Abstract
Background and Aim
Severe alcoholic hepatitis (SAH) is a serious condition with few treatments. By modifying the gut–liver axis, fecal microbiota transplantation (FMT) was proposed as a treatment for SAH. The purpose of this meta‐analysis was to evaluate the efficacy of FMT versus the standard of care (SOC) in improving SAH patient survival rates.
Methods
A thorough search of electronic databases was conducted till September 2023. The survival rates of SAH patients undergoing FMT versus SOC were compared. Using Review Manager 5.4, odds ratios (ORs) with 95% confidence intervals (CIs) were calculated.
Results
The meta‐analysis consisted of six studies with a total of 371 patients with SAH. Patients who received FMT had significantly higher survival rates at 1 and 3 months compared to those who received SOC, with pooled OR of 2.91 (95% CI: 1.56–5.42, P = 0.0008) and 3.07 (95% CI: 1.81–5.20, P < 0.0001), respectively. However, the survival advantage disappeared after 6 months (OR: 2.96, 95% CI: 0.99–8.85, P = 0.05) and 1 year of follow‐up (OR: 1.81, 95% CI: 0.44–7.46, P = 0.41).
Conclusion
This meta‐analysis highlights the potential of FMT to significantly improve short‐term survival rates in SAH patients. However, the survival benefit did not last 6–12 months. These findings call for additional research into the effectiveness of FMT over the long term, along with strategies for extending the survival benefit.
Keywords: corticosteroids, fecal microbiota transplantation, gut microbiota, hepatic ascites, hepatic encephalopathy, meta‐analysis, pentoxifylline, severe alcoholic hepatitis, standard of care, survival rates
Impact of Fecal Microbiota Transplantation in Severe Alcoholic Hepatitis: A Systematic Review and Meta‐Analysis.

Introduction
A severe manifestation of alcoholic liver disease (ALD) with a high incidence rate that primarily affects young people is severe alcoholic hepatitis (SAH). 1 A score of >20 on the Model for End‐Stage Liver Disease (MELD) scale or a Maddrey discriminant fraction (MDF) of >32 are additional criteria for SAH. It has a 28‐day mortality rate that ranges from 30 to 50%. 2 , 3 ALD encompasses multiple pathological mechanisms, including ethanol‐induced hepatocyte injury, an inflammatory response to the injury, and disruptions in intestinal permeability caused by imbalances in the gut microbiota. 4
The diagnosis of SAH requires the presence of specific criteria, including recent or ongoing excessive alcohol consumption exceeding minimal thresholds (≥40 g per day or 3 drinks for women and ≥50–60 g per day or 4 drinks for men), the onset of severe jaundice within the past 3 months (total bilirubin ≥5 mg/dL), and ideally, a liver biopsy demonstrating characteristic histological features. These features typically include macrovesicular steatosis alongside other findings such as ballooning hepatocytes, Mallory–Denk bodies, neutrophil infiltration, and intrasinusoidal fibrosis. 1
SAH is a serious condition with few effective treatment options. The current therapeutic approaches mainly focus on alcohol cessation, nutritional therapy, corticosteroids, pentoxifylline, a combination of corticosteroids with the anti‐oxidant N‐acetylcysteine, and, in severe instances, liver transplantation. 5 , 6
Gut microbiota is a complex of microorganisms residing in the gastrointestinal tract, forming a complex ecosystem comprised of various species of bacteria, archaea, fungi, protists, and viruses. It has an impact on a number of bodily functions and processes, including the production of microbial enzymes and vitamins (B and K), detoxification, the production of general protective factors, phagocytosis, and the stimulation of cytokine and interferon production by colonocytes. 7 The disruption of gut microbiota and increased gut permeability, triggering the release of inflammatory cytokines via the gut–liver axis, are acknowledged as key factors in hepatic injury. 8 The gut–liver axis represents the interconnected relationship between the gut microbiome and hepatocytes, facilitated by the portal system and biliary tract. 9
So, preserving a healthy gut barrier is essential to prevent toxins from reaching the gut–liver axis. Any disruption in the gut microbiome can compromise the gut barrier and lead to hepatic inflammation. Therefore, restoring gut symbiosis, which can be achieved through fecal microbiota transplantation (FMT), is a crucial and promising, cost‐effective treatment option. 10 FMT is an increasingly popular method of modifying gut microbiota during disease. It involves transplanting a healthy donor's intestinal microbiota, obtained from fecal matter, into the patient's gastrointestinal tract. 11
The objective of our meta‐analysis is to evaluate the effectiveness of FMT compared to that of the standard of care (SOC) in enhancing SAH patient survival rates.
