Abstract
Trichothecene mycotoxins are a family of potent translational inhibitors that are associated with foodborne outbreaks of human and animal gastroenteritis in which vomiting is a clinical hallmark. Deoxynivalenol (DON, vomitoxin) and other Type B trichothecenes have been previously demonstrated to cause emesis in the mink (Neovison vison) and this response has been directly linked to secretion of both the satiety hormone peptide YY3–36 (PYY3–36) and neurotransmitter 5-hydroxytryptamine (5-HT). Here, we characterized the emetic responses in the mink to T-2 toxin (T-2) and HT-2 toxin (HT-2), two highly toxic Type A trichothecenes that contaminate cereals, and further compared these effects to those of emetine, a natural alkaloid that is used medicinally and also well-known to block translation and cause vomiting. Following intraperitoneal (IP) and oral exposure, all three agents caused vomiting with evident dose-dependent increases in both duration and number of emetic events as well as decreases in latency to emesis. T-2 and HT-2 doses causing emesis in 50% of treated animals (ED50s) were 0.05 and 0.02 mg/kg BW following IP and oral administration, respectively, whereas the ED50s for emetine were 2.0 and 1.0 mg/kg BW for IP and oral exposure, respectively. Importantly, oral administration of all three toxins elicited marked elevations in plasma concentrations of PYY3–36 and 5-HT that corresponded to emesis. Taken together, the results suggest that T-2 and HT-2 were much more potent than emetine and that emesis induction by all three translational inhibitors co-occurred with increases in circulating levels of PYY3–36 and 5-HT.
Keywords: mycotoxin, trichothecene, emesis, T-2 toxin;HT-2 toxin, emetine
Introduction
Trichothecenes are a family of structurally related sesquiterpenoid mycotoxins produced by Fusarium that can contaminate wheat, barley, maize and rice (Pestka 2010b). Since trichothecenes are stable during milling and processing (Jackson and Bullerman 1999), they can be present in cereal-based foods and therefore have the potential to harm humans and animals. Exposure of experimental animals to trichothecenes elicits a spectrum of adverse effects including emesis, anorexia, growth retardation, neuroendocrine changes, and immunotoxicity (Pestka 2010a). Investigations of trichothecene food poisoning in humans have identified emesis to be a hallmark effect (Luo 1994; Ueno 1987; Yoshizawa 1983). Since trichothecene-induced emesis has the potential to adversely affect human and animal growth and survival by disrupting of normal nutrition, hydration, and electrolyte balance, it is essential to know the comparative emetic potencies of this mycotoxin family as well as to understand the underlying mechanisms for these effects.
Emesis represents a complex process that integrates hormones, neurotransmitters, visceral afferent neurons and the brain (Andrews and Horn 2006; Hornby 2001). The central pattern generator (CPG) located in the medulla oblongata of the hindbrain coordinates the whole neuronal network of emesis induction. Hormones and neurotransmitters bind to specific receptors located on vagal afferent terminals or the area postrema of the medulla, activate the CPG and eventually trigger emesis. Our laboratory recently developed a small animal model for emesis induction employing the mink (Neovison vison) and successfully applied it to the comparison of the emetic potency of deoxynivalenol (DON, vomitoxin) to that of other Type B trichothecenes (Wu et al. 2013a; Wu et al. 2013b; Wu et al. 2014). Emetic thresholds for DON in this model closely mimicked those in larger animal species including the pig, dog and cat. The mink was further used to demonstrate that DON-induced emesis involves secretion of both the satiety hormone peptide YY3–36 (PYY3–36) and the monoamine neurotransmitter 5-hydroxytryptamine (5-HT, serotonin) (Wu et al. 2013b).
While Type A trichothecenes such as T-2 toxin (T-2) and HT-2 toxin (HT-2) are also associated with grain contamination at lower frequencies and concentrations than the Type B trichothecenes (Schothorst and van Egmond 2004), the former are considered to be much more toxic (JECFA 2002; SCF 2002). T-2 toxicoses in farm animals have been reported in many countries including Canada, United States and Japan (Greenway and Puls 1976; Hsu et al. 1972; Ueno 1977). T-2 induces emesis in several animal species following oral, sublingual, intravenous, subcutaneous and intraperitoneal (Borison and Goodheart 1989; Ellison and Kotsonis 1973; Fairhurst et al. 1987; Gaige et al. 2014; Lutsky and Mor 1981; Sato et al. 1975) exposure. HT-2 is a closely-related Type A trichothecene that differs fromT-2 only by the functional group at the C-4 position (Fig.1). HT-2 also induces emesis in animals (Ellison and Kotsonis 1974) and appears to have comparable toxicity to T-2 (Ueno et al. 1973). To date, it is unclear as to what the comparative emetic potencies of Type A and B trichothecenesare or whether the underlying mechanisms of emesis are shared by the two families.
Figure 1. Structures of T-2 toxin, HT-2 toxin and emetine.
Emetine is a natural alkaloid produced from ipecacuanha (Fig. 1) and has been used pharmacologically to induce emesis against toxic agent ingestion, anorexia, anti-cough and anti-amoebiasis (Bhargava et al. 1961; Neal 1983). Moreover, emetine has the potential to block protein synthesis by binding to the 40S subunit of the ribosome (Gupta and Siminovitch 1977). Since the physiological and biochemical effects of emetine are very similar to trichothecenes, it is of fundamental interest to compare the emetic potencies and mechanisms of action of these structurally unrelated compounds.
The objective of this study was to test the hypothesis that, like the Type B trichothecenes, Type A trichothecenes and emetine will induce emesis in the mink and that this will correspond to elevated PYY3–36 and 5-HT secretion. To address this hypothesis, we employed the above-described mink model to: 1) characterize the dose response and kinetics of emetic responses to T-2 and HT-2 toxin, 2) compare those effects to emetine and 3) relate emesis induction by the three toxins to changes in plasma concentrations of PYY3–36 and 5-HT.
Materials and methods
Chemicals
T-2 toxin, HT-2 toxin and emetine were purchased from Enzo Life Sciences with purity ≥98%. T-2 toxin and HT-2 toxin were dissolved in 1% dimethyl sulfoxide (DMSO) in filter-sterilized phosphate buffered saline (PBS). Emetine was dissolved in filter-sterilized PBS.
