Abstract
Introduction
Ewing sarcoma (ES), is a rare cancer affecting children, adolescents and adults. After VIDE (vincristine-ifosfamide-doxorobucin-etoposide) induction chemotherapy, Busulfan-Melphalan (BuMel) high-dose chemotherapy followed by autologous hematopoietic stem cells transplantation improved outcomes in unfavourable localized ES, but with more toxicities than conventional chemotherapy (VAI: Vincristine-dactinomycin-Ifosfamide). We evaluated whether the risk of acute toxicity associated with BuMel compared to VAI varied according to age in patients recruited in the R2Loc and R2Pulm randomised trials of the Euro-E.W.I.N.G.99 and Ewing-2008 trials.
Methods
We included patients with a localized high-risk disease, or pulmonary or pleural metastasis. We analysed the risk of severe toxicity according to randomised treatment group (VAI versus BuMel) and age group (<12 years, 12–17 years, 18–24 years, ≥25 years). We evaluated the heterogeneity of treatment effects by age group using interaction terms in logistic multivariable models.
Results
The analysis included 243 patients treated with VAI and 205 with BuMel. Overall, BuMel was associated with a higher risk of severe acute toxicity than VAI particularly haematological, gastrointestinal, liver, sinusoidal occlusive syndrome, and infections. Severe haematological toxicity and lower general condition were significantly more frequent in younger patients, whatever treatment. We did not observe any significant heterogeneity in terms of the excess risk of severe toxicities associated with BuMel compared to VAI according to age group.
Conclusion
The excess of acute toxicity associated with BuMel compared to VAI does not vary significantly with age, suggesting the feasibility of BuMel across all age groups.
Keywords: Ewing sarcoma (ES), High dose chemotherapy, Safety, Autologous hematopoietic stem cells transplantation
Highlights
-
•
We compared toxicity after BuMel high-dose regimen vs VAI conventional chemotherapy
-
•
Severe acute toxicity (haemato, GI, liver, infection) is more frequent after BuMel.
-
•
Haemato-toxicity and lower general condition are more frequent in younger patients.
-
•
Excess risk of severe toxicity of BuMel / VAI appears homogeneous across age groups
-
•
This suggests the feasibility of BuMel whatever the age of patients up to 50 years.
1. Introduction
Ewing sarcoma (ES) is a rare cancer characterized by small round cells and pathognomonic fusion transcripts that arise from chromosomal translocations between FET and ETS family protein-encoding genes, [1] occurring in bone or soft tissue, with varied age-related site distribution. [2], [3], [4] The mean age of onset is 15 years. The latest Eurocare-5 program showed that adolescents with ES, similarly to some other cancers prevalent across the age spectrum, still had an overall survival (OS) worse than children, without any improvement in recent decades. The reasons for that are complex and poorly understood, and may include biological differences, psychosocial specificities, multiple care structures with different practices (paediatric and adult) in intensification and supportive care [5], [6].
The standard treatment of ES combines chemotherapy and surgery (+/- radiotherapy) of the primary tumour. The international Euro-E.W.I.N.G.99 (NCT00020566) and Ewing 2008 (NCT00987636) trials both compared, in terms of efficacy and safety, high-dose chemotherapy (HDC) with Busulfan-Melphalan and autologous hematopoietic stem-cell transplantation (HSCT) versus standard chemotherapy for patients up to 50 years, with an unfavourable localized ES (R2loc trial) or patients with pulmonary or pleural metastases (R2Pulm trial). [3], [4] Based on the R2loc trial, authors concluded that event-free survival (EFS) and overall survival (OS) were significantly improved with high-dose chemotherapy versus standard chemotherapy in patients with an unfavourable localized sarcoma after VIDE (vincristine-ifosfamide-doxorobucin-etoposide) induction chemotherapy. [7] Nevertheless, HDC did not improve EFS nor OS in patients with pulmonary/pleural metastases (R2Pulm trial). [8] While the authors observed higher acute toxicity in the HDC-arm compared to the standard arm, HDC has become a standard, in several countries, for Ewing sarcoma patients with unfavourable localized disease after induction chemotherapy [7], [8], [9].
In the setting of conventional chemotherapy, AYAs were reported to have toxicities different than younger children. [10] Juergens et al. already reported increased toxicity for children (<12 years) than adolescents (12–18 years) and adults (19–50 years) during the VIDE-induction. [11] Toxicities could also be related to pubertal status especially for female as suggested by van den Berg et al. [12].
In adult oncology, HSCT with HDC is less common in solid tumors due to limited indications. Excess toxicity often occurs in adults treated with HDC, with weaker evidence compared to children, highlighting the need for better evaluation of age-related impacts on toxicity [13].
In this study, based on R2Loc and R2pulm randomized trials, we evaluated the risk of severe toxicity in patients treated with HDC compared to conventional chemotherapy, according to four age groups: < 12 years, 12–17 years, 18–24 years, and ≥ 25 years.
2. Methods
2.1. Study design and participants
Euro-E.W.I.N.G-99 and Ewing2008 were international, randomized, superiority trials comparing different consolidation chemotherapy regimens in several arms. Eligible patients were younger than 50 years and enrolled at diagnosis for a newly diagnosed biopsy-proven Ewing sarcoma [7], [8]. Two arms evaluated the efficacy and toxicity of HDC with Busulfan-Melphalan (BuMel) followed by HSCT in high-risk patients: R2loc recruited patients with a localized disease but a poor histological response after induction chemotherapy (viable residual cells ≥10 %) for those who underwent surgery after induction chemotherapy, or a large tumour (volume ≥200 mL at diagnosis) in other cases (tumour resected at diagnosis, or unresected tumour); R2pulm recruited patients with pulmonary or pleural metastases only. The R2loc trial compared BuMel followed by HSCT versus 7 courses of conventional VAI chemotherapy (vincristine-dactinomycin-ifosfamide). The R2Pulm trial compared BuMel+HSCT without lung irradiation versus VAI consolidation chemotherapy followed by whole lung irradiation. For the current analysis, we selected all patients who started treatment allocated by randomization, pooling data from R2loc and R2Pulm trials. Written informed consent was obtained from all patients and/or their parents/guardians before enrolment in the randomised trials. Study protocols were approved by an independent ethics committee and the appropriate institutional review boards. The trials were conducted in accordance with the ethical principles of the Declaration of Helsinki and with Good Clinical Practice guidelines.
2.2. Treatment
Induction chemotherapy consisted of six VIDE-courses (Fig. 1). Local therapy was tailored to patient and tumour characteristics and included surgery, radiotherapy, or combination of both. After one VAI-course waiting for the assessment of histological response, allocated consolidation treatment was either seven VAI-courses or BuMel chemotherapy.
Fig. 1.
Study design and treatment regimens of the R2Loc and R2Pulm randomization trials of the Euro-E.W.I.N.G. 99 VIDE: Vincristine, Ifosfamide, Doxorubicin and Etoposide. VAI: Vincristine, Dactinomycin and Ifosfamide. BuMel: Busulfan and Melphalan, with intravenous Melphalan (140 mg/m² on day −2 before stem cell re-infusion) and Busulfan four daily doses over 4 days (days −6 to −3). (1) Busulfan was given per os before 2006 (cumulative dose of 600 mg/m² or 16 mg/kg if ≥60 kg) and intravenously afterwards with 0.8 mg/kg for patients with a body weight (BW) > 34 kg, 0.95 mg/kg if BW > 23 to 34 kg, 1.1 mg/kg if BW 16–23 kg, 1.2 mg/kg if BW 9 to < 16 kg, and 1 mg/kg if BW< 9 kg. Consolidation treatment was one VAI-course waiting for the assessment of histological response, allocated consolidation treatment was either seven VAI-courses (with 21-day intervals) or BuMel chemotherapy. Randomisation was balanced and stratified according to cooperative group, sex, age (younger than 25 years), and local treatment (resection after chemotherapy alone with or without postoperative radiotherapy versus initial surgery versus resection after chemotherapy and radiotherapy versus radiotherapy only). Centralized randomization software was used in all data centres, ensuring the concealment of the next patient allocation. The GPOH data centre used permuted blocks of four. In the other data centres, randomization was also balanced by the treating centre using dynamic allocation of treatment (minimization with a random factor set at 0.8).
2.3. Endpoints
Primary endpoint for the current analyses was the occurrence of a severe toxicity following consolidation regimen and radiotherapy if any. Acute toxicity was evaluated after each consolidation course for VAI group and during hospitalization for BuMel group, using a predefined list of items, including neutropenia, thrombocytopenia, infection, mucositis, diarrhoea, general condition, renal, bladder, liver (including liver sinusoidal occlusive syndrome, and other liver toxicities), cardiac, skin, or neurologic toxicities. Adverse events were graded according to the NCI-CTCAE version-2.0 and Bearman’s criteria for sinusoidal obstruction syndrome. Grade 4 hematologic toxicities and grade 3 or higher for all non-haematological toxicities were considered severe. For each toxicity category, we considered the worst reported grade during the consolidation treatment phase.