Methods
Based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA), the study was conducted. The study protocol has been registered with the PROSPERO International Registry for Systematic Reviews (CRD42023467250).
Search strategy
A thorough literature search using PubMed, Web of Science, and SCOPUS was conducted from inception to September 2023. The purpose of this study was to compare the effectiveness and results of FMT compared to that of the SOC in enhancing SAH patient survival rates. The Medical Subject Headings (MeSH) terms and keywords associated with “Fecal Microbiota Transplantation,” “Severe Alcoholic Hepatitis,” and “SAH” were used to create search strategies. Table S1, Supporting information includes a detailed search strategy of each database searched.
Study selection
The inclusion criteria were as follows: randomized controlled trials (RCTs) and observational studies; the participants were diagnosed with SAH; the interventions included FMT; the comparators received SOC; the outcomes included survival rates; and the studies were reported with full text in English. We excluded studies that haven't fulfilled the above criteria.
Two impartial reviewers conducted the preliminary screening based on the study's title and abstract before reading the full text to decide whether to include it or not in accordance with the inclusion and exclusion criteria. A third researcher would be consulted if there was any inconsistency in the study selection.
Data extraction
Using a standardized data extraction form, two researchers independently extracted the data in accordance with the Cochrane Handbook. The following information was extracted: study ID, country, study design, sample size, patient demographics (age, sex, follow‐up duration, and MELD score at baseline), and survival rates. A third researcher was consulted if there were any discrepancies in the data extraction.
Quality assessment
Two researchers independently assessed the included studies' risk of bias using Cochrane's “Risk of Bias” tool, described in the Cochrane Handbook for Systematic Reviews of Interventions. The studies included in this review were RCTs and observational studies. We used the Newcastle–Ottawa Scale for observational cohorts 12 and ROB 2 for appraisal of RCT studies. 13 Any disagreement was resolved through discussion.
Data synthesis and statistical analysis
Using Review Manager 5.4, we pooled data to calculate odds ratios (ORs) with 95% confidence intervals (CIs). Heterogeneity in each pairwise comparison was with the I 2 statistic. P value <0.05 indicated statistical significance. In the absence of heterogeneity among the included studies, a fixed effect model was employed. Otherwise, we used the random effect model.
Results
Literature search
As shown in Figure 1, a total of 449 records were retrieved from our literature search, including 8 records from PubMed, 435 records from Scopus, and 6 records from Web of Science. Then, 12 records were deleted. A total of 430 were rejected after screening on the basis of the evaluation of the title and abstract. After one study was excluded from the meta‐analysis due to having the incorrect population, six studies remained.
Figure 1.

Prisma flow chart for the inclusion and exclusion of the reviewed studies.
The included studies' characteristics
This meta‐analysis included six studies that were carried out from 2017 to 2023. As shown in Table 1, which summarizes the characteristics of the included studies, one was RCT, one was open‐label CT, one was a pilot study, and the other three were cohort studies. All the studies were conducted in India with the aim of assessing the efficacy of FMT versus other alternative multiple interventions that vary between the reviewed studies. All the studies had one group for FMT intervention against the SOC group. SOC included prednisolone, corticosteroids, pentoxifylline, and nutrition. All the studies had survival rate as their primary outcome, while the secondary outcome included resolution of hepatic encephalopathy (HE), resolution of ascites, and gut microbiota improvement. As shown in Table 1, the follow‐up duration of the enrolled studies varied, including 1, 3, and 6 months, while three studies had a follow‐up duration of 12 months. The total combined sample size was 371 patients, whereas the Pande et al. study had the largest sample size (60 included patients in each arm).