Animals
Animal treatment followed National Institutes of Health guidelines and were approved by the Michigan State University Institutional Animal Care and Use Committee (MSU-IACUC). Standard dark, female mink (Neovison vison) of 1 to 2 years of age (average weight = 1.3 ± 0.1 kg) were obtained from the Michigan State University (MSU) Experimental Fur Farm. Animals were housed singly in wire cages (62 cm long×25 cm wide×38 cm high) and provided with a nest box (24 cm long×24 cm wide×29 cm high) with aspen shavings or excelsior (wood wool) within an open-sided pole barn. Temperature (daily average = 59 to 74 °F), humidity and photoperiod were dependent on the ambient environment. Housing conditions met those specified in the Standard Guidelines for the Operation of Mink Farms in the United States (Fur Commission USA 2010).
Study 1: Dose response effects and kinetics of T-2, HT-2 and emetine-induced emesis in mink
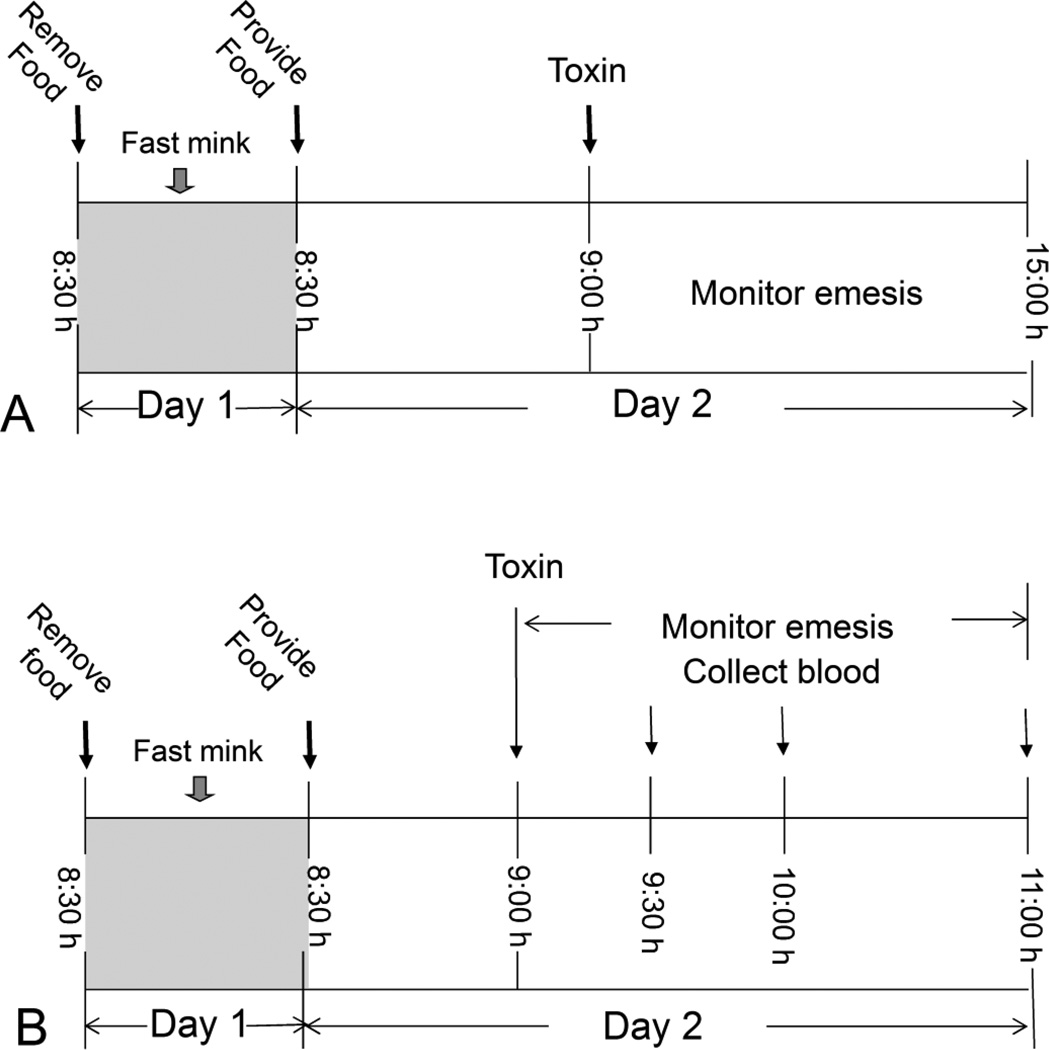
The mink emesis bioassay (Fig. 2A) was based on prior studies employing this species (Wu et al. 2013a; Wu et al. 2013b). Beginning at 8:30 am on the day prior to the experiment, mink were fasted for 24 h with water available ad lib. On the day of the experiment, animals were provided 50 g of feed at 8:30 h and allowed to eat for 30 min to assure a constant volume of gastric contents in each animal. At 9:00 h, mink (n=4) were given T-2 toxin, HT-2 toxin, emetine or PBS in a volume of 1 ml/kg BW by IP injection using a sterile 20-G, 2.54-cm needle or oral gavage using a sterile 16-G, 5-cm stainless steel gavage tube. After dosing, animals were returned to individual cages and monitored for emesis over the subsequent 6 h. Each individual retch or vomit was counted as described previously (Wu et al. 2013a) and the counts were combined to yield total emetic events. Vomiting was characterized as rhythmic abdominal contraction with oral expulsion of either solid or liquid material, whereas, retching was defined as responses that mimicked vomiting but without any material being expelled.
Figure 2.
(A) Experimental design for comparing emetic effects of T-2, HT-2 and emetine in mink. (B) Experimental design for relating emetic effects of oral exposure to T-2, HT-2 and emetine to plasma PYY3–36 and 5-HT concentrations in mink.