Toxicity of any grade and dose-intensity of alkylating agents were second endpoints [13].
2.4. Statistical analysis
We assessed safety by treatment arm (VAI versus BuMel) and by age group (considering four age groups when possible: <12 years versus 12–17 versus 18–24 years versus ≥25 years old; and pooling all patients ≥18 years if needed).
The risk of severe acute toxicity was evaluated separately for the main categories of toxicity. For each toxicity type, the risk of severe toxicity was modelled using a multivariable logistic regression to estimate the odds ratio and its 95 % confidence interval (95 %CI) associated with the covariates (treatment group and age group). An interaction term was included in the model to evaluate whether the treatment effect varied according to age group. Results were illustrated by the mean of forest plots. The primary analysis was performed considering acute toxicity reported over the whole maintenance treatment duration. We performed a sensitivity analysis focusing on the first two courses to limit the possible bias related to dose-reductions in the subsequent courses. We also performed a secondary analysis considering different cut-offs for age (<15 years versus 15–24 years versus ≥25 years old).
All tests were two-sided. Analyses were performed using SAS 9.4 software (SAS Institute, Inc., Cary, NC).
3. Results
A total of 527 patients fulfilling the predefined high-risk criteria were enrolled in R2Loc or R2Pulm randomized trials of the Euro-E.W.I.N.G-99 or Ewing 2008 studies between February 2000 and December 2015: 261 were allocated to VAI (R2Loc: 118; R2Pulm: 143) and 266 to BuMel (R2Loc: 122; R2Pulm: 144) (Fig. 2). We excluded 59 patients who did not receive treatment allocated by randomization including five patients with failure of peripheral blood stem cell harvest, and 20 due to missing data on safety evaluation. Consequently, the study population included 243 patients treated with VAI, and 205 with BuMel.
Fig. 2.
Participant flowchart of the study population VAI: Vincristine, Dactinomycin and Ifosfamide. BuMel: Busulfan and Melphalan. PBC: Peripheral Blood collection. WLI: Whole lung irradiation.
3.1. Baseline characteristics (Table-1)
Overall, the study population included 129 patients < 12 years (28.8 %), 181 patients 12–17 years (40.2 %), 73 patients 18–24 years (16.3 %) and 65 patients ≥ 25 years (14.6 %).
The distribution of age differed significantly between R2Loc and R2pulm arms (p = <0.001): 47 patients < 12 years (23 %), 36 patients 12–17 years (36 %), 38 patients 18–24 years (19 %) and 47 patients ≥ 25 years (23 %) in R2Loc, versus 82 (34 %), 108 (44 %), 35 (14 %) and 18 (7 %), respectively, in R2Pulm.
Overall, the disease was mainly osseous, axial, with a large primary tumour (≥200 mL) and metastatic.
As expected due to the randomised design of each trial, the baseline characteristics did not significantly differ between the two consolidation groups, in particular, the distribution of age (Wilcoxon test, p = 0.68), with a median age of 16 and 14 years, respectively. .
Table 1.
Baseline demographic and clinical characteristics in the overall population according to the randomisation group.
| VAI group (n = 243) | Bu-Mel group (n = 205) | p | |
|---|---|---|---|
| Randomization | 0.73 | ||
| R2Loc | 113 (47 %) | 92 (45 %) | |
| R2Pulm | 130 (53 %) | 113 (55 %) | |
| Gender (female) | 97 (40 %) | 80 (39 %) | 0.85 |
| Age at diagnosis, median (range) | 16 (0; 44) | 14 (2;48) | 0.38 |
| Age category | 0.64 | ||
| < 12 years old | 64 (26 %) | 65 (32 %) | |
| 12-17 years old | 100 (41 %) | 81 (40 %) | |
| 18-24 years old | 42 (17 %) | 31 (15 %) | |
| ≥ 25 years old | 37 (15 %) | 28 (14 %) | |
| Recruiting group | 0.99 | ||
| EORTC | 14 (6 %) | 11 (5 %) | |
| UKCCLG | 41 (17 %) | 37 (18 %) | |
| SFCE/GSF/Unicancer | 71 (29 %) | 64 (30 %) | |
| COG | 34 (14 %) | 26 (13 %) | |
| GPOH – EE99 | 63 (26 %) | 51 (25 %) | |
| GPOH – Ewing 2008 | 20 (8 %) | 16 (8 %) | |
| Involvement of primary tumour | 0.27 | ||
| Osseous lesion with or without soft tissue component | 207 (85 %) | 181 (88 %) | |
| Extra-osseous lesion | 36 (15 %) | 23 (11 %) | |
| Unknown origin(1) | 0 | 1 (0 %) | |
| Primary tumour site | 0.69 | ||
| Limb | 105 (43 %) | 92 (45 %) | |
| Axis | 138 (57 %) | 112 (55 %) | |
| Unknown origin(1) | 0 | 1 (0 %) | |
| Tumour volume (MD=5) | 0.93 | ||
| < 200 mL | 105 (44 %) | 88 (43 %) | |
| ≥ 200 mL | 135 (56 %) | 115 (56 %) | |
| Metastatic sites at diagnosis | 0.78(2) | ||
| No metastases | 111 (46 %) | 91 (44 %) | |
| Lung metastases only | 128 (53 %) | 113(3) (55 %) | |
| Metastases other than lung(4) | 2 (1 %) | 1 (0 %) | |
| Metastases. Unknown site | 2 (1 %) | 0 | |
| Local treatment of the primary tumour (MD=8) | 0.18(5) | ||
| No local treatment of primary site | 2 (1 %) | 7 (3 %) | |
| RT alone | 29 (12 %) | 26 (13 %) | |
| Surgery of primary site | 92 (39 %) | 89 (44 %) | |
| Surgery followed by RT | 115 (48 %) | 80 (39 %) | |
| Histological response (MD = 8(6)) | 0.73 | ||
| Good Response | 75 (32 %) | 69 (34 %) | |
| Poor Response | 120 (51 %) | 96 (47 %) | |
| Not evaluable (no surgery, early surgery, prior RT) | 41 (17 %) | 39 (19 %) |
NOTE. Data are No (%) unless otherwise specified.
BuMel, busulfan and melphalan; COG, Children’s Oncology Group; EORTC, European Organization for Research and Treatment of Cancer; GPOH, Gesellschaft fuer Paediatrische Onkologie und Haematologie; GSF, French Sarcoma Group; MD, missing data; RT, radiotherapy; SFCE, French society of Paediatric Oncology; UKCCLG, Children’s Cancer and Leukaemia group; VAI, vincristine, dactinomycin and ifosfamide.
(1) One patient with lung metastases, for whom origin of primary tumour is unknown
(2) P-value of the test comparing No metastases versus Yes
(3) Two patients from R2Pulm had lung metastases and others metastases whom localisation was unknown
(4) One patient from R2Loc and two patients from R2Pulm had metastases other than lung at diagnosis. They were enrolled in the randomized trials, although they were ineligible.
(5) After pooling the first categories “No local treatment of primary site” and “RT alone” because of small number of patients
(6) Information about histological response of primary tumour was missing for 8 patients from R2Pulm.
3.2. Feasibility of VAI consolidation according to age and BuMel intensification
In the VAI-group (Table 2), 14 % patients received less than 8 maintenance courses, and this proportion significantly increased with age: 8 %, 8 %, 26 % and 28 % in the four age groups, respectively (p = 0.0005). Main reason for early treatment discontinuation was disease progression (n = 18). Toxicity was the reported reason for only one patient. The median treatment duration between courses 1 and 8 was 24 or 25 weeks in all age groups. The median cumulative dose of equivalent-Ifosfamide did not statistically differ between age groups (p = 0.17), nor the proportion of patients with a reduced relative dose intensity (RDI<80 %) (p = 0.64). Among the 34 patients with a documented reason for dose reduction, toxicity was responsible for dose reduction of alkylating agents with a relative dose intensity < 80 % for 4/4 children, 8/16 patients of 12–17 years, 4/7 patients of 15–24 years, and 7/7 patients ≥ 25 years.
Table 2.