Table 1.
Baseline characteristics of the included studies.
| ID | Group | Country | Study design | Sample size | Age (years), mean (SD) | Sex (male), n | Follow‐up duration (months) | FMT method |
|---|---|---|---|---|---|---|---|---|
| Pande et al. 2023 14 | FMT | India | RCT | 60 | 43.2 (8.73) | 60 | 3 | A fresh fecal slurry from 30 g of stool was infused daily for 7 days via a nasoduodenal tube, with a 3‐h gap maintained between FMT and meals |
| Prednisolone | 60 | 40.8 (7.91) | 57 | 3 | ||||
| Philips et al. 2017 15 | FMT | India | Pilot | 8 | — | 8 | 12 | Thirty grams of donor stool, rigorously screened from consenting family members, were homogenized with 100 mL of sterile normal saline in a blender, filtered through sterile gauze, and infused in small aliquots via a nasoduodenal tube daily for 7 days |
| SOC | 18 | — | 18 | 12 | ||||
| Philips et al. 2018 16 | FMT | India | Cohort | 16 | 47.6 (8.2) | 16 | 3 | Stool samples weighing approximately 30 g were collected 6 h before the procedure. These were mixed with 100 mL of sterile normal saline and homogenized in a blender for 2–4 min. The resulting mixture was strained and filtered, and 100 mL was administered daily for 7 days through a nasoduodenal tube placed under fluoroscopy guidance the day before the FMT |
| Nutrition (SOC) | 17 | 49.6 (8.3) | 17 | 3 | ||||
| Corticosteroids | 8 | 48.7 (11.8) | 8 | 3 | ||||
| Pentoxifylline | 10 | 43.6 (9.9) | 10 | 3 | ||||
| Philips et al. 2022 17 | FMT | India | Cohort | 47 | 45.2 (10.2) | — | 6 | One hundred milliliters of manually filtered stool were delivered daily for 7 days through a nasoduodenal tube, which was placed under fluoroscopy guidance 1 day prior to the FMT. The recipient was required to fast for at least 4 h before each stool instillation |
| Pentoxifylline | 25 | 47.7 (9.9) | — | 6 | ||||
| Philips et al. 2023 18 | FMT | India | Cohort | 34 | — | — | 12 | NA |
| Corticosteroids | 35 | — | — | 12 | ||||
| Sharma et al. 2022 19 | FMT | India | OCT | 13 | 53.3 (4.1) | 13 | 3 | Thirty grams of fresh stool collected from pre‐identified donors 6 h before the procedure were homogenized with 100 mL of sterile normal saline in a blender for 2–4 min and filtered through gauze. The filtered product was then instilled in a single session through a nasojejunal tube placed under endoscopic guidance |
| Nutrition (SOC) | 20 | 51.8 (6.4) | 20 | 3 |
FMT, fecal microbiota transplantation; NA, not available; OCT, open‐label controlled trial; RCT, randomized controlled trial; SOC, standard of care.
Results of quality assessments
Regarding methodological quality, the included RCTs raised some concerns, as shown in Figure 2.
Figure 2.

Risk of bias assessment using Cochrane RoB 2 tool of the included reviewed studies.
All of the studies were of good quality, with two receiving scores of 7 and one receiving a 6, according to the New Castle Scale for evaluating study quality (Table 2).
Table 2.
