Abstract
Cannabis has been used in rheumatic diseases as therapy for chronic pain or inflammatory conditions. Herein, the authors systematically review the rheumatological diseases in which cannabis has been studied: systemic sclerosis, fibromyalgia, osteoarthritis, rheumatoid arthritis, osteoporosis, polymyalgia rheumatica, gout, dermatomyositis, and psoriatic arthritis. We systematically searched PubMed for articles on cannabis and rheumatic diseases between 1966 and March 2023. Twenty-eight articles have been selected for review. Most of them (n=13) were on fibromyalgia and all of them but one showed important reduction in pain; sleep and mood also improved. On rheumatoid arthritis, two papers displayed decrease in pain and in one of them a reduction in inflammatory parameters was found. In scleroderma there was a case description with good results, one study on local use for digital ulcers also with good outcomes and a third one, that disclosed good results for skin fibrosis. In dermatomyositis a single study showed improvement of skin manifestations and in osteoarthritis (3 studies) this drug has demonstrated a good analgesic effect. Several surveys (n=5) on the general use of cannabis showed that rheumatological patients (mixed diseases) do use this drug even without medical supervision. The reported side effects were mild. In conclusion, cannabis treatment is an interesting option for the treatment of rheumatological diseases that should be further explored with more studies.
Keywords: Cannabidiol, cannabis, marijuana, pain, rheumatic diseases
Highlight key points
Cannabis has been used in rheumatic diseases as therapy for chronic pain or inflammatory conditions.
This article systematically reviewed the rheumatological diseases in which cannabis has been studied: systemic sclerosis, fibromyalgia, osteoarthritis, rheumatoid arthritis, osteoporosis, polymyalgia rheumatica, gout, dermatomyositis, and psoriatic arthritis.
Most of studies were on fibromyalgia and all of them but one showed important improvement of the diseases.
Cannabis sativa has been associated with human society since ancient times, possibly initiating about 10,000 BC [1]. It has been used as a therapy for arthritis as described by historical documents from the Chinese emperor Shen Nung, in the year 2,700 BC, from the Assyrians in the 9th century BC, and, more recently, in 1924 in the Sajous’s Analytic Cyclopedia of Practical Medicine [2]. Due to its content of tetrahydrocannabinol (THC) - the toxic and psychoactive element of the plant, cannabis has been used as a recreational drug being considered illicit in several countries [1, 2]. THC causes euphoria, hallucinations, lightheadedness, coordination, and recent memory impairment [2].
The discovery of the human endocannabinoid system consisting of cannabinoid (CB) receptors and their endogenous ligands (endocannabinoids) as well as their modulating enzymes has rekindled interest in cannabis as a potential therapy and its use is gradually becoming legalized throughout the world [1, 2]. Cannabinoids and cannabis are usually studied as analgesics, although recently they have been studied as immune-modulatory agents, turning this drug into an interesting therapeutic agent for rheumatological diseases [3, 4]. However, the studies in this context are scarce and difficult to interpret as they used different formulations as well as different indications; routes of administration also vary a lot.
Considering this, the objective of this article is to perform a systematic review of the safety and efficacy of existing data on cannabis use in rheumatic diseases.
Literature Review
A systematic search of articles published in PubMed/MEDLINE, Web of Sciences, LILACS, and Scielo from 1966 to March 2023 using the following MeSH entry terms: “cannabis” OR “marijuana” OR “cannabidiol” OR “nabilone” OR “nabiximol” OR “dronabinol” OR “lenabasum” AND “rheumatic” OR “rheumatologic” OR “fibromyalgia” OR “rheumatoid arthritis” OR “spondyloarthritis” OR “Sjögren’s syndrome” OR “myositis” OR “systemic sclerosis” OR “ vasculitis” OR “osteoarthritis” OR “ gout” was performed. Equivalent strategies were used in other databases. No language restriction was established. The reference lists in the selected papers were analyzed to identify other publications. Initially, two authors (JFC and TLS) performed the literature search and independently selected the study abstracts. In the second stage, the same reviewers independently read the full-text articles selected by abstracts. Finally, a third reviewer solved disagreements arising in consensus meetings. The authors followed PRISMA guidelines [5]. A uniform form to extract information from pertinent articles regarding authors, year of publication, number of studied patients, demographic data, disease duration, study follow-up, pre- and post-intervention results, cannabis posology, outcomes, and side effects was designed.