Description of the treatment in the VAI group (N = 243).
| < 12 years old N = 64 | 12-17 years old N = 100 | 18-24 years old N = 42 | ≥ 25 years old N = 37 | p(1) | |
|---|---|---|---|---|---|
| Total number of maintenance courses administered (MD=4) | 0.0005(2) | ||||
| 7 and fewer | 5 (8 %) | 8 (8 %) | 11 (26 %) | 10 (28 %) | |
| 8 | 54 (87 %) | 84 (84 %) | 29 (69 %) | 25 (69 %) | |
| 9 and more | 3 (5 %) | 7 (7 %) | 2 (6 %) | 1 (3 %) | |
| Courses of VAI administered (MD=3) | 0.05 | ||||
| 7 and fewer | 17 (27 %) | 18 (18 %) | 15 (36 %) | 12 (33 %) | |
| 8 and more | 46 (72 %) | 81(81 %) | 27 (64 %) | 24 (67 %) | |
| Courses of VAC administered (MD=3) | 0.37 | ||||
| No | 53 (84 %) | 90 (91 %) | 38 (90 %) | 34 (94 %) | |
| Yes (one or more) | 10 (16 %) | 9 (9 %) | 4 (9 %) | 2 (6 %) | |
| Courses other than VAI/VAC (MD=5)(3) | 0.36 | ||||
| No | 58 (94 %) | 91 (92 %) | 39 (95 %) | 36 (100 %) | |
| Yes (one or more) | 4 (6 %) | 8 (8 %) | 2 (5 %) | 0 | |
| Time interval between 1stVAI1 and 8thVAI (weeks) (MD=2) | 0.009 | ||||
| Median (range) | 25 (6; 44) | 26 (10; 38) | 24 (6; 45) | 24 (6; 37) | |
| Cumulative dose of equivalent-Ifosfamide VAI1-VAI8 (g/m²)(4) (MD=2) | 0.17 | ||||
| Median | 46.6 | 47.1 | 45.8 | 45.2 | |
| Range | (6.0; 52.5) | (12.1; 49.6) | (11.6; 48.2) | (9.3; 49.1) | |
| Relative Dose intensity of alkylating agents in maintenance courses (%)(5) (MD=3) | 0.39 | ||||
| Median (range) | 89 (7; 109) | 90 (56; 107) | 93 (41; 105) | 92 (27; 104) | |
| RDI<80 % (MD=3) | 0.64 | ||||
| No | 52 (83 %) | 75 (77 %) | 31 (74 %) | 30 (81 %) | |
| Yes | 11 (17 %) | 23 (23 %) | 11 (26 %) | 7 (19 %) | |
| Reported reason for treatment modification (MD = 18) | |||||
| Toxicity | 4 | 8 | 4 | 7 | |
| Progression | 0 | 3 | 0 | 0 | |
| Patient’s choice | 0 | 1 | 1 | 0 | |
| Physician’s choice | 0 | 1 | 1 | 0 | |
| Other | 0 | 3 | 1 | 0 | |
| Reason for early stop of treatment | |||||
| Toxicity | 0 | 0 | 1 | 0 | |
| Progression | 5 | 5 | 4 | 5 | |
| Patient’s choice | 0 | 0 | 1 | 2 | |
| Physician's choice | 0 | 1 | 5 | 2 | |
| Unknown reason | 0 | 2 | 1 | 1 |
MD, missing data; RDI: Relative Dose Intensity; VAI: Vincristine-Dactinomycin-Ifosfamide; VAC: Vincristine-Dactinomycin-Cyclophosphamide.
(1) P-value of the test comparing < 12 years versus 12-17 years old versus ≥ 18 years (pooling the two groups of patients ≥ 18 years because of the limited number of patients)
(2) P-value of the test comparing ≤ 7 versus ≥ 8 maintenance courses
(3) The courses other VAI/VAC consisted in dactinomycin alone in all but three courses, 1 course of vincristine + dactinomycin, 1 course of vincristine + ifosfamide, and 1 course of dactinomycin + ifosfamide
(4) The cumulative dose of equivalent-Ifosfamide was computed from the 1st to the 8th course (or less if treatment permanently discontinued before the 8th course), considering dose of ifosfamide or cyclophosphamide, assuming that 1.5 g/m² of cyclophosphamide was equivalent to 6 g/m² of ifosfamide, as assumed when comparing ifosfamide to cyclophosphamide in standard risk patients (Euro-E.W.I.N.G-99 R1 trial 21). Consequently, cumulative dose = cumulative dose of ifosfamide (g/m²) + 4 x cumulative dose of cyclophosphamide (g/m²)
(5) The dose intensity (DI) of alkylating agent was computed as the cumulative dose of alkylating agents over the first eight maintenance courses (or less if treatment permanently discontinued before the 8th course) divided by the corresponding treatment duration (including 20 days after the last course). The relative dose-intensity of alkylating agents (RDI) was finally computed as the ratio between the observed DI and the theoretical dose intensity (6 g/m² every 3 weeks, equivalent to 2 g/m²/week).
BuMel treatment is detailed by age category in Table 3. The median time between the last VAI-course and HDC did not significantly differ between age groups (p = 0.14), nor the percentage of patients with a delayed start of HDC after the last VAI (time interval >35 days) (p = 0.39). Overall, 26 patients (13 %) had a significant dose reduction (>20 %) of Busulfan, with no clear trend with age: 5 %, 19 %, 10 % and 20 % in the four age groups, respectively (difference between groups, p = 0.04), whereas no patient had a significant dose reduction of Melphalan.
Table 3.
Description of the treatment in the BuMel group according to the age category (N = 205).
| < 12 years old (n = 65) | 12-17 years old (n = 81) | 18-24 years old (n = 31) | > =25 years old (n = 28) | p(1) | |
|---|---|---|---|---|---|
| ECOG Performance status (MD = 53) | 0.26(2) | ||||
| 0 | 36 (67 %) | 33 (54 %) | 13 (65 %) | 6 (35 %) | |
| 1 | 9 (17 %) | 17 (28 %) | 5 (25 %) | 8 (47 %) | |
| 2 | 8 (15 %) | 5 (8 %) | 0 | 0 | |
| 3 | 1 (2 %) | 6 (10 %) | 2 (10 %) | 3 (18 %) | |
| Tumour status before HDC (MD=5) | 0.55(3) | ||||
| CR | 39 (62 %) | 46 (58 %) | 13 (42 %) | 12 (46 %) | |
| PR | 18 (29 %) | 24 (30 %) | 10 (32 %) | 8 (31 %) | |
| SD | 3 (5 %) | 7 (9 %) | 7 (23 %) | 3 (12 %) | |
| Not evaluable | 1 (2 %) | 1 (1 %) | 0 | 2 (8 %) | |
| Not evaluated | 2 (3 %) | 2 (3 %) | 1 (3 %) | 1 (4 %) | |
| Time interval between last VAI course and HD (MD = 13) | 0.14 | ||||
| Median (range) | 28 (20; 67) | 29 (19; 64) | 31 (21; 58) | 30 (21; 85) | |
| Time interval between last VAI course and HDC>35 days (MD=13) | 0.39 | ||||
| No | 49 (80 %) | 58 (73 %) | 20 (71 %) | 16 (67 %) | |
| Yes | 12 (20 %) | 21 (27 %) | 8 (29 %) | 8 (33 %) | |
| Reported reason for delayed HDC (MD=20) | |||||
| Surgical complications | 1 | 1 | 0 | 0 | |
| Problems in stem cell collection | 3 | 3 | 0 | 1 | |
| Decreased general status | 0 | 1 | 0 | 0 | |
| Other | 3 | 11 | 3 | 2 | |
| Number of CD34 + stem cells infused (106/kg) (MD = 46) | 0.18 | ||||
| Median (range) | 4.4 (1.8; 10) | 4.7 (2.2; 10) | 5.6 (3; 9.8) | 5 (2.3; 9.6) | |
| Number of CD34 + stem cells infused (in categories) | 0.09(4) | ||||
| < 2 106/kg | 1 (1 %) | 0 | 0 | 0 | |
| [2-3[106/kg | 8 (17 %) | 14 (22 %) | 0 | 3 (16 %) | |
| ≥3 106/kg | 39 (81 %) | 51 (78 %) | 27 (100 %) | 16 (84 %) | |
| Duration of grade 4 leukopenia from stem cell rescue (days) (MD=3) | 0.15 | ||||
| Median (range) | 11 (0; 55) | 11.5 (0; 60) | 11 (2; 26) | 11 (0; 97) | |
| Duration of grade 4 neutropenia from stem cell rescue (days) (MD=18) | 0.02 | ||||
| Median (range) | 12 (0; 55) | 12 (0; 378) | 11 (0; 42) | 10 (6; 18) | |
| Dose modification for Busulfan > 20 % (MD = 5) | 0.04 | ||||
| No | 60 (95 %) | 64 (81 %) | 28 (90 %) | 20 (80 %) | |
| Yes | 3 (5 %) | 15 (19 %) | 3 (10 %) | 5 (20 %) | |
| Preventive treatment of SOS (MD=19) | 0.02(5) | ||||
| None | 21 (34 %) | 35 (50 %) | 20 (65 %) | 13 (54 %) | |
| Heparin | 10 (16 %) | 11 (16 %) | 2 (6 %) | 6 (25 %) | |
| Ursodiol | 23 (38 %) | 22 (31 %) | 6 (19 %) | 4 (17 %) | |
| Both | 7 (11 %) | 2 (3 %) | 3 (10 %) | 1 (4 %) |
MD, missing data; CR, Complete response; PR, Partial response; SD, Stable disease; SOS, Sinusoidal occlusive syndrome
(1) P-value of the test comparing < 12 years versus 12-17 years old versus ≥ 18 years (pooling the two groups of patients ≥ 18 years because of the limited number of patients)
(2) P-value of the test comparing ECOG 0 versus ECOG 1,2 or 3
(3) P-value of the test comparing CR versus PR or SD. Patients with tumour status non evaluable or not evaluated are excluded for the comparison
(4) P-value of the test comparing < 3 106/kg versus ≥ 3 106/kg
(5) P-value of the test comparing Preventive treatment of SOS No versus Yes (any type)
3.3. Acute toxicity analysis
When considering the risk of severe toxicity over the whole treatment duration, as already published, more severe toxicities were observed in the BuMel-group than during VAI-consolidation, with more haematological (p < 0.0001) toxicities, lower general condition (p = 0.0002), and more gastro-intestinal (p = <0.0001), liver (p = 0.0003), sinusoidal occlusive syndrome (p = 0.03) and infections (p < 0.0001) toxicities. VAI was associated with significantly more severe renal toxicities (p = 0.02) (Supplementary Table-S1). We observed no significant difference for skin, lung, neurological, or cardiac toxicities.