Newcastle–Ottawa scale for quality assessment of cohort studies
| Study ID | Philips et al. 2018 16 | Philips et al. 2022 17 | Philips et al. 2023 18 |
|---|---|---|---|
| Representativeness of the exposed cohort | — | — | — |
| Selection of the nonexposed cohort | ★ | ★ | ★ |
| Ascertainment of exposure | ★ | ★ | ★ |
| Demonstration that the outcome of interest was not present at the start of the study | ★ | — | ★ |
| Comparability of cohorts on the basis of the design or analysis | ★ | ★ | ★ |
| Assessment of outcome | ★ | ★ | ★ |
| Was follow‐up long enough for outcomes to occur? | ★ | ★ | ★ |
| Adequacy of follow‐up of cohorts | ★ | ★ | ★ |
Study outcomes
Survival rates following 1 month of follow‐up
The pooled OR of the survival rates for the FMT‐treated patients included in five of the studies was 2.91 (1.56, 5.42) significantly (P = 0.0008) after 1 month of follow‐up, as shown in Figure 3a. The overall survival rates were reported as 88% and 68% in the FMT group and the SOC group, respectively. The heterogeneity among the studies was insignificant (I 2 = 0%, P = 0.57).
Figure 3.
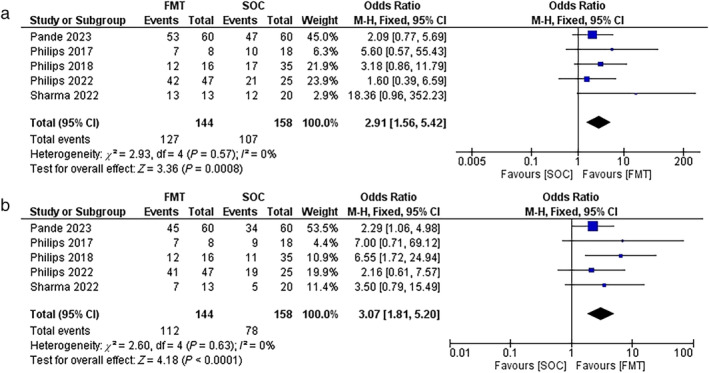
(a) Forest plot of the survival rate in the fecal microbiota transplantation (FMT) group versus the standard of care (SOC) group at 1 month. (b) Forest plot of the survival rate in the FMT group versus the SOC group at the 3 months.
Three‐month follow‐up after FMT intervention
As shown in Figure 3b, after 3 months of follow‐up, the pooled OR value for the survival rates was 3.07 (0.79, 15.49) with significance (P < 0.0001). The overall survival rates in the FMT group were 78% versus 49% in the SOC group. The heterogeneity factor between the studies was insignificant (P = 0.63, I 2 = 0%).
Six‐month follow‐up after FMT intervention
Only three studies had a follow‐up duration of 6 months. The pooled OR value was 2.96 (0.99, 8.85), with insignificance (P = 0.05). As shown in Figure 4a, the overall survival rates were 71% and 49% in the FMT and SOC groups, respectively. The heterogeneity factor between the reviewed studies was significant (P = 0.08) with a high I 2 value (59%).
Figure 4.
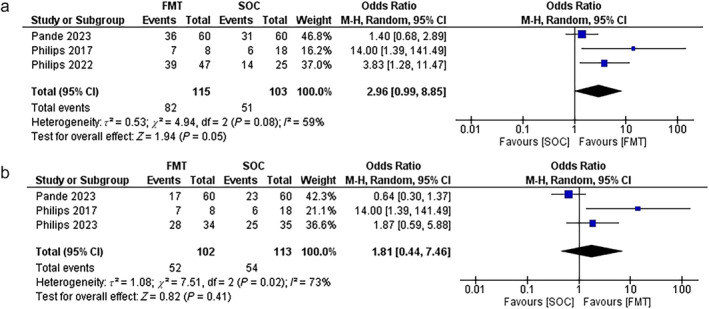
(a) Forest plot of survival rates in the fecal microbiota transplantation (FMT) group versus the standard of care (SOC) group at 6 months. (b) Forest plot of survival rates in FMT groups versus SOC groups at 1 year.
One‐year follow‐up after FMT intervention
As shown in Figure 4b, the pooled OR value is 1.81 (0.44, 7.46), with insignificance (P = 0.41). The overall survival rates were 51% and 48% in the FMT group and the SOC group, respectively. The heterogeneity factor was significant (P = 0.02) with I 2 value = 73%.