Results
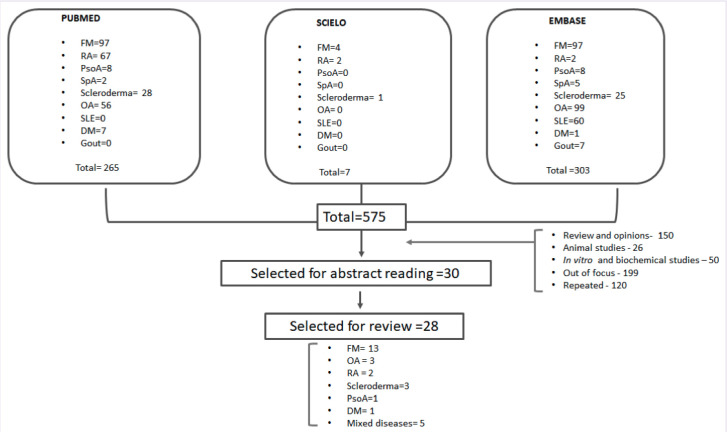
Figure 1 shows the flowchart on the articles’ selection. Appendix 1 includes all studies included in this systematic review that evaluated cannabis therapy in fibromyalgia [6–18].
Figure 1.
Flow-chart on articles’ selection: Cannabis in rheumatic diseases.
The disease best studied in this context is fibromyalgia with 13 studies: 8 prospectives; 3 cross-sectionals, and 2 retrospectives. They originate from Canada (n=4); Israel (n=4); Italy (n=2) and Netherlands, Brazil, and Spain (n=1 for each) and encompassed 1,817 individuals. The most evaluated outcomes were pain [6 – 15], sleep [7, 8, 10, 12, 14, 16], fibromyalgia impact questionnaire revised (FIQ-R) [12, 17], quality of life [6, 10, 15], mood [7, 14–16], and disability [9, 12]. In almost all studies the treatment offered benefits; only one of them did not observe improvement in pain, quality of life (QoL) and mood, although it did observe sleep improvement [15]. Appendix 1 shows the details of these studies.
Appendix 2 displays the findings in the 15 studies on other rheumatological conditions: 5 randomized double-blind studies; 5 surveys, 3 case reports, 1 prospective open study and 1 cross-sectional [19–33]. Their origin was: USA (n=6), Canada (n=4), Denmark, France, Israel, Italy and UK (one for each).
Osteoarthritis was the second disease more studied, with osteoarthritis patients being included in seven of mentioned studies [20–24, 26); followed by rheumatoid arthritis, studied in five papers [19, 25–27, 31] and scleroderma in four [22, 28–30]. Psoriatic arthritis was studied in three papers [24, 25, 31] and there was one study in dermatomyositis [32]. In osteoarthritis cannabis use has been shown to improve pain and functionality [20, 21, 23, 26] allowing reduction in baseline pain medication [23, 26]. In rheumatoid arthritis, the work by Blake et al. [19], has shown that this drug improved not only pain and functionality but also disease activity measured by DAS-28. In scleroderma, it improved skin fibrosis clinically and histologically [28] and promoted healing of digital ulcers when used topically [29]. In dermatomyositis it favored the improvement of skin manifestations and showed reduction in the expression of interferon in immune-stained skin sections [32].
Several authors studied the prevalence and motivation of non-prescription and prescription cannabis use among patients with a broad spectrum of rheumatological diseases [20, 21, 23, 26] showing that cannabis use in this population may be higher than that reported for the general population of similar age [25].