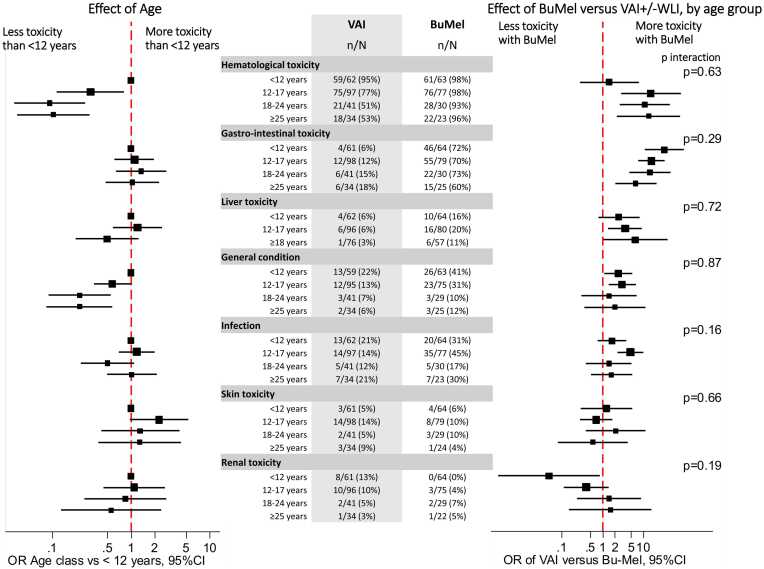
As illustrated by Fig. 3 and detailed in Supplementary Table-S2, we did not observe any significant heterogeneity in terms of the risk of severe toxicities of BuMel compared to VAI, across the age groups. Overall, haematological toxicities were more frequent in younger patients (<12 years: 96 %) than older ones (12–17 years: 87 %, 18–24 years: 69 %, and ≥25 years: 70 %) (p < 0.0001). These differences appeared more clear-cut in the VAI-group (95 %, 77 %, 51 %, and 53 %, respectively), whereas differences between age groups were not obvious in the BuMel-group. However, the interaction test was not significant (p = 0.63). A low general condition was more frequently observed in younger patients (p = 0.0007), but without significant interaction between treatment and age group (p = 0.87). We observed slightly more gastro-intestinal toxicity associated with BuMel in younger patients, but without significant interaction between treatment and age group (p = 0.29).
Fig. 3.
Risk of severe toxicity considering the whole treatment duration: Effect of age and heterogeneity of the treatment effect according to age group. Left and right panels represent age effect and treatment effect according to age, respectively. Odds ratios were estimated in the multivariable models including treatment, age, treatment-by-age interaction. Each effect was estimated for a toxicity category, separately.
Results were similar when considering all grade toxicities (Supplementary Figure-S1), as well as in the sensitivity analysis focusing on the first two courses in the VAI-group (Supplementary Figure-S2).
3.4. Treatment-related deaths
Overall, six patients died from treatment-related toxicity in the BuMel-group (details in Supplementary Table-S3): 2/59 patients ≥ 18 years (3.3 %) vs 4/146 < 18 years (2.7 %) (p = 1.0). Four patients died from Acute Respiratory Distress Syndrome, one from pulmonary veno-occlusive syndrome, and one from myelopathy related to radiation therapy; all deaths but one occurred before the protocol amendment stating that patients who were expected to receive radiotherapy of ≥ 30 Gy to the spinal cord or ≥ 45 Gy to large intestinal volume were no longer eligible. No treatment-related death occurred in the VAI-group.
4. Discussion
This study assessed the impact of age on the safety of BuMel versus VAI consolidation based on the data of Euro-E.W.I.N.G-99 and Ewing2008 arms concerning patients with an unfavourable localized ES or patients with pulmonary or pleural metastases at diagnosis. [7], [8] The excess of acute toxicity associated with BuMel compared to VAI did not vary significantly with age, suggesting the feasibility of BuMel across all age groups.
BuMel was associated with a higher risk of toxic death, unrelated to age. [14] A Treosulfan-based HDC has also been explored in the Ewing2008-R3 trial without any HDC-related death [15].
Similarly to Juergens et al. evaluating toxicity during VIDE chemotherapy, we observed more severe haematological toxicity in younger patients, especially in the VAI-group [11].
However, it is noteworthy that we observed a reduction in the number of VAI maintenance courses in patients over 18 years (p = 0.0005), possibly influencing a difference in toxicity.
To facilitate the comparison with previous published data on adolescents [2], [16], [17] whose definition is ambiguous [5], [18], we also performed analyses considering 3 groups of age: < 15, 15–24, and ≥ 25 years. We found essentially the same results with no significant heterogeneity in toxicity among age groups < 15, 15–24, and ≥ 25 years (Supplementary Table-S4, S5 and Figure-S3).
The feasibility and relative safety of BuMel in young adults as well as in younger patients are very important to claim because BuMel intensification has proven to improve the OS for patients with a localized high-risk Ewing sarcoma with 8-year OS of 64.5 % (54.4–73.5) in BuMel arm versus 55.6 % (45.8–65.1) for VAI arm, with an hazard ratio of 0.63 (0.41–0.95, p = 0.028). [7].
Overall, HDC+HSCT is not a common indication in adult solid tumour oncology regarding the incidence of cancers. [19], [20] We found no significant difference in treatment-related mortality between children and young adults, with an estimated risk comparable to autograft literature in multiple myeloma. [21], [22] In the context of ES, proposing intensification for high-risk patients should not be hindered by age.
Alternatively, enhancing survival in chemosensitive cancers like bone sarcoma may involve intensifying standard chemotherapy. In the Euro-EWING-2012 phase-III trial, Vincristine-Doxorubicin-Cyclophosphamide/Ifosfamide-Etoposide (VDC/IE) induction regimen with an increased dose-intensity (2-week cycles) and decreased doses of alkylating agents was compared to VIDE-induction. [8], [23], [24] VDC/IE chemotherapy was found superior to VIDE with 3-year EFS of 67 % versus 61 %, and 3-year OS of 82 % versus 74 %, without excess of toxicity. [25] Very few BuMel intensifications were performed in this study (2 % and 7 % in VDC/IE-arm and VIDE-arm, respectively) raising the issue of the benefit of HDC. Various polychemotherapy combinations, including topotecan addition with anthracycline dose reduction, and targeted therapies like ganitumab, showed no superiority results. [3], [26] Another trial is exploring the addition of regorafenib during induction (ClinicalTrials.gov Identifier: NCT05830084), but results are still pending. Nevertheless, we must keep in mind that polychemotherapy can lead to acute toxicities as it has been shown by Pieper et al. for older patients suffering from Ewing’s tumours. For these patients older than 40 years, no treatment delays were reported for consolidation while there were some for the VIDE chemotherapy. Thirteen patients performed HDC by BuMel without any toxicity-related mortality [27].
Our study has some limitations. It is challenging to disentangle risk of toxicity and treatment modifications, which both may vary with age. The observed differences in treatment exposure according to age must be interpreted with caution. Although adults received fewer VAI courses, the cumulative dose of alkylating agents remains similar. Moreover, we observed more haematological toxicity in children than in adults even when focusing on the first two courses, so cumulative dose of chemotherapy does not explain this difference (Supplementary data Figure-S2). Furthermore, the few parameters with significant differences do not show a linear trend across age category or within the same age subgroup. Another limitation is that we only considered the maximum toxicity over the whole consolidation treatment, ignoring possible repeated toxicities in the VAI-arm. Finally, our study focused solely on acute toxicities, neglecting long-term effects, which may be age-related. For example, fertility is a detrimental side effect of HDC and ifosfamide, posing more intricate prevention challenges in prepubertal children. Corvest et al. compared the long-term toxicities of consolidation chemotherapies, revealing higher renal toxicity with VAI, emphasizing the importance of limiting cumulative doses of alkylating agents and considering mixed regimens like VIDE-VAC or VDC/IE. [28] The long-term lung toxicity of WLI or busulfan should also be investigated to optimize consolidation strategies.
In conclusion, the acute toxicity of consolidation treatment (BuMel intensification compared to VAI) did not appear to be different according to age and the use of high-dose chemotherapy seems to be a feasible option whatever the age of patients up to 50 years. The need of HDC in the era of neoadjuvant compressed VDC/IE, continues to be debated in term of efficacy.