Study 2: Relation of T-2-, HT-2-and emetine-induced emesis to plasma PYY3–36 and 5-HT concentrations
Groups of fasted mink (n=4) were given 50 g feed at 8:30 h on the day of dosing and then allowed to eat for 30 min (Fig. 2B). At 9:00 h, mink were orally gavaged with T-2 toxinorHT-2 toxinat 0.5 mg/kg BW or with emetine at 5 mg/kg BW in 100 µl PBS or vehicle alone. Emetic events were recorded over the next 2 h. After 0-, 0.5-, 1-and 2-h intervals, mink groups were anesthetized by intramuscular injection with ketamine (Ketaset III, ketamine HCl, 100 mg/ml) at 10mg/kg BW). Blood was collected by heart puncture into vacutainers containing EDTA as anticoagulant, and mink were immediately euthanized by CO2 exposure. Blood was centrifuged at 1000× g for 10 min. Resultant plasma, which was platelet free, was frozen at −80°C until subsequent ELISA analyses for PYY3–36 (mouse, rat, porcine, and canine specific; Phoenix Pharmaceuticals, Burlingame, CA) and 5-HT (Enzo Life Sciences, Plymouth Meeting, PA).
Statistics
Data were plotted and statistically analyzed using SigmaPlot 11 for Windows (Jandel Scientific; San Rafael, CA) with the exception of emetic dose (ED), which was calculated with Proc Probit using SAS (Version 9.2, SAS, Cary, NC). Means were considered significantly different at p < 0.05. Fisher’s Exact Test was used for incidence, and one-way ANOVA using the Holm-Sidak method was used tor latency, duration, retching, vomiting, total emetic events. A two-way ANOVA using the Holm-Sidak method was used to assess significant differences in mean cumulative emetic events and kinetics of plasma PYY3–36 and5-HT in mink.
Results
Study 1
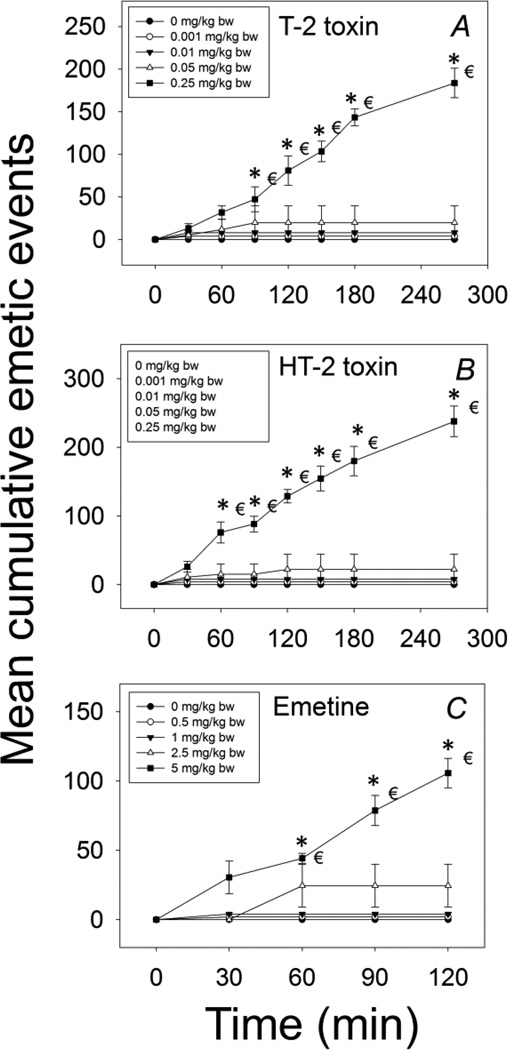
The effects of IP exposure to T-2, HT-2 and emetine on emesis induction were compared in mink. The lowest dose at which T-2 toxin induced emesis in mink following IP exposure was 0.05 mg/kg BW, with 25% of the animals responding (Table 1, Fig 3A). After exposure to T-2 toxin at0.25 mg/kg BW, all animals vomited. Exposure to T-2 at 0.001 and 0.01 mg/kg BW had no effect. The majority of emetic episodes occurred within 30 min, although new emetic events were detectable up to 270 min at the 0.25 mg/kg BW dose.
Table 1.
Comparison of emetic responses in mink following IP Exposure to T-2, HT-2 and emetine
| Toxin | Dose (mg/kg BW) |
Incidence (Responding/Tested) |
Latency to emesis (min)A,B |
Emesis duration (min)A,B |
Emetic eventsC | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Retching | Vomiting | Total | |||||
|
|
|
||||||
| T-2 | 0 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 0.001 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 0.01 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 0.05 | 1/4 | 30 ± 0a | 55 ± 0a | 18 ± 18 | 2 ± 2 | 20 ± 20 | |
| 0.25* | 4/4 | 27 ± 3a | 207 ± 7b | 161 ± 15 | 23 ± 2 | 184 ± 17 | |
| HT-2 | 0 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 0.001 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 0.01 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 0.05 | 1/4 | 28 ± 0a | 71 ± 0a | 20 ± 20 | 3 ± 3 | 22 ± 22 | |
| 0.25* | 4/4 | 24 ± 2a | 242 ± 7b | 210 ± 22 | 28 ± 2 | 238 ± 22 | |
| Emetine | 0 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 0.5 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 1 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 2.5 | 2/4 | 40 ± 5a | 17 ± 4a | 21 ± 13 | 3 ± 2 | 25 ± 15 | |
| 5* | 4/4 | 23 ± 5a | 82 ± 6b | 92 ± 9 | 14 ± 2 | 106 ± 11 | |
Average of positive responders only.
If animals failed to retch or vomit, the latency and duration of emesis are shown as “-”.
Average of both responders and non-responders. Data represent the mean ± SEM. Values with an asterisk indicate significant differences at p < 0.05 relative to the control for incidence, retching, vomits and total emetic events. Values for each compound with different superscript within a column indicate significant differences at p < 0.05.
Figure 3. Mean cumulative emetic events in mink following IP exposure to T-2, HT-2 and emetine.
Data are averages for both responders and nonresponders and represent mean ± SEM (n=4/group). Two-way ANOVA using the Holm-Sidak method was used to assess significant differences in mean cumulative emetic events as compared with the control. Symbols: *indicates statistically significant differences in cumulative emetic episodes compared with the control (p < 0.05) and € indicates a statistically difference relative to the 0-min time point within a given dose (p < 0.05).
Responses to IP exposure with HT-2 were remarkably similar to those observed for T-2. The minimum emetic dose for HT-2 was 0.05 mg/kg BW, with 25% of the treated mink experiencing emesis (Table 1, Fig. 3B). When the dose was increased to 0.25 mg/kg BW, all mink vomited within 30 min and no emetic events were recorded after 270 min.