Concerning side effects most works described here show that the drug was well tolerated and safe, with mild side effects. Somnolence, dizziness, dryness, gastrointestinal complaints, and mucosal irritation were among the most common. The work of Habib et al. [7] observed 8% of dependence.
DISCUSSION
Cannabis sativa, one of the oldest cultivated plants, contains 538 chemical compounds, of which just 100 are natural phytocannabinoids and they link with the cannabinoid receptors that are present in the entire body. The most abundant are tetrahydrocannabinol (THC) and cannabidiol (CBD) [3, 4]. THC is found in great quantity in drug-type cannabis chemovars and is responsible for the drug psychoactive effects; in addition, it may have beneficial effects on pain control, sleep disorders, anxiety, and anorexia. CBD also has anti-epileptic, analgesic, anxiolytic, and sedative properties. Both phytocannabinoids have anti-inflammatory effects, which appear to be more significant when THC and CBD are combined [1].
The preparations containing cannabis may be used by vaporization and inhalation, ingestion, or topical applications; it may also be administered sublingual, vaginal or rectal [3]. Due to the great variability possible in composition and concentration as well as the diverse ways of administration of the compounds, it is difficult to compare the existing studies among themselves. Medical cannabis (MC) refers to the plant or to an extract used for medical purposes and with a specific quantity of THC and CBD [4] Nevertheless, this sort of compound is available only in certain countries.
Survey studies have shown that rheumatic patients may use this drug more frequently than the general population even when it is obtained through illicit ways [21, 25–27]. In one of the studies, it was found to be twice as common [25]. One may hypothesize that the chronic pain may be a stimulus to try this treatment option.
On the other side, the work by Rampakakis et al. [25] has also revealed that - in a country in which MC is available- the rheumatologists are not prepared to prescribe the drug, choosing to refer patients to specialized clinics.
The cannabis analgesic properties make this drug an interesting alternative for diseases such as fibromyalgia and osteoarthritis in which pain dominates the clinical picture. Moreover, the existent armamentarium to treat these two diseases is not as effective as desired. According to the most of studies described here, cannabis was able to reduce pain, improve sleep and had few adverse events but none of the revised studies addressed the long-term side effects. In individuals with nociplastic pain, the endocannabinoid system seems to be dysfunctional justifying the mechanism of action of this drug [34].
There are two well-characterized endocannabinoid receptors in animals: CB1 and CB2; CB1 is mainly found in the central nervous system, while CB2 is found in many tissues and organs, especially those with immune-related activities [1]. Blake et al. [19] showed that this drug was able to reduce not only pain but also inflammatory activity measured by DAS-28 in rheumatoid arthritis patients. The immunosuppressant effects of cannabis reach both the specific and the innate immune systems [35]. Cannabis-based products can suppress pro-inflammatory cytokine production and production of antimicrobial components of activated immune cells; inhibit the immune effector cells activation and their functions and lead to a wide range of immune cells to apoptosis [36]. Studies in vitro, with human synovial samples of rheumatoid arthritis patients undergoing joint replacement, disclosed that CBD reduces the synovial fibroblasts cell viability, interleukin (IL)-6, IL-8 and metalloproteinase (MMP)-3 production [37].
Regarding the action of cannabis on fibrosis, the double-blind randomized trial by Spiera et al. [28] showed promising results. The CB receptors appear to have diverse actions on fibrosis. While CB1 inactivation exerted antifibrotic effects as they controlled leukocyte infiltration and production of transforming growth factor beta 1 (TGFβ1), the activation of CB2 receptors decreases tissue fibrosis in various rodent models of organ fibrosis, including skin, heart, and liver [38, 39]. More studies are needed in this setting.
Finally, in dermatomyositis this product was studied for the treatment of refractory skin lesions with favorable results; it also reduced the levels of IFN-β and IFN-γ in the injured skin [32].