Funding
The study was supported by. Bone Cancer Research Trust;. Association Enfants et Santé, Société Française de Lutte Contre les Cancers et les Leucémies de l′Enfant et de l′Adolescent, Unicancer, Ligue Nationale contre le Cancer;.
Cancer Research UK (CRUK/02/014), National Institute for Health Research, University College London Hospitals, Biomedical Research Centre;.
Deutsche Krebshilfe (70–2551-Jue3 and 108128), and Bundesministerium für Bildung und Forschung (BMBF 01GM0869; BMBF/Era-Net 01KT1310);.
National Cancer Institute, Bethesda, MD (U10CA180899, U10CA180886).
CRediT authorship contribution statement
Bernadette Brennan: Writing – review & editing, Writing – original draft, Validation, Project administration, Investigation, Data curation. Séverine Risbourg: Writing – review & editing, Writing – original draft, Validation, Project administration, Investigation, Formal analysis, Data curation. Xavier Choderlos de Laclos: Writing – review & editing, Writing – original draft, Validation, Project administration, Methodology, Formal analysis, Conceptualization. Douglas S. Hawkins: Writing – review & editing, Writing – original draft, Validation, Project administration, Investigation, Data curation. Hans Gelderblom: Writing – review & editing, Writing – original draft, Validation, Project administration, Investigation, Data curation. Nathalie Gaspar: Writing – review & editing, Writing – original draft, Validation, Project administration, Investigation, Formal analysis, Data curation. François Bertucci: Writing – review & editing, Validation, Project administration, Investigation, Data curation. Mark D Krailo: Writing – review & editing, Writing – original draft, Validation, Project administration, Investigation, Data curation. Virginie Gandemer: Writing – review & editing, Writing – original draft, Validation, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Bernd Kasper: Writing – review & editing, Writing – original draft, Validation, Project administration, Investigation, Data curation. Uta Dirksen: Writing – review & editing, Writing – original draft, Validation, Project administration, Investigation, Data curation. Heribert Juergens: Writing – review & editing, Writing – original draft, Validation, Project administration, Investigation, Data curation. Rachael Windsor: Writing – review & editing, Writing – original draft, Validation, Project administration, Investigation, Data curation. Katherine Janeway: Writing – review & editing, Writing – original draft, Validation, Project administration, Investigation, Data curation. Markus Metzler: Writing – review & editing, Writing – original draft, Validation, Project administration, Investigation, Data curation. Martin McCabe: Writing – review & editing, Writing – original draft, Validation, Project administration, Investigation, Data curation. Perrine Marec-Bérard: Writing – review & editing, Writing – original draft, Validation, Project administration, Investigation, Data curation. Marie-Cecile Le Deley: Writing – review & editing, Writing – original draft, Validation, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Marie-Dominique Tabone: Writing – review & editing, Writing – original draft, Validation, Project administration, Investigation, Data curation. Sandra Strauss: Writing – review & editing, Writing – original draft, Validation, Project administration, Investigation, Data curation. Andreas Ranft: Writing – review & editing, Writing – original draft, Validation, Project administration, Investigation, Data curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We are indebted to:
- all the patients and parents who accepted to participate in this study;.
- Carolyn Douglas, Sue Ablett, Paul Donachie, CCLG Data Centre, Leicester, United Kingdom;, Davina Scott, Jennifer Anderton, Nicola Fenwick, Keith Wheatley, Veronica Moroz, Cancer Research UK Clinical Trials Unit, University of Birmingham, United Kingdom; Susanne Ahrens, Martina Blankschän, Gabriele Braun-Munzinger, Susanne Jabar, Andreas Ranft, GPOH data centre, Münster, Germany; Universitätsklinikum Münster, sponsor of the trial in Germany; Eva Sorz, Austrian-GPOH data centre, Vienna, Austria; Anthony Mangin, Jean-François Leforestier, Yulia Belikova, Noel Ny Tovo, Zakia Idir, Fatima Bizeul, Julien Marandet, Muriel Wartelle, Gwénaël Le Teuff, Institut Gustave-Roussy, Villejuif; Marta Jimenez, Céline Mahier Aït Oukhatar, Naïma Bonnet, Jessy Delaye, Jean Genève, Unicancer, sponsor of the trial in France, Paris, France; Christine Olungu, Anne Kirkpatrick, Saskia Litiere, Sandrine Marreaud, EORTC data centre, Brussels, Belgium; for data management and study coordination assistance;.
- all the members of the Euro-Ewing Consoritum (EEC) and especially Abigail Evans, for their support.
- Pr Robert Souhami, Pr Paul Meyers and Abdel Babiker for participating in the Independent Data Monitoring Committee;.
- all investigators who participated in the trial;.
From Austria: Ch. Urban, University Children's Hospital, Graz; B. Meister and FM. Fink, University Children's Hospital, Innsbruck; R. Kerbl, Leoben Clinical Centre, Children's Hospital Leoben; O. Stollinger, Children’s Hospital Barmherzige Schwestern, Linz; K. Schmitt and G. Ebetsberger, Linz Clinical Centre, Children's Hospital, Linz; N. Jones, University Hospital, Salzburg, R. Ladenstein and H. Gadner, St. Anna Children's Hospital, Wien; B. Ausserer, Children`s Hospital, Dornbirn;.
From Belgium: P. Maes, AZM Children’s Cancer Centre, Antwerpen; B. Brichard, University Children's Hospital, Brussels; F. Mazzeo, University Hospital, Clinical Oncology, Brussels; T. Gil, Hôpitaux universitaires Bordet-Erasme, Brussels; C. Dhooge, Universitair Ziekenhuis, Gent; J.B. Vermorken, Universitair Ziekenhuis, Antwerpen; A. Klein, Hôpital Universitaire des Enfants Reine Fabiola, Brussels;.
From Czech Republic: J. Kruseova, University Hospital Motol, Pragua;.
From Denmark: A. Krarup-Hansen, Herlev Hospital-University, Copenhagen; O.Nielsen, Aarhus University Hospital, Aarhus.
From Eire: M. Capra, Our Lady's Children's Hospital, Dublin;.
From Finland: J. Kanerva, Paediatric Oncology, Helsinki University Children’s Hospital, Helsinki, Finland;.
From France: C. Devoldere, University Hospital, Amiens; I. Pellier, University Hospital, Angers; O. Collard, Institut de Cancerologie de l′Ouest, Saint Priest en Jarez; P. Soulie, Centre Paul Papin, Angers; E. Plouvier, University Hospital, Besançon; C. Vérité , University Hospital, Bordeaux; B. Bui N′guyen, Institut Bergonié , Bordeaux; G. Dabouis, University Hospital, Nantes; F. Aubier, Hopital d′enfants Margency, Margency; O. Minckes, University Hospital, Caen; C. Delcambre, Centre François Baclesse, Caen; J.O. Bay, Centre Jean Perrin, Clermont Ferrand; J. Kanold, University Hospital, Clermont-Ferrand; E. Colomb, University Hospital, Dijon; N. Isambert, Centre Jean François Leclerc, Dijon; D. Plantaz, University Hospital, Grenoble; A.S Defachelles, Centre Oscar Lambret, Lille; C. Piguet, University Hospital, Limoges; P. Marec-Bérard, Centre Léon-Bérard, Lyon; J.Y. Blay, Centre Léon-Bérard, Lyon; J.C. Gentet, University Hospital, Marseille; F. Duffaud, University Hospital, Marseille; F. Bertucci, Institut Paoli-Calmettes, Marseille; J.P Lotz, Hopital Tenon, Paris; L. Saumet, University Hospital, Montpellier; C. Schmitt, University Hospital, Nancy; M. Rios, Centre Alexis Vautrin, D. Cupissol, ICM Val d′Aurelle, Montpellier; Nancy; N. Corradini, University Hospital, Nantes; F. Rolland, Centre René Gauducheau, Nantes; A. Deville, University Hospital, Nice; A. Thyss, Centre Antoine Lacassagne, Nice; J. Michon, Institut Curie, Paris; M.D. Tabone, Trousseau University Hospital, Paris; V. Laurence, Institut Curie, Paris; F. Millot, University Hospital, Poitiers; S. Gorde GrosJean, University Hospital, Reims; S. Taque, University Hospital, Rennes; J.P. Vannier, University Hospital, Rouen; C. Guillemet, Centre Henri Becquerel, Rouen; L. Chauvenet, Hotel Dieu, Paris; E. Brain, Centre René Huguenin, St Cloud; C. Berger, University Hospital, St Etienne; P. Lutz, University Hospital, Strasbourg M.P. Castex, University Hospital, Toulouse; H. Roche, Institut Claudius Regaud, Toulouse; O. Lejars, University Hospital, Tours; C. Linassier, University Hospital, Tours; O. Oberlin, Institut Gustave-Roussy, Villejuif; N. Gaspar, Institut Gustave-Roussy, Villejuif; A. Le Cesne, Institut Gustave-Roussy, Villejuif;.