Resolution of hepatic encephalopathy and ascites as a secondary outcome
In 2017, Philips et al. reported that six and five patients out of eight had resolution of their ascites and hepatic encephalopathy, respectively. After 6 months of FMT therapy, Philips et al. in 2022 reported that only 10% of the patients in the FMT arm still had hepatic encephalopathy, compared to 40% in the pentoxifylline arm. According to Sharma et al., hepatic encephalopathy resolution went from 100% to 56.17% and from 100% to 40% (FMT vs SOC) for ascites. 19
Discussion
FMT, formerly known as fecal bacteriotherapy, has been used as a treatment for dysbiosis of the gut microbiota that is accompanied by various diseases for many years. 20 This meta‐analysis emphasized the value of FMT for SAH patients and its effectiveness in significantly lowering mortality rates. According to the findings, FMT's effectiveness in terms of survival rates was significant after 1 and 3 months of follow‐up post‐treatment. These findings suggest that the restoration of the gut microbiota in patients with SAH has a beneficial impact on disease enhancement. However, our analysis did not find any significant difference between FMT groups and SOC groups in terms of survival rates after 6 and 12 months post‐treatment. This finding suggests that the initial effects of FMT observed in short‐term follow will not be sustained for a longer period of time. Our findings were found to be aligned with the findings of the previous studies. One of the reviewed studies provides evidence in support of our findings, where the effectiveness of FMT in 60 SAH patients was compared to prednisolone treatment in 60 SAH patients. 14 Prednisolone demonstrated effectiveness in SAH patients in terms of 28‐day survival over the placebo 21 ; however, in Pande et al. study, FMT demonstrated superior survival rates among SAH patients after 3 months of follow‐up post‐treatment, with 75% of FMT group survivorship achieved compared to 56.6% in the prednisolone group. 14 In contrast, after 6 months post‐treatment, it demonstrated comparable efficacy between the two groups with negligible differences, and after 12 months post‐treatment, there was no significant difference in reported survival rates between the two groups. 14
In a different study, the effects of FMT intervention were compared in 8 SAH patients to SOC administered to 18 SAH patients. 15 Only six patients survived in the SOC group, compared to seven patients in the FMT group who survived up to 1 year. On the other hand, a study compared patients receiving FMT treatment to those receiving SOC, corticosteroids, and pentoxifylline treatment. 16 In comparison to the other groups, it was reported that FMT was more effective up to 3 months, with 12 patients out of 16 surviving versus 5/17, 3/8, and 3/10 in the SOC, corticosteroid, and pentoxifylline groups, respectively. 16
In one of the reviewed studies, pentoxifylline was the only medication compared to FMT. The results showed that FMT was significantly more effective than pentoxifylline after 6 months of follow‐up post‐treatment, when survival rates were 83% versus 56%, respectively. The advantage of this study over the previous two studies was the larger sample size (47 in the FMT group versus 25 in the pentoxifylline group). 17
Philips et al. evaluated the alterations in the gastrointestinal microbiota linked to alcohol abuse in both the FMT‐treated patients and corticosteroid‐treated patients over the course of a year of follow‐up in one study. According to the study, 25 out of 35 patients who received corticosteroids survived, compared to 28 out of 34 who received FMT. Furthermore, it was noted that the FMT group experienced significantly fewer alcohol relapses than the other group, with only 20% of the FMT group experiencing such relapses compared to 70% of the other group. 18 Further evidence for the significance of the effect of FMT against SOC was provided by Sharma et al. study, in which survival rates were 100% versus 60% and 53.84% versus 25% after 1 and 3 months of follow‐up post‐treatment, respectively. 19
The FMT effect may deteriorate over lengthy follow‐up periods for a variety of reasons. One of these factors may be the failure to maintain the therapy through the use of antibiotics or dietary changes. Additionally, it is possible that the variance in immune reactions and environmental factors among the patients has an impact on FMT maintenance.