Some limitations were observed in this study. For instance, no comparison between cannabis and classical treatments used in rheumatic diseases was available in the literature. In addition, the studies had low number of participants and the period of observation was short. Future studies should include large patient samples with more long-term follow-ups, enabling a better understanding of the course of cannabis in rheumatic conditions. Few rheumatic diseases have been evaluated until now; a significant number of different rheumatological conditions should be included in future trials. On the other hand, this is the first study to systematically review the therapeutic effects of cannabis in rheumatic diseases. The study strengths are: (1) The inclusion of studies with patients with international criteria for rheumatic diseases; and (2) The inclusion of all kinds of study designs of the use of cannabis in rheumatic diseases, except in vivo and in vitro studies, and review articles.
Conclusion
There are few studies in the literature evaluating cannabis in rheumatological diseases and include systemic sclerosis, fibromyalgia, osteoarthritis, rheumatoid arthritis, osteoporosis, polymyalgia rheumatica, gout, dermatomyositis, and psoriatic arthritis. However, almost all analyzed studies demonstrated that cannabis use seems efficacious in treating signs and symptoms of rheumatic diseases, with a few minor side effects detected during the observation.
Appendices
https://jag.journalagent.com/nci/abs_files/NCI-43669/NCI-43669_(0)_appendices.pdf
Footnotes
Cite this article as: de Carvalho JF, Ribeiro MFLDS, Skare T. Cannabis therapy in rheumatological diseases: A systematic review. North Clin Istanb 2024;11(4):361–366.
Authorship Contributions
Concept – JFC; Design – JFC, TS; Supervision – JFC; Fundings – JFC; Data collection and/or processing – JFC, MR, TS; Analysis and/or interpretation – JFC, TS; Literature review – JFC, MR, TS; Writing – JFC, TS; Critical review – JFC, TS.
Conflict of Interest
No conflict of interest was declared by the authors.
Use of AI for Writing Assistance
Not declared.
Financial Disclosure
The authors declared that this study has received no financial support.
Peer-review
Externally peer-reviewed.
References
- 1.Warf B. High points: an historical geography of Cannabis. Geogr Rev. 2014;104:414–38. [Google Scholar]
- 2.Zuardi AW. History of cannabis as a medicine: a review. Braz J Psychiatry. 2006;28:153–7. doi: 10.1590/s1516-44462006000200015. [DOI] [PubMed] [Google Scholar]
- 3.Giorgi V, Marotto D, Batticciotto A, Atzeni F, Bongiovanni S, Sarzi-Puttini P. Cannabis and autoimmunity: possible mechanisms of action. Immunotargets Ther. 2021;10:261–71. doi: 10.2147/ITT.S267905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonen T, Amital H. Cannabis and cannabinoids in the treatment of rheumatic diseases. Rambam Maimonides Med J. 2020;11:e0007. doi: 10.5041/RMMJ.10389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagy I, Bar-Lev Schleider L, Abu-Shakra M, Novack V. Safety and efficacy of medical cannabis in fibromyalgia. J Clin Med. 2019;8:807. doi: 10.3390/jcm8060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habib G, Avisar I. The consumption of cannabis by fibromyalgia patients in Israel. Pain Res Treat. 2018;2018:7829427. doi: 10.1155/2018/7829427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habib G, Khazin F, Artul S. The effect of medical cannabis on pain level and quality of sleep among rheumatology clinic outpatients. Pain Res Manag. 