From Germany (EORTC): S. Bauer, P. Ebeling and M. Flasshove, Essen University Hospital, Essen; J.T. Hartmann, Eberhard Karls University, Tuebingen; P. Reichardt, Charite Universitaetsmedizin, Campus Berlin- Bush, Berlin,
From Germany (GPOH): R. Mertens, U. Kontny, University Children's Hospital, Aachen; R. Osieka, University Hospital, Medical Oncology, Aachen; A. Gnekow, Augsburg Clinical Centre, Children’s Hospital, Augsburg; G. Schlimok, Medical Oncology, Augsburg Clinical Centre, Augsburg; G. Henze, P. Hundsdörfer, Charité Virchow Clinical Centre, Children's Hospital, Berlin; P. Thuss-Patience, A. Kunitz, Charité Virchow Clinical Centre, Berlin; L. Wickmann, L. Schweigerer, Berlin-Buch Children’s Hospital, Berlin; P. Reichardt, Medical Oncology, Berlin-Buch Clinical Centre, Berlin; K. Possinger, Medical Oncology, Charité Central Campus, Berlin; J. Potenberg, Ev. Waldkrankenhaus, Berlin; P. Reichardt, Charite Universitaetsmedizin, Campus Berlin- Buch, Berlin; N. Jorch, Ev. Children’s Hospital Bielefeld; U. Krümpelmann, Medical Oncology Ev. Hospital, Bielefeld; Weh, Medical Oncology, Franziskus Hospital, Bielefeld; J. Baier, Medical Oncology, St Josef’s Hospital, Bochum; W. Schmiegel, Medical Oncology, Ruhr-University Hospital, Bochum; U. Bode, D. Dilloo, University Children's Hospital, Bonn; I.G.M. Schmidt-Wolf, V. Janzen, Medical Oncology I, University Hospital Bonn; J. Nolting, University Hospital, Medical Oncology III, Bonn; W. Eberl, Children’s Hospital, Braunschweig; B. Wörmann, Medical Oncology, City Hospital, Braunschweig; W. Hoffmann, Radiology and Radiotherapy, City Hospital, Braunschweig; Th. Wolff, J. Kullmer, Ev. Diakonie Hospital, Bremen; A. Pekrun, Bremen Mitte Children’s Hospital, Bremen; A. Hofmann, Children’s Hospital, Chemnitz; G. Geißler, Internal Medicine III, Chemnitz; E. Hohlfeld; Carl-Thiem-Hospital, Cottbus; W. Andler, T. Wiesel, Children's Hospital, Datteln; D. Schneider, B. Bernbeck, Children's Hospital, Dortmund; I. Lauterbach, M. Suttorp, University Children’s Hospital, Dresden; S. Richter, G. Ehninger, University Hospital Dresden; P. Zickler, Children’s Hospital, Duisburg; U. Göbel, A. Borkhardt, University Children's Hospital, Düsseldorf; J.-N- Machatschek, Medical Oncology, University Hospital, Düsseldorf; A. Lemmer, A. Sauerbrey, Children's Hospital, Erfurt; W. Holter, M. Metzler, University Children's Hospital, Erlangen; N. Meidenbauer, University Hospital, Erlangen; Seeber, S. Bauer, P. Ebeling and M. Flasshove, University Hospital, Essen; A. Eggert, G. Fleischhack, University Children's Hospital, Essen; T. Klingebiel, University Children's Hospital Frankfurt, M. Ahrens University Hospital Frankfurt; C. Niemeyer, J. Rößler University Children's Hospital, Freiburg; J. Heinz, Medical Oncology, University Hospital, Freiburg; A. Reiter, W. Wössmann University Children's Hospital, Giessen; M. Rummel, W. Blau, Medical Oncology, University Hospital, Giessen; V. Runde, Goch Clinical Centre, Goch; M. Lakomek, C. Kramm University Children's Hospital, Göttingen; L. Trümper, D. Hertramph, Medical Oncology, University Hospital, Göttingen; J. Beck, H. Lode University Children's Hospital, Greifswald; G. Dölken, Medical Oncology, University Hospital, Greifswald; H.-W. Lindemann, Hagen Clinical Centre, Hagen; G. Günther, D. Körholz, T. Bernig, University Children's Hospital, Halle; H.-J. Schmoll, Medical Oncology, University Hospital, Halle; R. Schneppenheim, W. A. Hassenpflug UKE University Children's Hospital, Hamburg; D.K. Hossfeld, C. Bokemeyer, Medical Oncology, UKE University Hospital, Hamburg; W. Alberti, Radiology and Radiotherapy, UKE University Hospital, Hamburg; H. Keles, Medical Oncology, Asklepios Hospital Altona, Hamburg; N. Schmitz, Haematology, Asklepios Hospital St. Georg, Hamburg; N. Brüllke, Medical Oncology, Asklepios Hospital Barmbek, Hamburg; H. Schmidt, Internal Medicine, Regional Hospital, Hameln; C. Dürk, Medical Oncology, St. Marien Hospital, Hamm; K. Welte, C. Klein, B. Maecker-Kohlhoff, Paediatric Oncology, Hannover Medical School, Hannover; Ch. Reuter, Medical Oncology, Hannover Medical School, Hannover; H. Kirchner, Medical Oncology III, Siloah Hospital, Hannover; A. Kulozik, W. Behnisch University Children's Hospital, Heidelberg; G. Egerer, Medical Oncology, University Hospital, Heidelberg; Ewerbeck, Orthopedic, University Hospital, Heidelberg; M. Thomas, Medical Oncology, Thorax Hospital, Heidelberg; J. Cyran, Clinical Centre, Heilbronn; Kachel, Paediatrics, SLK Hospital, Heilbronn; U. Martens, Paediatric, Haematology, SLK Hospital Heilbronn; Ch. Tautz, General Hospital, Herdecke; D. Strumberg, St. Marien Hospital, Herne; W. Freier, Ocology Practice, Hildesheim; U. Kaiser, Medical Oncology II, St. Bernward, Hildesheim; N. Graf, University Children's Hospital, Homburg; M. Pfreundschuh, Medical Oncology, University Hospital, Homburg; F. Zintel, B. Gruhn, University Children's Hospital, Jena; E. Eigendorff, University Hospital, Jena; H. Link, Medical Oncology, Westpfalz Hospital, Kaiserslautern; R. Germann, A. Leipold, Children's Hospital, Karlsruhe; Th. Fischer, M. Bentz, Medical Oncology, Karlsruhe Clinical Centre, Karlsruhe; J. Mezges, Medical Oncology, St. Vincentius Hospital, Karlsruhe; H. Wehinger, M. Nathrath Children's Hospital Kassel; M. Wolf, Medical Oncology, Kassel Hospital, Kassel; H. Meye, Radiotherapy, Kassel Hospital, Kassel; Prümmer, Internal Medicine III, Kempten Ober-Allgäu Hospital, Kempten; M. Schrappe, A. Claviez University Children's Hospital, Kiel; M. Lamprecht, Medical Oncology II, University Hospital, Kiel; H. Nolte, Kemperhof Clinical Centre, Koblenz; M. Rister, M. Jakob, Kemperhof Koblenz; F. Berthold, T. Simon University Children's Hospital, Cologne; Diehl, J. Wolf, Medical Oncology, University Hospital, Cologne; W. Sternschulte, A. Prokop, Children's Hospital, Cologne; E. Stoelben, Pulmonary Hospital, Cologne; S. Voelpel, T. Imschweiler Children’s Hospital, Krefeld; T. Frieling, Medical Oncology, Krefeld Clinical Centre, Krefeld; J. Moessner, D. Niederwiese, Medical Oncology, University Hospital, Leipzig; U. Bierbach, H. Christiansen, University Children’s Hospital, Leipzig; J. Bennek, Paediatric Surgery, University Hospital, Leipzig; L. Mantovani, Medical Oncology, St. Georg Hospital, Leipzig; D. Selle, St. Annastift Children's Hospital, Ludwigshafen; P. Bucsky, T. Langer, University Children’s Hospital, Lübeck; H. Bartels, Medical Oncology, Clinical Centre Lübeck South, Lübeck; Th. Wagner, Medical Oncology I, University Hospital, Lübeck; G. Heil, Medical Oncology, Reginal Hospital, Lüdenscheid; M. Mohren, Th. Fischer, E. Schalk, Medical Oncology, University Hospital, Magdeburg; U. Kluba, P. Vorwerk, University Children's Hospital Magdeburg; P. Gutjahr, M. Dittrich, J. Faber, University Children's Hospital, Mainz; H.