Mink dosed IP with emetine at 0.5 and 1 mg/kg BW did not exhibit emesis, whereas doses of2.5 and 5 mg/kg BW induced emesis in 50 and 100% of the animals within these groups (Table 1, Fig. 3C). The 2.5 mg/kg BW dose induced emesis within 40 min, which continued up to 60 min post-dosing while 5 mg/kg BW initiated emesis within 30 min that persisted up to120 min post-dosing.
Emesis induction was also assessed in mink following oral administration with T-2, HT-2 and emetine. Regarding T-2, no effects were observed at 0.005 mg/kg BW, whereas the 0.05 mg/kg BW dose caused 3 out of 4 of the exposed mink to vomit (Table 2, Fig.4A). Increasing the dose to 0.25 or 0.5 mg/kg BW caused emesis in all animals. Most emetic events were observed within 30 min and continued up to 270 min.
Table 2.
Comparison of emetic responses in mink following oral exposure to T-2, HT-2 and emetine
| Toxin | Dose (mg/kg BW) |
Incidence (Responding/Tested) |
Latency to emesis (min)A,B |
Emesis duration (min)A,B |
Emetic eventsC | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Retching | Vomiting | Total | |||||
|
|
|
||||||
| T-2 | 0 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 0.005 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 0.05 | 3/4 | 23 ± 3a | 52 ± 15a | 66 ± 23 | 8 ± 3 | 74 ± 26 | |
| 0.25* | 4/4 | 20 ± 3a | 108 ± 11a | 130 ± 17 | 17 ± 1 | 147 ± 18 | |
| 0.5* | 4/4 | 13 ± 3a | 171 ± 22b | 191 ± 50 | 21 ± 3 | 212 ± 53 | |
| HT-2 | 0 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 0.005 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 0.05 | 3/4 | 27 ± 7a | 50 ± 11a | 60 ± 24 | 7 ± 3 | 68 ± 27 | |
| 0.25* | 4/4 | 22 ± 4a | 121 ± 21a | 157 ± 24 | 18 ± 2 | 175 ± 26 | |
| 0.5* | 4/4 | 19 ± 2a | 201 ± 18b | 222 ± 29 | 23 ± 3 | 245 ± 31 | |
| Emetine | 0 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 0.5 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 1 | 2/4 | 53 ± 15a | 12 ± 6a | 8 ± 5 | 2 ± 1 | 9 ± 6 | |
| 2.5 | 3/4 | 40 ± 8a | 54 ± 11a | 33 ± 14 | 5 ± 2 | 38 ± 15 | |
| 5* | 4/4 | 36 ± 5a | 109 ± 16b | 82 ± 16 | 13 ± 2 | 95 ± 18 | |
Average of positive responders only.
If animals failed to retch or vomit, the latency and duration of emesis are shown as “-”.
Average of both responders and non-responders. Data represent the mean ± SEM. Values with an asterisk indicate significant differences at p < 0.05 relative to the control for incidence, retching, vomits and total emetic events. Values for each compound with different superscript within a column indicate significant differences at p < 0.05.
Figure 4. Mean cumulative emetic events in mink following oral exposure to T-2, HT-2 and emetine.
Experiment was conducted and data analyzed as described in Figure 3 legend.
Following oral dosing with HT-2 toxin at 0.05, 0.25, and 0.5 mg/kg BW, 3 out of 4, and 4 out of 4 mink experienced emesis, respectively, whereas no effects were observed at 0.005 mg/kg BW (Table 2, Fig. 4B). As observed for T-2 toxin, most emetic events were observed within 30 min and continued up to 270 min (Fig.4B).
Oral dosing with 1, 2.5 and 5 mg/kg BW emetine induced emesis in 50, 75 and 100% of the mink, respectively, whereas 0.5 mg/kg BW had no effect (Table 2). The majority of emetic episodes occurred within the first60 min, and continued up to 150 and 210 min after dosing at 2.5 and 5 mg/kg BW, respectively (Fig.4C).
Study 2
To relate toxin-induced emesis to PYY3–36 and 5-HT release, mink were treated orally with T-2, HT-2 and emetine at doses that caused emesis in all animals and then plasma concentrations of the two hormones were monitored over 2 h. When mink were orally dosed with 0.5 mg/kg BW T-2 39, 25 and 36% of emetic events were observed during the 0 to 0.5 h, 0.5 to 1 h, and 1 to 2 h periods, respectively (Fig. 5A).). Plasma PYY3–36 increased steadily throughout the 2 h post-dosing period with a statistically significant difference relative to the control group being evident at 2 h (Fig. 5B). For 5-HT, PYY3–36 was highest after 1 h and returned to basal level at 2 h after treatment (Fig. 5C).
Figure 5. Relationship of T-2 -induced emetic response to plasma PYY3–36 and 5-HT following oral exposure in mink.
Mink were orally gavaged with either 1% DMSO (solid lines) or 0.5 mg/kg BW T-2 (broken lines).(A) Mean cumulative emetic events (total retches and vomits) in mink following oral exposure to T-2. Data are averages for both responders and nonresponders. Kinetics ofT-2 -induced plasma (B) PYY3–36 and (C) 5-HT elevation in mink. Two-way ANOVA using the Holm-Sidakm was used to assess significant differences in mean cumulative emetic events and kinetics of plasma PYY3–36 and5-HT in mink. Symbols: *indicates difference in mean cumulative emetic events and plasma PYY3–36 or 5-HT concentration relative to the control at specific time point (p < 0.05) and € indicates difference in mean cumulative emetic events and plasma PYY3–36 or 5-HT concentration relative to the 0h time point (p < 0.05).
Similar to T-2, oral exposure to HT-2 at 0.5 mg/kg BW resulted in 38, 23 and 39% of emetic events during the 0 to 0.5, 0.5 to 1 h, and 1 to 2 h periods, respectively (Fig. 6A). Plasma PYY3–36 concentrations were increased at 0.5 h, peaked at 1 h, and still markedly elevated at 2 h (Fig. 6B). In contrast, a trend toward elevated 5-HT was evident only at 2 h (Fig. 6C).
Figure 6. Relationship of HT-2 -induced (A) emetic response to plasma (B) PYY3–36 and (C) 5-HT following oral exposure in mink.
Mink were orally gavaged with either 1% DMSO (solid lines) or 0.5mg/kg BW HT-2 (broken lines). Experiment was conducted and data analyzed as described in Figure 5 legend.