2021;2021:1756588. doi: 10.1155/2021/1756588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazza M. Medical cannabis for the treatment of fibromyalgia syndrome: a retrospective, open-label case series. J Cannabis Res. 2021;3:4. doi: 10.1186/s42238-021-00060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiz J, Durán M, Capellà D, Carbonell J, Farré M. Cannabis use in patients with fibromyalgia: effect on symptoms relief and health-related quality of life. PLoS One. 2011;6:e18440. doi: 10.1371/journal.pone.0018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Donk T, Niesters M, Kowal MA, Olofsen E, Dahan A, van Velzen M. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain. 2019;160:860–9. doi: 10.1097/j.pain.0000000000001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yassin M, Oron A, Robinson D. Effect of adding medical cannabis to analgesic treatment in patients with low back pain related to fibromyalgia: an observational cross-over single centre study. Clin Exp Rheumatol. 2019;37(Suppl 116):13–20. [PubMed] [Google Scholar]
- 13.Skrabek RQ, Galimova L, Ethans K, Perry D. Nabilone for the treatment of pain in fibromyalgia. J Pain. 2008;9:164–73. doi: 10.1016/j.jpain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Sotoodeh R, Waldman LE, Vigano A, Moride Y, Canac-Marquis M, Spilak T, et al. Predictors of pain reduction among fibromyalgia patients using medical cannabis: a long-term prospective cohort study. Arthritis Care Res (Hoboken) 2023;75:1588–94. doi: 10.1002/acr.24985. [DOI] [PubMed] [Google Scholar]
- 15.Ware MA, Fitzcharles MA, Joseph L, Shir Y. The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Anesth Analg. 2010;110:604–10. doi: 10.1213/ANE.0b013e3181c76f70. [DOI] [PubMed] [Google Scholar]
- 16.Giorgi V, Bongiovanni S, Atzeni F, Marotto D, Salaffi F, Sarzi-Puttini P. Adding medical cannabis to standard analgesic treatment for fibromyalgia: a prospective observational study. Clin Exp Rheumatol. 2020;38(Suppl 123):53–9. [PubMed] [Google Scholar]
- 17.Chaves C, Bittencourt PCT, Pelegrini A. Ingestion of a THC-rich cannabis oil in people with fibromyalgia: a randomized, double-blind, placebo-controlled clinical trial. Pain Med. 2020;21:2212–8. doi: 10.1093/pm/pnaa303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzcharles MA, Rampakakis E, Sampalis JS, Shir Y, Cohen M, Starr M, et al. Use of medical cannabis by patients with fibromyalgia in Canada after cannabis legalization: a cross-sectional study. Clin Exp Rheumatol. 2021;39(Suppl 130):115–9. doi: 10.55563/clinexprheumatol/qcyet7. [DOI] [PubMed] [Google Scholar]
- 19.Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford) 2006;45:50–2. doi: 10.1093/rheumatology/kei183. [DOI] [PubMed] [Google Scholar]
- 20.Wang A, Lo A, Ubhi K, Cameron T. Small and transient effect of cannabis oil for osteoarthritis-related joint pain: a case report. Can J Hosp Pharm. 2021;74:156–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Fader L, Scharf Z, DeGeorge BR. Jr.-Assessment of medical cannabis in patients with osteoarthritis of the thumb basal joint. J Hand Surg Am. 2023;48:257–62. doi: 10.1016/j.jhsa.2021.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Maurer GE, Imperato NS, Juybari CM, Kincaid H, Koons A. The utility of cannabis-based medicine in chronic pain management: a case report. Cureus. 2022;14:e31555. doi: 10.7759/cureus.31555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renslo B, Greis A, Liu CS, Radakrishnan A, Ilyas AM. Medical cannabis use reduces opioid prescriptions in patients with osteoarthritis. Cureus. 2022;14:e21564. doi: 10.7759/cureus.21564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vela J, Dreyer L, Petersen KK, Arendt-Nielsen L, Duch KS, Kristensen S. Cannabidiol treatment in hand osteoarthritis and psoriatic arthritis: a randomized, double-blind, placebo-controlled trial. Pain. 2022;163:1206–14. doi: 10.1097/j.pain.0000000000002466. [DOI] [PubMed] [Google Scholar]
- 25.Rampakakis E, Thorne C, Cesta A, Movahedi M, Li X, Mously C, et al. Medical cannabis use by rheumatology patients in routine clinical care: results from The Ontario Best Practices Research Initiative. Clin Exp Rheumatol. 2023;41:118–25. doi: 10.55563/clinexprheumatol/b85xu5. [DOI] [PubMed] [Google Scholar]