-J. Beck, Medical Oncology III, Univeristy Hospital, Mainz; S. Reiter, B. Kasper, Medical Oncology III, University Hospital, Mannheim; M. Dürken, University Children’s Hospital, Mannheim; A. Neubauer, J. Beyer, Medical Oncology, University Hospital, Marburg; B. Schütz, H. Christiansen, University Children's Hospital, Marburg; B. Erdlenbruch, Paediatrics Johannes Wesling Hospital, Minden; M. Griesshammer, Medical Oncology, Johannes Wesling Hospital, Minden; Reis, Medical Oncology, St,. Franziskus Hospital, Mönchengladbach; S. Burdach, A. Wawer, TU Clinical Centre, Children's Hospital, München; I. Schmid, LMU Children's Hospital, Munich; C. Meyer zum Büschenfelde, C. Peschel, S. Lorenzen, Medical Oncology, TU Clinical Centre, Munich; B. Emmerich, M. Schlemmer, F. Oduncu, R. Issels, Medical Oncology, LMU Clinical Centre, Munich; H. Jürgens, University Children's Hospital, Münster; W. Berdel, A. Kerkhoff, Medical Oncology, University Hospital, Münster; H. Held, Medical Oncology, Clinical Centre, Neumünster; G. Hofmann-Wackersreuther, W. Scheurlen, M. Augustin, Medical Oncology, Clinical Centre, Nürnberg; H. Müller, Children’s Hospital, Oldenburg; C.-H. Köhne, D. Krämer, Medical Oncology, Oldenburg Hospital, Oldenburg; A. Rickers, Peadiatrics, Marienhospital, Osnabrück; T. Wolff, Medical Oncology, St. Josef Hospital, Paderborn; U. Loss, Internal Medicine, Knappschaftshorpital, Recklinghausen; O. Peters, St. Hedwig Children's Hospital, Regensburg; R. Andreesen, S. Krause, M. Grube, Medical Oncology,University Hospital, Regensburg; Kreuser, Medical Oncology, Barmherzige Brüder Hospital, Regensburg; S. Corbacioglu, University Children’s Hospital, Regensburg; C.-F. Classen, University Childrens’s Hospital, Rostock, M. Freund, Medical Oncology, University Hospital Rostock; J. Potratz, F. Heits, Medical Oncology, Rotenburg Clinical Centre, Rotenburg; R. Geib-König, Winterberg Clinical Centre, Children's Hospital, Saarbrücken; J. Weis, Radiology and Radiotherapy, Saarbrcken Hospital, Saarbrücken; M. Kasbohm, Helios Clinical Centre, Children’s Hospital, Schwerin; D. Hähling, Medical Oncology, Helios Hospital, Schwerin; D. Bürger, R. Burghard, DRK Children's Hospital, Siegen; R. Dickerhoff, H. Reinhard, Asklepios Clinical Centre, Children's Hospital, St. Augustin; S. Bielack, K. Apel, S. Simon-Klingenstein, Stuttgart Cancer Center, Pediatrics 5 (Oncology, Hematology, Immunology), Klinikum Stuttgart – Olgahospital, Stuttgart; H.G. Mergenthaler, G. Illerhaus, Stuttgart Cancer Center, Medical Oncology, Klinikum Stuttgart - Katharinenhospital, Stuttgart; C. Denzlinger, Internal Medicine III, Marienhospital, Stuttgart; I. Feddersen, S. Weis, Rauh Mutterhaus der Borromäerinnen, Children's Hospital, Trier; M.R. Clemens, Hospital "Barmherzige Brüder", Trier; Waladkhani, Medical Oncology I, Mutterhaus der Borromäerinnen, Trier; H. Kirchen, Medical Oncology, Barmherzige Brüder Hospital, Trier; R. Handgretinger, M. Ebinger, University Children's Hospital, Tübingen; Brossart, J.T. Hartmann, H.-G. Kopp, Eberhard Karls Universitaet, Tuebingen;; K.-M. Debatin, University Children's Hospital, Ulm; Döhner, R. Mayer-Steinacker, University Cancer Centre, Ulm; M. Müller, A. Schoengen, Internal Medicine I, Bundeswehrkrankenhaus, Ulm; St. Brettner, Medical Oncology, Regional Hospital, Waldbröhl; N. Frickhofen, Internal Medicine III, Dr.-Horst-Schmidt-Hospital, Wiebaden; Dohrn, Paediatrics, HELIOS Hospital, Wuppertal; M. Sandmann, Medical Oncology, St. Antonius Hospital, Wuppertal; P. Schlegel, University Children's Hospital, Würzburg;.
From Hong-Kong: V. Lee and K-W Chik, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong;.
From Hungary: P. Hauser, Semmelweis University Children’s Hospital, Budapest, Hungary;.
From the Netherlands: H. van den Berg, AMC Emma Children's Hospital/Academic Medical Centre, Amsterdam; S. Rodenhuis, The Netherlands Cancer Institute-Antoni Van Leeuwenhoekziekenhuis, Amsterdam; A.J. Gelderblom, J. Anninga, Leiden University Medical Centre, Cancer Centre, Leiden; P. Hoogerbrugge and JPM. Bökkerink, UMC St Radboud, Nijmegen; R. Pieters, Sophia Children Hospital - Erasmus Medical Centre, Rotterdam;.
From New-Zealand: R, Corbett, Christchurch Hospital, Christchurch.
From Sweden: G. Österlundh, Queen Silvia’s Children’s Hospital, Sahlgrenska Hospital, Gothenburg; M. Behrendtz and B.-M. Holmqvist, Department of paediatrics, Linköping University Hospital, Linköping; L. Hjorth, Children’s Hospital, Skåne University Hospital, Lund; C. Petersen and Å. Jakobson, Astrid Lindgren’s Children’s Hospital, Karolinska Hospital, Stockholm; U. Hjalmars, Department of paediatrics, Umeå University Hospital, Umeå; G. Ljungman, Academic Children’s Hospital, Academic Hospital, Uppsala;.
From Switzerland: R. Angst, Kantonsspital Aarau; T. Kühne and M. Paulussen, University Children's Hospital, Basel; S. Leyvraz, Lausanne Cancer Centre, Lausanne; J. Rischewski, Luzerner Kantonsspital, Luzern; A. Feldges and J. Greiner, Ostschweizer Kinderspital, St. Gallen, U. Hess, Clinical Centre, Medical Oncology, St. Gallen; G.U. Exner, Orthopedic Hospital Balgrist, Zürich; F. Niggli, University Children’s Hospital, Zürich; A. Knuth, University Hospital, Medical Oncology, Zürich;.
From United-Kingdom: D. King and H. Bishop, Royal Aberdeen Children's Hospital, Aberdeen; A. McCarthy, Royal Belfast Hospital for Sick Children, Belfast; P. Henry, Belfast City Hospital, Belfast; B. Morland, Birmingham Children's Hospital, Birmingham; H. Rees, University Hospitals Bristol NHS Foundation Trust, Bristol; J. Nicholson, Addenbrooke’s Hospital, Cambridge; H. Traunecker, Children's Hospital for Wales, Cardiff; H. Wallace, Royal Hospital for Sick Children, Edinburgh; M. Ronghe and E. Simpson, Royal Hospital for Sick Children, Glasgow; F. Cowie and J. White, NHS Greater Glasgow and Clyde-Western Infirmary, Glasgow; S. Picton and I. Lewis, Leeds General Infirmary, Leeds; M. Leahy, D Stark and PJ. Selby, St. James University Hospital, Leeds; D. Heney and E. Ross, Leicester Royal Infirmary, Leicester; B. Pizer and H. McDowell, Royal Liverpool Children's Hospital NHS Trust, Alder Hey, Liverpool; A. Michalski, Great Ormond Street Hospital for Children, London; J. Whelan, University College Hospital NHS Trust, London; J. Chisholm and K. Pritchard-Jones, Royal Marsden NHS Trust, London; I. Judson, Royal Marsden Hospital, London; B. Brennan, Royal Manchester Children's Hospital and The Christie NHS Foundation Trust, Manchester; J. Hale and Q. Campbell-Hewson, Royal Victoria Infirmary, Newcastle upon Tyne; M. Verrill, Newcastle-Upon-Tyne, Newcastle; D. Walker, Queens Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham; M. Sokal, Nottingham University Hospital NHS Trust, Nottingham; K. Wheeler, Oxford Radcliffe Hospitals, Oxford; V. Lee and M. Gerrard, Sheffield Children's Hospital, Sheffield; P. Woll, P. Lorigan and M. Robinson, Weston Park Hospital, Sheffield; J. Kohler and R. Ramanujachar, Southampton General Hospital, Southampton.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ejca.2024.114229.
Contributor Information
Xavier Choderlos de Laclos, Email: x.choderlos-de-laclos@rennes.unicancer.fr.
Séverine Risbourg, Email: s-risbourg@o-lambret.fr.
Bernadette Brennan, Email: Bernadette.Brennan@mft.nhs.uk.
François Bertucci, Email: BERTUCCIF@ipc.unicancer.fr.
Nathalie Gaspar, Email: Nathalie.GASPAR@gustaveroussy.fr.
Hans Gelderblom, Email: a.j.gelderblom@lumc.nl.
Douglas S. Hawkins, Email: doug.hawkins@seattlechildrens.org.
Katherine Janeway, Email: Katherine_Janeway@dfci.harvard.edu.
Heribert Juergens, Email: jurgh@ukmuenster.de.
Bernd Kasper, Email: Bernd.Kasper@medma.uni-heidelberg.de.
Mark D. Krailo, Email: mkrailo@childrensoncologygroup.org.
Marie Cécile Le Deley, Email: m-ledeley@o-lambret.fr.