Following oral administration with emetine at 5 mg/kg BW, 16, 34 and 50% of emetic events were observed during the 0 to 0.5 h, 0.5 to 1 h, and 1 to 2 h periods, respectively (Fig. 7A). Significant elevation of plasma PYY3–36 was observed at 1 h and further increased 2 h after treatment (Fig. 7B), whereas, 5-HT concentration was significantly elevated only at 2 h (Fig. 7C).
Figure 7. Relationship of emetine-induced (A) emetic response to plasma (B) PYY3–36 and (C) 5-HT following oral exposure in mink.
Mink were orally gavaged with either PBS (solid lines) or 5mg/kg BW emetine (broken lines). Experiment was conducted and data analyzed as described in Figure 5 legend.
Discussion
Although gastroenteritis following exposure to common foodborne trichothecenes has long been of major human and animal health concern, there have been relatively few investigations systematically comparing their effects in a common animal model until recently (Wu et al. 2013a; Wu et al. 2013b;Wu et al. 2014). This study is the first to characterize the emetogenic potentials of T-2 toxin and HT-2 toxin in the mink, an established animal model for emesis (Qian et al. 2010a; Qian et al. 2010b; Qian et al. 2009), and to further relate these emetogenic potentials to that of emetine, a medicinal alkaloid that, like the trichothecene mycotoxins, inhibits translation. The results revealed several novel findings. First, T-2 toxin-and HT-2 toxin-induced emetogenic effects were virtually indistinguishable relative to dose-response, latency, duration, intensity and number of emetic events. Second, emetine was less potent than either T-2 orHT-2 at eliciting emesis. Third, oral exposure to T-2 toxin, HT-2 toxin and emetine was more effective in evoking emesis than IP administration based on incidence, duration and intensity. Finally, elicitation of emesis by all three toxins corresponded to elevated plasma concentrations of PYY3–36 and 5-HT, hormones with known emetic effects.
As summarized in Table 3, emesis incidence data were used to determine each compound’s no-observed adverse effect level (NOAEL) and lowest observed effect level (LOAEL) as well as estimate the ED20, ED50 and ED80 values. The results reveal that these values are similar for T-2 and HT-2. These similarities might reflect the observation that T-2 is very rapidly and almost completely biotransformed to HT-2 after exposure (Sintov et al. 1986). Besides incidence, other key endpoints for characterizing potencies of emesis-inducing compounds are latency, duration and intensity of emetic events (Kris et al. 2006). Latency reflects each compound’s rate of activation for emesis and/or absorption, whereas the duration provides insight into the rate of elimination for each compound. Again, T-2 and HT-2 were very similar with respect to latency (13 to 27 min), persistent duration (171 to 242 min) and number of emetic responses (>180 times). The short latency to emetic response suggests these toxins rapidly reach the active site. T-2 was reported to reach maximum plasma concentrations at about 30 min after exposure (Kalantari and Moosavi 2010). Although the long duration for emesis could indicate that they are eliminated slowly, many studies indicate T-2 and HT-2 have short plasma half-lives in several species (Beasley et al. 1986; Osselaere et al. 2013; Sintov et al. 1986). So, the persistent duration of emesis for T-2 and HT-2 might not only depend on their elimination but on their inherently higher toxicity or ability of low concentrations to sustain the response.
Table 3.
Summary of NOAELs, LOAELs, ED20s, ED50s and ED80s for emetic effects of T-2, HT-2 and emetine in mink
| Toxin (mg/kg BW) |
IP | Oral | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| NOAELA | LOAELB | ED20C | ED50D | ED80E | NOAELA | LOAELB | ED20C | ED50D | ED80E | |
| T-2 | 0.01 | 0.05 | 0.03 | 0.05 | 0.09 | 0.005 | 0.05 | 0.01 | 0.02 | 0.04 |
| HT-2 | 0.01 | 0.05 | 0.03 | 0.05 | 0.09 | 0.005 | 0.05 | 0.01 | 0.02 | 0.04 |
| Emetine | 1 | 2.5 | 1.36 | 1.96 | 2.83 | 0.5 | 1 | 0.58 | 1.03 | 1.85 |
NOAEL = no observed adverse effect level.
LOAEL = lowest observed adverse effect level.
ED20 = Dose causing emesis in 20% of the animals tested.
ED50 = Dose causing emesis in 50% of the animals tested.
ED80 = Dose causing emesis in 80% of the animals tested. ED20, ED50 and ED80 values were determined using a Proc Probit model.
Oral administration ofT-2, HT-2 and emetine induced stronger emetic effects than IP administration based on NOAEL, LOAEL, ED20, ED50 and ED80 values (Table 3). These findings correspond with our previous findings relative to Type B trichothecene-induced emesis in mink (Wu et al. 2013a). The observations are consistent with the observations that LOAELs for T-2 toxin-induced emesis in cat by IP and oral exposure were 2 and 0.08 mg/kg BW, respectively (Borison and Goodheart 1989; Lutsky and Mor 1981). Other reports suggest that oral exposure is less effective than IP exposure in emesis induction by DON in pigs (Forsyth et al. 1977; Pestka et al. 1987). It is further notable that with exception of the cat, the LOAELs of T-2 toxin and HT-2 toxin in the present emesis study differ somewhat from those reported previously in studies with other species (Table 4). Possible reasons for these divergent findings might include different experimental designs (fasted or unfasted animals) and inherent species differences in intestinal transit time, absorption, metabolism, distribution, and bioavailability of trichothecenes for the IP and oral exposure routes. In addition, it is possible that trichothecenes need to bind to specific receptors located in the gastrointestinal tract to evoke emesis (Wu et al. 2013b).
Table 4.