- 26.Frane N, Stapleton E, Iturriaga C, Ganz M, Rasquinha V, Duarte R. Cannabidiol as a treatment for arthritis and joint pain: an exploratory cross-sectional study. J Cannabis Res. 2022;4:47. doi: 10.1186/s42238-022-00154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright S, Ware M, Guy G. The use of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology. 2006;45:781. doi: 10.1093/rheumatology/kel114. [DOI] [PubMed] [Google Scholar]
- 28.Spiera R, Hummers L, Chung L, Frech TM, Domsic R, Hsu V, et al. Safety and efficacy of Lenabasum in a phase II, randomized, placebo-controlled trial in adults with systemic sclerosis. Arthritis Rheumatol. 2020;72:1350–60. doi: 10.1002/art.41294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spinella A, de Pinto M, Baraldi C, Galluzzo C, Testoni S, Lumetti F, et al. Topical cannabidiol in the treatment of digital ulcers in patients with scleroderma: comparative analysis and literature review. Adv Skin Wound Care. 2023;36:18–23. doi: 10.1097/01.ASW.0000891856.08360.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nogueira AR, Shoenfeld Y, Amital H. Cannabis sativa as a potential treatment for systemic sclerosis. Isr Med Assoc J. 2019;21:217–8. [PubMed] [Google Scholar]
- 31.Ouatah H, Authier N, Tournadre A, Fan A, Soubrier M, Mathieu S. Frequency of cannabis use in patients with rheumatoid arthritis or spondyloarthropathies: a single-center study. Clin Exp Rheumatol. 2023;41:198. doi: 10.55563/clinexprheumatol/yb4f0n. [DOI] [PubMed] [Google Scholar]
- 32.Werth VP, Hejazi E, Pena SM, Haber J, Zeidi M, Reddy N, et al. Safety and efficacy of Lenabasum, a cannabinoid receptor type 2 agonist, in patients with dermatomyositis with refractory skin disease: a randomized clinical trial. J Invest Dermatol. 2022;142:2651–9. doi: 10.1016/j.jid.2022.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsang J, Silverberg O, Machhar R, Pollock R, Pereira D, Sutton M, et al. Exploring cannabis use and perspectives among psoriatic disease patients. Clin Rheumatol. 2022;41:1431–7. doi: 10.1007/s10067-022-06066-6. [DOI] [PubMed] [Google Scholar]
- 34.Fitzcharles MA, Petzke F, Tölle TR, Häuser W. Cannabis-based medicines and medical cannabis in the treatment of nociplastic pain. Drugs. 2021;81:2103–16. doi: 10.1007/s40265-021-01602-1. [DOI] [PubMed] [Google Scholar]
- 35.Lowin T, Tingting R, Zurmahr J, Classen T, Schneider M, Pongratz G. Cannabidiol (CBD): a killer for inflammatory rheumatoid arthritis synovial fibroblasts. Cell Death Dis. 2020;11:714. doi: 10.1038/s41419-020-02892-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khoury M, Cohen I, Bar-Sela G. “The two sides of the same coin”-medical cannabis, cannabinoids and immunity: pros and cons explained. Pharmaceutics. 2022;14:389. doi: 10.3390/pharmaceutics14020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Río C, Ruiz-Pino F, Prados ME, Fiebich BL, Tena-Sempere M, Muñoz E. Cannabidiol markedly alleviates skin and liver fibrosis. Front Pharmacol. 2022;13:981817. doi: 10.3389/fphar.2022.981817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Servettaz A, Kavian N, Nicco C, Deveaux V, Chéreau C, Wang A, et al. Targeting the cannabinoid pathway limits the development of fibrosis and autoimmunity in a mouse model of systemic sclerosis. Am J Pathol. 2010;177:187–96. doi: 10.2353/ajpath.2010.090763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mai P, Yang L, Tian L, Wang L, Jia S, Zhang Y, et al. Endocannabinoid system contributes to liver injury and inflammation by activation of bone marrow-derived monocytes/macrophages in a CB1-dependent manner. J Immunol. 2015;195:3390–401. doi: 10.4049/jimmunol.1403205. [DOI] [PubMed] [Google Scholar]