One of the treatment options for SAH patients is early liver transplantation; however, it has some drawbacks, such as organ failures, transplant‐related contraindications, recurrent alcohol relapses, and donor scarcity. 22 In comparison to liver transplantation, FMT demonstrated ease of use, improvement in alcohol relapse, and effectiveness in terms of survival rates.
Infections were the primary cause of death in the Pande et al. study, accounting for 22.2% and 47.8% of all fatalities in the FMT and prednisolone arms, respectively. Another cause of death among FMT patients was cardiac arrest, along with kidney failure. 14 According to Sharma et al.'s study, organ failures, pneumonia, refractory septic shock, and massive upper gastrointestinal bleeding were the main causes of death. 19
According to Pande et al., the recipient's gut microbiota began to improve on day 28 following FMT, and by day 90, it was nearly identical to that of the donors. Furthermore, Philips et al. discovered in their study that the FMT‐treated patients had higher relative abundances of Bifidobacterium, Bacteroides, and Citrobacter than the pentoxifylline‐treated patients after 3 months of follow‐up. Following a 6‐month follow‐up, patients who received FMT had significantly higher levels of Bifidobacterium, whereas those who received pentoxifylline had significantly higher levels of Aerococcaceae.
As far as we are aware, this is the first meta‐analysis to compare the outcomes of survival rates from six studies to assess the efficacy of FMT in SAH patients in comparison to SOC. Along with data collection and stratification according to the follow‐up period, another strength of this meta‐analysis is the exact inclusion and exclusion criteria used during the literature search. The majority of the studies we reviewed, however, had small sample sizes; for example, one study only enrolled 13 patients for an FMT intervention, another only enrolled 16 patients, and a third only enrolled 34 patients. The studies with the largest sample sizes, however, were one that enrolled 60 FMT‐treated patients and another that enrolled 47 FMT‐treated patients. Moreover, our analysis focused mainly on survival outcomes and did not consider any other relevant clinical outcomes, such as disease remission rates. As a result, the results of our meta‐analysis were uncertain. Additionally, all the studies under review were carried out in India.
Additionally, all the studies included in our review were conducted in India, a nation with a single population which may limit the generalizability of the findings to other populations. Moreover, four out of the six studies were led by the same primary investigator, which could potentially introduce bias and affect the diversity of the study designs and methodologies employed. Due to the limited number of studies included in this analysis, we were unable to perform subgroup analyses or meta‐regression. This limitation restricts our ability to adjust for potential confounding factors and to explore more detailed stratifications among the study variables. To accurately evaluate the effects of various SOC therapies, future research with a bigger sample size and more consistent SOC criteria would be beneficial. Additionally, this will make it possible to conduct more thorough subgroup analyses, which can shed light on the ways in which particular SOC types affect the efficacy of FMT.
Conclusion
This meta‐analysis emphasized that FMT has promising short‐term benefits for survival rates at 1‐ and 3‐month post‐treatment. However, no significant difference was observed between the FMT‐treated and SOC‐treated groups at 6 and 12 months. Further future research is needed to identify strategies for optimizing long‐term treatment efficacy.
Supporting information
Table S1. Search strategies.
Declaration of conflict of interest: The authors have no conflict of interest to declare.