Summary of previously reported emetic effects of T-2, HT-2 and emetine
| Toxin | Species | Route | NOAEL (mg/kg BW) | LOAEL (mg/kg BW) | Study |
|---|---|---|---|---|---|
| T-2 | Pigeon | sublingual | n.d. | 0.2 | Fairhurst et al., 1987 |
| T-2 | Pigeon | oral | n.d. | 0.72 | Ellison et al., 1973 |
| Pigeon | iv | n.d. | 0.15 | ||
| Dog | iv | n.d. | 0.25 | ||
| T-2 | Cat | oral | n.d. | 0.08 | Lutsky et al., 1981 |
| T-2 | Cat | ip | n.d. | 2 | Borison et al., 1989 |
| Cat | iv | n.d. | 2 | ||
| T-2 | Cat | sc | n.d. | 0.5 | Sato et al., 1975 |
| T-2 | Pig | intra-aortal | n.d. | 1.2 | Beasley et al., 1986 |
| HT-2 | Pigeon | iv | n.d | 0.68 | Ellison et al., 1974 |
| Emetine | Ferret | oral | n.d | 5 | Hasegawa et al., 2002 |
| Emetine | Pigeon | sc | n.d | 20 | Ferrari et al., 1998 |
| Emetine | Pigeon | im | 1 | 5 | Wolff et al., 1995 |
| Emetine | House shrew | sc | n.d | 40 | Ueno et al., 1987 |
| Emetine | Dog | iv | n.d | 3 | Bhargava et al., 1961 |
| Dog | intraventricular | n.d | 0.1 | ||
| Emetine | Dog | oral | n.d. | 1 | Boyd et al., 1964 |
| Emetine | Cat | oral | n.d. | 0.35 | Child et al., 1964 |
| Cat | sc | 1.4 | 2.8 |
Emetine is a primary alkaloid found in ipecacuana plants. Ipecac, a syrup extract of this plant, has pharmaceutical properties that include induction of vomiting for gastrointestinal decontamination as well as treatment of amoebiasis and malaria (Yen and Ewald, 2012). Because people with eating disorders (anorexia nervosa, bulimia) have used ipecac as a drug of abuse, it is not available over the counter in the U.S. Emetine blocks ribosomal binding of T-2 toxin in eukaryotic cells (Leatherman and Middlebrook 1993a; Leatherman and Middlebrook 1993b) and inhibits translation by interacting with the E-site of the ribosomal subunit and interfering with mRNA/tRNA translocation (Wong et al. 2014). Therefore it is possible that emetine and other natural translational inhibitors elicit emesis in analogous fashion to T-2, HT-2 and other trichothecenes. Our results are consistent with a previous study in the closely related ferret, in which all of the animals vomited after oral exposure to 5 mg/kg BW emetine within 40 min (Hasegawa et al.2002). As shown here, emetine had transient latency (23 to 36 min), short duration (82 to 109 min) and moderate emetic response (95 to 106 times) compared with T-2 and HT-2. The ED50 values observed here for T-2/HT-2 (0.05 and 0.02 mg/kg BW for IP and oral exposure, respectively) and emetine (1.96 and 1.03 mg/kg BW for IP and oral exposure) suggested that emetic potencies followed approximate rank orders of T-2=HT-2 > emetine for both IP and oral exposure.
The NOAELs and LOAELs of emetine reported here in mink (1 and 2.5 mg/kg BW for IP exposure, 0.5 and 1 mg/kg BW for oral exposure, respectively) were different than those reported previously for other species. For example, in the pigeon, the NOAEL and LOAEL of emesis were 1 and 5 mg/kg BW following intramuscular exposure (Wolff and Leander 1995), respectively, and 1.4 and 2.8 mg/kg BW in the cat following subcutaneous exposure (Child et al. 1964), respectively. Moreover, the LOAELs for emetine-induced emesis by oral administration were 5, 1 and 0.35 mg/kg BW in ferret, dog and cat, respectively (Boyd and Knight 1964; Child et al. 1964; Hasegawa et al. 2002). Both dose selection as well as species differences in absorption, distribution, metabolism, and excretion of emetine might explain the differences in these values.
It was particularly notable that elicitation of emesis by all three toxins corresponded to elevated plasma concentrations of PYY3–36 and 5-HT, hormones with known emetic effects. The neurologic and physiologic basis for emesis is a complex process integrating hormones, neurotransmitters, and visceral afferent neurons (Andrews and Horn 2006; Hornby 2001). In a prior study, the contributions of PYY3–36 and 5-HT in DON-induced emesis were investigated (Wu et al. 2013b). IP administration of DON at 0.1 and 0.25 mg/kg BW induced emesis within 15 to 30 min and continued for up to 120 min. Increased plasma PYY3–36 was observed by 30 to 60 min and by 60 min for 5-HT. Pharmacological antagonism of neuropeptide Y2 receptor, which is activated by PYY3–36, suppressed both DON-and PYY3–36-induced emesis. Blocking of 5-HT3 receptor with granisetron completely inhibited induction of vomiting by DON as well as cisplatin, an inducer of 5-HT. PYY3–36 alone induced emesis that could be partially blocked with granisetron, thus indicating a possible upstream role for PYY3–36 in 5-HT release. Therefore, both PYY3–36 and 5-HT appear to contribute to emesis induction by DON. Both 5-HT and PYY3–36 have been implicated in cytotoxic chemical-induced emesis (Endo et al. 2000; Perry et al. 1994) and 5-HT has been shown to contribute to DON’s emetic effect (Prelusky and Trenholm 1993). Therefore, the observations here that T-2, HT-2 and emetine also elicited early PYY3–36 and later 5-HTelevationsin mink plasma following oral exposure, suggest that as found for 1) these two hormones might be similarly involved in emesis evoked by all three agents d 2) PYY might contribute to the increase in plasma 5-HT.
In conclusion, the results presented herein indicate that T-2, HT-2 and emetine dose-dependently evoked emetic responses in the mink and furthermore, these effects corresponded with robust increases in plasma concentrations of the hormones PYY3–36 and 5-HT. From human and animal health perspectives, the data presented here should be useful for establishing toxic potencies to other trichothecenes. Moreover, studies such as this will improve our understanding of how these compounds adversely affect hormone release that impact nutritional homeostasis and energy lance. Finally, future investigations based on this research could provide new perspectives into how translational inhibitors per se induce hormone excytosis as well as into linkages to subsequent emetic responses.
Acknowledgements
We would like to acknowledge the assistance of Angelo Napolitano, Erica Clark and Mary Rosner. This study was supported by USDA NIFA Award (2011-0635), USDA Wheat and Barley SCAB Initiative Award 59-0206-9-058 and by Public Health Service Grant ES03553 from the National Institutes of Health. WW was supported by National Natural Science Foundation of China (31402268), the Priority Academic Development Program of Jiangsu Higher Education Institutions, Natural Science Foundation of Jiangsu Province of China (BK20140691).
