Initiative (IMI) covered by the European Union (EU) and the European Federation of Pharmaceutical Industries and Associations (EFPIA).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIAL
The contribution of each variable to components 1 and 2.
References
- 1.Global Initiative for Asthma. Global strategy for asthma management and prevention [Internet] place unknown: Global Initiative for Asthma; 2017. [cited 2018 Nov 12]. Available from: https://ginasthma.org/ [Google Scholar]
- 2.Jie Y, Isa ZM, Jie X, Ju ZL, Ismail NH. Urban vs. rural factors that affect adult asthma. Rev Environ Contam Toxicol. 2013;226:33–63. doi: 10.1007/978-1-4614-6898-1_2. [DOI] [PubMed] [Google Scholar]
- 3.Ortega VE, Meyers DA. Implications of population structure and ancestry on asthma genetic studies. Curr Opin Allergy Clin Immunol. 2014;14:381–389. doi: 10.1097/ACI.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Federico MJ, Covar RA, Brown EE, Leung DY, Spahn JD. Racial differences in T-lymphocyte response to glucocorticoids. Chest. 2005;127:571–578. doi: 10.1378/chest.127.2.571. [DOI] [PubMed] [Google Scholar]
- 5.Lemanske RF, Jr, Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010;362:975–985. doi: 10.1056/NEJMoa1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naqvi M, Tcheurekdjian H, DeBoard JA, Williams LK, Navarro D, Castro RA, et al. Inhaled corticosteroids and augmented bronchodilator responsiveness in Latino and African American asthmatic patients. Ann Allergy Asthma Immunol. 2008;100:551–557. doi: 10.1016/S1081-1206(10)60055-5. [DOI] [PubMed] [Google Scholar]
- 7.Garcia E, Serban N, Swann J, Fitzpatrick A. The effect of geographic access on severe health outcomes for pediatric asthma. J Allergy Clin Immunol. 2015;136:610–618. doi: 10.1016/j.jaci.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 8.Krstić G. Asthma prevalence associated with geographical latitude and regional insolation in the United States of America and Australia. PLoS One. 2011;6:e18492. doi: 10.1371/journal.pone.0018492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menzies-Gow A, Canonica GW, Winders TA, Correia de Sousa J, Upham JW, Fink-Wagner AH. A charter to improve patient care in severe asthma. Adv Ther. 2018;35:1485–1496. doi: 10.1007/s12325-018-0777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 12.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 13.Shaw DE, Sousa AR, Fowler SJ, Fleming LJ, Roberts G, Corfield J, et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015;46:1308–1321. doi: 10.1183/13993003.00779-2015. [DOI] [PubMed] [Google Scholar]
- 14.Lima-Matos A, Ponte EV, de Jesus JP, Almeida PC, Lima VB, Kwon N, et al. Eosinophilic asthma, according to a blood eosinophil criterion, is associated with disease severity and lack of control among underprivileged urban Brazilians. Respir Med. 2018;145:95–100. doi: 10.1016/j.rmed.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Kim TB, Jang AS, Kwon HS, Park JS, Chang YS, Cho SH, et al. Identification of asthma clusters in two independent Korean adult asthma cohorts. Eur Respir J. 2013;41:1308–1314. doi: 10.1183/09031936.00100811. [DOI] [PubMed] [Google Scholar]
- 16.Park SY, Jung HW, Lee JM, Shin B, Kim HJ, Kim MH, et al. Novel trajectories for identifying asthma phenotypes: a longitudinal study in Korean asthma cohort, COREA. J Allergy Clin Immunol Pract. 2019;7:1850–1857.e4. doi: 10.1016/j.jaip.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Park SY, Jung H, Kim JH, Seo B, Kwon OY, Choi S, et al. Longitudinal analysis to better characterize Asthma-COPD overlap syndrome: Findings from an adult asthma cohort in Korea (COREA) Clin Exp Allergy. 2019;49:603–614. doi: 10.1111/cea.13339. [DOI] [PubMed] [Google Scholar]