Author contribution: Amira Mohamed Taha was responsible for conceptualization, methodology, project administration, formal analysis, and investigation. Khaled Abouelmagd and Abdelrahman Mohamed Mahmoud handled data creation. Dang Nguyen, Sarah A. Nada, Sadish Sharma and Mandy Elewa prepared the original draft. Amira Mohamed Taha and Khaled Abouelmagd reviewed and edited the writing. All authors participated in data interpretation and agreed on the submitted manuscript.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N. Engl. J. Med. 2009; 360: 2758–2769. [DOI] [PubMed] [Google Scholar]
- 2. Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Mezey E, White RI. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978; 75: 193–199. [PubMed] [Google Scholar]
- 3. Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG clinical guideline: alcoholic liver disease. Am. J. Gastroenterol. 2018; 113: 175–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dunn W, Shah VH. Pathogenesis of alcoholic liver disease. Clin. Liver Dis. 2016; 20: 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frazier TH, Stocker AM, Kershner NA, Marsano LS, McClain CJ. Treatment of alcoholic liver disease. Therap. Adv. Gastroenterol. 2011; 4: 63–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitchell MC, Kerr T, Herlong HF. Current management and future treatment of alcoholic hepatitis. Gastroenterol. Hepatol. 2020; 16: 178. [PMC free article] [PubMed] [Google Scholar]
- 7. Martinez‐Guryn K, Leone V, Chang EB. Regional diversity of the gastrointestinal microbiome. Cell Host Microbe. 2019; 26: 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Albillos A, de Gottardi A, Rescigno M. The gut‐liver axis in liver disease: pathophysiological basis for therapy. J. Hepatol. 2020; 72: 558–577. [DOI] [PubMed] [Google Scholar]
- 9. Jung JH, Kim SE, Suk KT, Kim DJ. Gut microbiota‐modulating agents in alcoholic liver disease: links between host metabolism and gut microbiota. Front Med (Lausanne). 2022; 9: 913842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anirvan P, Panigrahi MK, Singh SP. Fecal microbiota transplantation in alcoholic hepatitis: new treatment paradigm or a shot in the dark? Hepatol Int. 2023; 17: 1318–1319. [DOI] [PubMed] [Google Scholar]
- 11. Antushevich H. Fecal microbiota transplantation in disease therapy. Clin. Chim. Acta. 2020; 503: 90–98. [DOI] [PubMed] [Google Scholar]
- 12. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur. J. Epidemiol. 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 13. Sterne JAC, Savović J, Page MJ et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 14. Pande A, Sharma S, Khillan V et al. Fecal microbiota transplantation compared with prednisolone in severe alcoholic hepatitis patients: a randomized trial. Hepatol. Int. 2023; 17: 249–261. [DOI] [PubMed] [Google Scholar]
- 15. Philips CA, Pande A, Shasthry SM et al. Healthy donor fecal microbiota transplantation in steroid‐ineligible severe alcoholic hepatitis: a pilot study. Clin. Gastroenterol. Hepatol. 2017; 15: 600–602. [DOI] [PubMed] [Google Scholar]
- 16. Philips CA, Phadke N, Ganesan K, Ranade S, Augustine P. Corticosteroids, nutrition, pentoxifylline, or fecal microbiota transplantation for severe alcoholic hepatitis. Indian J. Gastroenterol. 2018; 37: 215–225. [DOI] [PubMed] [Google Scholar]
- 17. Philips CA, Ahamed R, Rajesh S et al. Clinical outcomes and gut microbiota analysis of severe alcohol‐associated hepatitis patients undergoing healthy donor fecal transplant or pentoxifylline therapy: single‐center experience from Kerala. Gastroenterol. Rep. 2022; 10: goac074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Philips CA, Ahamed R, Abduljaleel JK, Rajesh S, Tharakan A, Augustine P. Significant gut microbiota related to patterns of drinking and alcohol relapse in patients with alcoholic hepatitis undergoing stool transplant or corticosteroid therapy. Indian J. Gastroenterol. 2023; 42: 1–7. [DOI] [PubMed] [Google Scholar]
- 19. Sharma A, Roy A, Premkumar M et al. Fecal microbiota transplantation in alcohol‐associated acute‐on‐chronic liver failure: an open‐label clinical trial. Hepatol. Int. 2022; 16: 433–446. [DOI] [PubMed] [Google Scholar]
- 20. Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat. Rev. Gastroenterol. Hepatol. 2012; 9: 88–96. [DOI] [PubMed] [Google Scholar]
- 21. Thursz MR, Richardson P, Allison M et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N. Engl. J. Med. 2015; 372: 1619–1628. [DOI] [PubMed] [Google Scholar]
- 22. Deng L, Sundaram V. Evidence for and against liver transplantation for acute‐on‐chronic liver failure. Curr. Treat. Options Gastroenterol. 2022; 20: 194–204. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search strategies.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