References
- Andrews PL, Horn CC. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton Neurosci. 2006;125(1):100–115. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley VR, Swanson SP, Corley RA, Buck WB, Koritz GD, Burmeister HR. Pharmacokinetics of the trichothecene mycotoxin, T-2 toxin, in swine and cattle. Toxicon : official journal of the International Society on Toxinology. 1986;24(1):13–23. doi: 10.1016/0041-0101(86)90161-3. [DOI] [PubMed] [Google Scholar]
- Bhargava K, Gupta P, Chandra O. Effect of ablation of the chemoreceptor trigger zone (CT zone) on the emetic response to intraventricular injection of apomorphine and emetine in the dog. J Pharmacol Exp Ther. 1961;134(3):329–331. [PubMed] [Google Scholar]
- Borison HL, Goodheart ML. Neural factors in acute emetic, cardiovascular, and respiratory effects of T-2 toxin in cats. Toxicol Appl Pharmacol. 1989;101(3):399–413. doi: 10.1016/0041-008x(89)90190-7. [DOI] [PubMed] [Google Scholar]
- Boyd EM, Knight LM. The expectorant action of cephaeline, emetine and 2 - dehydroemetine. J Pharm Pharmacol. 1964;16(2):118–124. doi: 10.1111/j.2042-7158.1964.tb07430.x. [DOI] [PubMed] [Google Scholar]
- Child K, Davis B, Dodds M, Tomich E. Toxicity and tissue distribution studies on the hydrochloride, bismuth iodide complex and a resinate of emetine. J Pharm Pharmacol. 1964;16(2):65–71. doi: 10.1111/j.2042-7158.1964.tb07423.x. [DOI] [PubMed] [Google Scholar]
- Ellison RA, Kotsonis FN. T-2 toxin as an emetic factor in moldy corn. Appl Microbiol. 1973;26(4):540–543. doi: 10.1128/am.26.4.540-543.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison RA, Kotsonis FN. In vitro metabolism of T-2 toxin. Appl Microbiol. 1974;27(2):423–424. doi: 10.1128/am.27.2.423-424.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Minami M, Hirafuji M, et al. Neurochemistry and neuropharmacology of emesis—the role of serotonin. Toxicol. 2000;153(1):189–201. doi: 10.1016/s0300-483x(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Fairhurst S, Marrs T, Parker H, Scawin J, Swanston D. Acute toxicity of T2 toxin in rats, mice, guinea pigs, and pigeons. Toxicol. 1987;43(1):31–49. doi: 10.1016/0300-483x(87)90072-2. [DOI] [PubMed] [Google Scholar]
- Forsyth D, Yoshizawa T, Morooka N, Tuite J. Emetic and refusal activity of deoxynivalenol to swine. Appl Environ Microbiol. 1977;34(5):547–552. doi: 10.1128/aem.34.5.547-552.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fur Commission USA. Standard guidelines for the operation of mink farms in the US. [last accessed November 18, 2014];2010 (http://www.maninnature.com/FCUSA/Members/Resources/Minkguide.pdf. [Google Scholar]
- Gaige S, Djelloul M, Tardivel C, et al. Modification of energy balance induced by the food contaminant T-2 toxin: a multimodal gut-to-brain connection. Brain Behav Immun. 2014;37:54–72. doi: 10.1016/j.bbi.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Greenway J, Puls R. Fusariotoxicosis from barley in British ColumbiaI. I. Natural occurrence and diagnosis. Canadian J Compar Med. 1976;40(1):12–15. [PMC free article] [PubMed] [Google Scholar]
- Gupta RS, Siminovitch L. The molecular basis of emetine resistance in Chinese hamster ovary cells: alteration in the 40S ribosomal subunit. Cell. 1977;10(1):61–66. doi: 10.1016/0092-8674(77)90140-4. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Sasaki T, Sadakane K, et al. Studies for the emetic mechanisms of ipecac syrup (TJN-119) and its active components in ferrets: involvement of 5-hydroxytryptamine receptors. Japanese J Pharmacol. 2002;89(2):113–119. doi: 10.1254/jjp.89.113. [DOI] [PubMed] [Google Scholar]
- Hornby PJ. Central neurocircuitry associated with emesis. Am. J. Med. 2001;111(8):106–112. doi: 10.1016/s0002-9343(01)00849-x. [DOI] [PubMed] [Google Scholar]
- Hsu I-C, Smalley E, Strong F, Ribelin WE. Identification of T-2 toxin in moldy corn associated with a lethal toxicosis in dairy cattle. Appl Microbiol. 1972;24(5):684–690. doi: 10.1128/am.24.5.684-690.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LS, Bullerman LB. Impact of Processing on Food Safety. Vol. 459. Springer; 1999. Effect of processing on Fusarium mycotoxins; pp. 243–261. [DOI] [PubMed] [Google Scholar]
- JECFA. Evaluation of Certain Mycotoxins in Food:56th Report of the Joint FAO/WHO Expert Committee on Food Additives WHO Technical Report Series. 2002;906:42–51. [PubMed] [Google Scholar]
- Kalantari H, Moosavi M. Review on T-2 toxin. Jundishapur Journal of Natural Pharmfcentical Prodacts. 2010;(5):26–38. [Google Scholar]
- Kris MG, Hesketh PJ, Somerfield MR, et al. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol. 2006;24(18):2932–2947. doi: 10.1200/JCO.2006.06.9591. [DOI] [PubMed] [Google Scholar]
- Leatherman DL, Middlebrook JL. Effect of emetine on T-2 toxin-induced inhibition of protein synthesis in mammalian cells. J Pharmacol Exp Ther. 1993a;266(2):741–748. [PubMed] [Google Scholar]
- Leatherman DL, Middlebrook JL. Effects of emetine on the specific association of T-2 toxin with mammalian cells. J Pharmacol Exp Ther. 1993b;266(2):732–740. [PubMed] [Google Scholar]
- Luo X. Proceedings of the Second Asian Conference on Food Safety Chatuchak. Vol. 129. Thailand: International Life Sciences Institute; 1994. Food poisoning caused by Fusarium toxins; p. 136. [Google Scholar]