- 18.Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017;195:302–313. doi: 10.1164/rccm.201602-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz AA, Riley JH, Bansal AT, Ponte EV, Souza-Machado A, Almeida PC, et al. Asthma similarities across ProAR (Brazil) and U-BIOPRED (Europe) adult cohorts of contrasting locations, ethnicity and socioeconomic status. Respir Med. 2020;161:105817. doi: 10.1016/j.rmed.2019.105817. [DOI] [PubMed] [Google Scholar]
- 20.Dunn RM, Busse PJ, Wechsler ME. Asthma in the elderly and late-onset adult asthma. Allergy. 2018;73:284–294. doi: 10.1111/all.13258. [DOI] [PubMed] [Google Scholar]
- 21.Park HW, Song WJ, Kim SH, Park HK, Kim SH, Kwon YE, et al. Classification and implementation of asthma phenotypes in elderly patients. Ann Allergy Asthma Immunol. 2015;114:18–22. doi: 10.1016/j.anai.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Song WJ, Sintobin I, Sohn KH, Kang MG, Park HK, Jo EJ, et al. Staphylococcal enterotoxin IgE sensitization in late-onset severe eosinophilic asthma in the elderly. Clin Exp Allergy. 2016;46:411–421. doi: 10.1111/cea.12652. [DOI] [PubMed] [Google Scholar]
- 23.Yáñez A, Cho SH, Soriano JB, Rosenwasser LJ, Rodrigo GJ, Rabe KF, et al. Asthma in the elderly: what we know and what we have yet to know. World Allergy Organ J. 2014;7:8. doi: 10.1186/1939-4551-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amelink M, de Nijs SB, de Groot JC, van Tilburg PM, van Spiegel PI, Krouwels FH, et al. Three phenotypes of adult-onset asthma. Allergy. 2013;68:674–680. doi: 10.1111/all.12136. [DOI] [PubMed] [Google Scholar]
- 25.Song WJ, Wong GW. Changing trends and challenges in the management of asthma in Asia. J Allergy Clin Immunol. 2017;140:1272–1274. doi: 10.1016/j.jaci.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Song WJ, Kang MG, Chang YS, Cho SH. Epidemiology of adult asthma in Asia: toward a better understanding. Asia Pac Allergy. 2014;4:75–85. doi: 10.5415/apallergy.2014.4.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SY, Kim JH, Kim HJ, Seo B, Kwon OY, Chang HS, et al. High prevalence of asthma in elderly women: findings from a Korean National health database and adult asthma cohort. Allergy Asthma Immunol Res. 2018;10:387–396. doi: 10.4168/aair.2018.10.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141:1169–1179. doi: 10.1016/j.jaci.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samuchiwal SK, Boyce JA. Role of lipid mediators and control of lymphocyte responses in type 2 immunopathology. J Allergy Clin Immunol. 2018;141:1182–1190. doi: 10.1016/j.jaci.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Song BM, Kim HC, Kim DJ, Ahn SV, Kim KM, Lee JM, et al. Aminotransferase levels, body mass index, and the risk of diabetes: a prospective cohort study. Ann Epidemiol. 2018;28:675–680.e6. doi: 10.1016/j.annepidem.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Chipps BE, Haselkorn T, Paknis B, Ortiz B, Bleecker ER, Kianifard F, et al. More than a decade follow-up in patients with severe or difficult-to-treat asthma: The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) II. J Allergy Clin Immunol. 2018;141:1590–1597.e9. doi: 10.1016/j.jaci.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Kynyk JA, Mastronarde JG, McCallister JW. Asthma, the sex difference. Curr Opin Pulm Med. 2011;17:6–11. doi: 10.1097/MCP.0b013e3283410038. [DOI] [PubMed] [Google Scholar]
- 33.Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev. 2012;33:1–47. doi: 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melgert BN, Postma DS. All men are created equal?: new leads in explaining sex differences in adult asthma. Proc Am Thorac Soc. 2009;6:724–727. doi: 10.1513/pats.200906-054DP. [DOI] [PubMed] [Google Scholar]
- 35.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–383. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The contribution of each variable to components 1 and 2.