Perrine Marec-Bérard, Email: perrine.marec-berard@ihope.fr.
Martin G. McCabe, Email: Martin.McCabe@manchester.ac.uk.
Markus Metzler, Email: Markus.Metzler@uk-erlangen.de.
Andreas Ranft, Email: Andreas.ranft@uk-essen.de.
Sandra Strauss, Email: sandra.strauss@nhs.net.
Marie-Dominique Tabone, Email: marie-dominique.tabone@aphp.fr.
Rachael Windsor, Email: rachael.windsor@nhs.net.
Uta Dirksen, Email: Uta.Dirksen@uk-essen.de.
Virginie Gandemer, Email: virginie.gandemer@chu-rennes.fr.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
References
- 1.Anderson W.J., Doyle L.A. Updates from the 2020 World Health Organization classification of soft tissue and bone tumours. Histopathology. 2021;78:644–657. doi: 10.1111/his.14265. [DOI] [PubMed] [Google Scholar]
- 2.Worch J., Ranft A., DuBois S.G., et al. Age dependency of primary tumor sites and metastases in patients with Ewing sarcoma. Pedia Blood Cancer. 2018;65 doi: 10.1002/pbc.27251. [DOI] [PubMed] [Google Scholar]
- 3.Leavey P.J., Laack N.N., Krailo M.D., et al. Phase III Trial Adding Vincristine-Topotecan-Cyclophosphamide to the Initial Treatment of Patients With Nonmetastatic Ewing Sarcoma: A Children’s Oncology Group Report. JCO. 2021;39:4029–4038. doi: 10.1200/JCO.21.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Applebaum M.A., Worch J., Matthay K.K., et al. Clinical features and outcomes in patients with extraskeletal ewing sarcoma. Cancer. 2011;117:3027–3032. doi: 10.1002/cncr.25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trama A., Botta L., Foschi R., et al. Survival of European adolescents and young adults diagnosed with cancer in 2000–07: population-based data from EUROCARE-5. Lancet Oncol. 2016;17:896–906. doi: 10.1016/S1470-2045(16)00162-5. [DOI] [PubMed] [Google Scholar]
- 6.Keegan T.H.M., Abrahão R., Alvarez E.M. Survival Trends Among Adolescents and Young Adults Diagnosed With Cancer in the United States: Comparisons With Children and Older Adults. JCO. 2024;42:630–641. doi: 10.1200/JCO.23.01367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whelan J., Le Deley M.-C., Dirksen U., et al. High-Dose Chemotherapy and Blood Autologous Stem-Cell Rescue Compared With Standard Chemotherapy in Localized High-Risk Ewing Sarcoma: Results of Euro-E.W.I.N.G.99 and Ewing-2008. JCO. 2018;36:3110–3119. doi: 10.1200/JCO.2018.78.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dirksen U., Brennan B., Le Deley M.-C., et al. High-dose chemotherapy compared with standard chemotherapy and lung radiation in ewing sarcoma with pulmonary metastases: results of the European Ewing tumour working initiative of national groups, 99 Trial and EWING 2008. JCO. 2019;37:3192–3202. doi: 10.1200/JCO.19.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ladenstein R., Pötschger U., Le Deley M.C., et al. Primary disseminated multifocal Ewing sarcoma: results of the Euro-EWING 99 Trial. JCO. 2010;28:3284–3291. doi: 10.1200/JCO.2009.22.9864. [DOI] [PubMed] [Google Scholar]
- 10.Bukowinski A.J., Burns K.C., Parsons K., et al. Toxicity of Cancer Therapy In Adolescents And Young Adults (AYAs) Semin Oncol Nurs. 2015;31:216–226. doi: 10.1016/j.soncn.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Juergens C., Weston C., Lewis I., et al. Safety assessment of intensive induction with vincristine, ifosfamide, doxorubicin, and etoposide (VIDE) in the treatment of Ewing tumors in the EURO-E.W.I.N.G. 99 clinical trial. Pedia Blood Cancer. 2006;47:22–29. doi: 10.1002/pbc.20820. [DOI] [PubMed] [Google Scholar]
- 12.van den Berg H., Paulussen M., Le Teuff G., et al. Impact of gender on efficacy and acute toxicity of alkylating agent -based chemotherapy in Ewing sarcoma: Secondary analysis of the Euro-Ewing99-R1 trial. Eur J Cancer. 2015;51:2453–2464. doi: 10.1016/j.ejca.2015.06.123. [DOI] [PubMed] [Google Scholar]
- 13.Gratwohl A., Baldomero H., Passweg J. Hematopoietic stem cell transplantation activity in Europe. Curr Opin Hematol. 2013;20:485–493. doi: 10.1097/MOH.0b013e328364f573. [DOI] [PubMed] [Google Scholar]
- 14.Bölling T., Dirksen U., Ranft A., et al. Radiation toxicity following busulfan/melphalan high-dose chemotherapy in the EURO-EWING-99-trial: Review of GPOH Data. Strahl Onkol. 2009;185:21–22. doi: 10.1007/s00066-009-1009-9. [DOI] [PubMed] [Google Scholar]
- 15.Koch R., Gelderblom H., Haveman L., et al. High-dose treosulfan and melphalan as consolidation therapy versus standard therapy for high-risk (Metastatic) Ewing Sarcoma. JCO. 2022;40:2307–2320. doi: 10.1200/JCO.21.01942. [DOI] [PubMed] [Google Scholar]
- 16.Wilhelm M., Dirksen U., Bielack S.S., et al. ENCCA WP17-WP7 consensus paper on teenagers and young adults (TYA) with bone sarcomas. Ann Oncol. 2014;25:1500–1505. doi: 10.1093/annonc/mdu153. [DOI] [PubMed] [Google Scholar]
- 17.Piperno-Neumann S., Le Deley M.-C., Rédini F., et al. Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17:1070–1080. doi: 10.1016/S1470-2045(16)30096-1. [DOI] [PubMed] [Google Scholar]
- 18.Ferlay J., Steliarova-Foucher E., Lortet-Tieulent J., et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Passweg J.R., Baldomero H., Ciceri F., et al. Hematopoietic cell transplantation and cellular therapies in Europe 2021. The second year of the SARS-CoV-2 pandemic. A Report from the EBMT Activity Survey. Bone Marrow Transpl. 2023;58:647–658. doi: 10.1038/s41409-023-01943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferlay J., Colombet M., Soerjomataram I., et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Gertz M.A., Ansell S.M., Dingli D., et al. Autologous Stem Cell Transplant in 716 Patients With Multiple Myeloma: Low Treatment-Related Mortality, Feasibility of Outpatient Transplant, and Effect of a Multidisciplinary Quality Initiative. Mayo Clin Proc. 2008;83:1131–1135. doi: 10.4065/83.10.1131. [DOI] [PubMed] [Google Scholar]
- 22.Attal M., Lauwers-Cances V., Hulin C., et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Engl J Med. 2017;376:1311–1320. doi: 10.1056/NEJMoa1611750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu E., Ryan C.W., Bassale S., et al. Feasibility of Treating Adults with Ewing or Ewing‐Like Sarcoma with Interval‐Compressed Vincristine, Doxorubicin, and Cyclophosphamide Alternating with Ifosfamide and Etoposide. Oncologist. 2020;25:150–155. doi: 10.1634/theoncologist.2019-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderton J., Moroz V., Marec-Bérard P., et al. International randomised controlled trial for the treatment of newly diagnosed EWING sarcoma family of tumours – EURO EWING 2012 Protocol. Trials. 2020;21:96. doi: 10.1186/s13063-019-4026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brennan B., Kirton L., Marec-Bérard P., et al. Comparison of two chemotherapy regimens in patients with newly diagnosed Ewing sarcoma (EE2012): an open-label, randomised, phase 3 trial. Lancet. 2022;400:1513–1521. doi: 10.1016/S0140-6736(22)01790-1. [DOI] [PubMed] [Google Scholar]
- 26.DuBois S.G., Krailo M.D., Glade-Bender J., et al. Randomized Phase III Trial of Ganitumab With Interval-Compressed Chemotherapy for Patients With Newly Diagnosed Metastatic Ewing Sarcoma: A Report From the Children’s Oncology Group. JCO. 2023;41:2098–2107. doi: 10.1200/JCO.22.01815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pieper S., Ranft A., Braun-Munzinger G., et al. Ewing’s Tumors over the Age of 40 – a Retrospective Analysis of 47 Patients Treated According to the International Clinical Trials EICESS 92 and EURO-E.W.I.N.G. 99. Oncol Res Treat. 2008;31:657–663. doi: 10.1159/000165361. [DOI] [PubMed] [Google Scholar]
- 28.Corvest V., Marec‐Bérard P., Lervat C., et al. Late toxicity comparison of alkylating‐based maintenance regimen with cyclophosphamide ( VAC) vs ifosfamide ( VAI) in Ewing sarcoma survivors treated in the randomized clinical trial Euro‐EWING99–R1 in France. Int J Cancer. 2023;152:1659–1667. doi: 10.1002/ijc.34326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material