- Lutsky I, Mor N. Alimentary toxic aleukia (septic angina, endemic panmyelotoxicosis, alimentary hemorrhagic aleukia): t-2 toxin-induced intoxication of cats. American J Pathol. 1981;104(2):189–191. [PMC free article] [PubMed] [Google Scholar]
- Neal R. Experimental amoebiasis and the development of anti-amoebic compounds. Parasitol. 1983;86(01):175–191. doi: 10.1017/s0031182000057279. [DOI] [PubMed] [Google Scholar]
- Osselaere A, Devreese M, Goossens J, et al. Toxicokinetic study and absolute oral bioavailability of deoxynivalenol, T-2 toxin and zearalenone in broiler chickens. Food Chem Toxicol. 2013;51:350–355. doi: 10.1016/j.fct.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Perry M, Rhee J, Smith W. Plasma levels of peptide YY correlate with cisplatin-induced emesis in dogs. J Pharm Pharmacol. 1994;46(7):553–557. doi: 10.1111/j.2042-7158.1994.tb03855.x. [DOI] [PubMed] [Google Scholar]
- Pestka J. Toxicological mechanisms and potential health effects of deoxynivalenol and nivalenol. World Mycotox J. 2010a;3(4):323–347. [Google Scholar]
- Pestka J, Lin W-S, Miller E. Emetic activity of the trichothecene 15-acetyldeoxynivalenol in swine. Food Chem Toxicol. 1987;25(11):855–858. doi: 10.1016/0278-6915(87)90264-x. [DOI] [PubMed] [Google Scholar]
- Pestka JJ. Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol. 2010b;84(9):663–679. doi: 10.1007/s00204-010-0579-8. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, Trenholm HL. The efficacy of various classes of anti - emetics in preventing deoxynivalenol - induced vomiting in swine. Nat Toxins. 1993;1(5):296–302. doi: 10.1002/nt.2620010508. [DOI] [PubMed] [Google Scholar]
- Qian Q, Chen W, Yue W, Yang Z, Liu Z, Qian W. Antiemetic effect of Xiao-Ban-Xia-Tang, a Chinese medicinal herb recipe, on cisplatin-induced acute and delayed emesis in minks. J Ethnopharmacol. 2010a;128(3):590–593. doi: 10.1016/j.jep.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Qian QH, Yue W, Chen WH, Yang ZH, Liu ZT, Wang YX. Effect of gingerol on substance P and NK1 receptor expression in a vomiting model of mink. Chin Med J (Engl) 2010b;123(4):478–484. [PubMed] [Google Scholar]
- Qian QH, Yue W, Wang YX, Yang ZH, Liu ZT, Chen WH. Gingerol inhibits cisplatin-induced vomiting by down regulating 5-hydroxytryptamine, dopamine and substance P expression in minks. Arch Pharm Res. 2009;32(4):565–573. doi: 10.1007/s12272-009-1413-9. [DOI] [PubMed] [Google Scholar]
- Sato N, Ueno Y, Enomoto M. Toxicological approaches to the toxic metabolites of Fusaria. VIII. Acute and subacute toxicities of T-2 toxin in cats. Japanese J Pharmacol. 1975;25(3):263–270. doi: 10.1254/jjp.25.263. [DOI] [PubMed] [Google Scholar]
- SCF. Part 6: Group evaluation of T-2 toxin, HT-2 toxin, nivalenol and deoxynivalenol European Commission. Brussels, Belgium: 2002. Opinion of the Scientific Committee on Food on Fusarium toxins. [Google Scholar]
- Schothorst RC, van Egmond HP. Report from SCOOP task 3.2.10 "collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states". Subtask: trichothecenes. Toxicol Lett. 2004;153(1):133–143. doi: 10.1016/j.toxlet.2004.04.045. [DOI] [PubMed] [Google Scholar]
- Sintov A, Bialer M, Yagen B. Pharmacokinetics of T-2 toxin and its metabolite HT-2 toxin, after intravenous administration in dogs. Drug Metab Dispo. 1986;14(2):250–254. [PubMed] [Google Scholar]
- Ueno Y. Mode of action of trichothecenes. Pure Appl Chem. 1977;49(11):1737–1745. [Google Scholar]
- Ueno Y. Trichothecenes in food. Mycotoxins in food. 1987:123–147. [Google Scholar]
- Ueno Y, Sato N, Ishii K, Sakai K, Tsunoda H, Enomoto M. Biological and chemical detection of trichothecene mycotoxins of Fusarium species. Appl Microbiol. 1973;25(4):699–704. doi: 10.1128/am.25.4.699-704.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MC, Leander JD. Comparison of the antiemetic effects of a 5-HT<sub>1A</sub> agonist, LY228729, and 5-HT<sub>3</sub> antagonists in the pigeon. Pharmacol Biochem Behav. 1995;52(3):571–575. doi: 10.1016/0091-3057(95)00142-j. [DOI] [PubMed] [Google Scholar]
- Wong W, Bai XC, Brown A, et al. Cryo-EM structure of the Plasmodium falciparum 80S ribosome bound to the anti-protozoan drug emetine. Elife. 2014;3 doi: 10.7554/eLife.03080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Bates MA, Bursian SJ, et al. Comparison of emetic potencies of the 8-ketotrichothecenes deoxynivalenol, 15-acetyldeoxynivalenol, 3-acetyldeoxynivalenol, fusarenon X and nivalenol. Toxicol Sci. 2013a;131(1):279–291. doi: 10.1093/toxsci/kfs286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Bates MA, Bursian SJ, et al. Peptide YY3–36 and 5-hydroxytryptamine mediate emesis Induction by trichothecene deoxynivalenol (vomitoxin) Toxicol Sci. 2013b;133(1):186–195. doi: 10.1093/toxsci/kft033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Zhou HR, Bursian SJ, et al. Comparison of anorectic and emetic potencies of deoxynivalenol (vomitoxin) to the plant metabolite deoxynivalenol-3-glucoside and synthetic deoxynivalenol derivatives EN139528 and EN139544. Toxicol Sci. 2014;142(1):167–181. doi: 10.1093/toxsci/kfu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen M, Ewald MB. Toxicity of weight loss agents. J. Med. Toxicol. 2012;8(2):145–152. doi: 10.1007/s13181-012-0213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T. Trichothecenes: chemical, biological, and toxicological aspects. Tokyo, Japan: Kodansha Ltd.; 1983. pp. 195–209. [Google Scholar]