Abstract
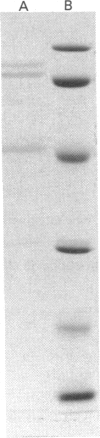
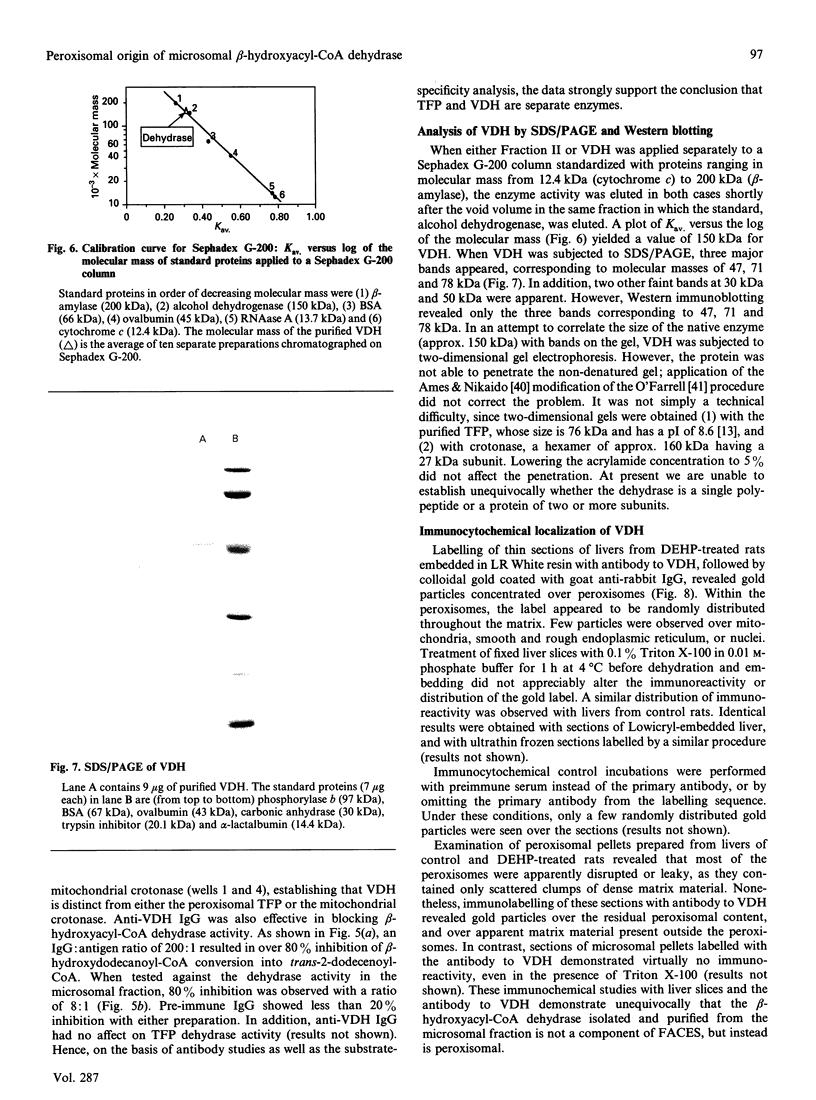
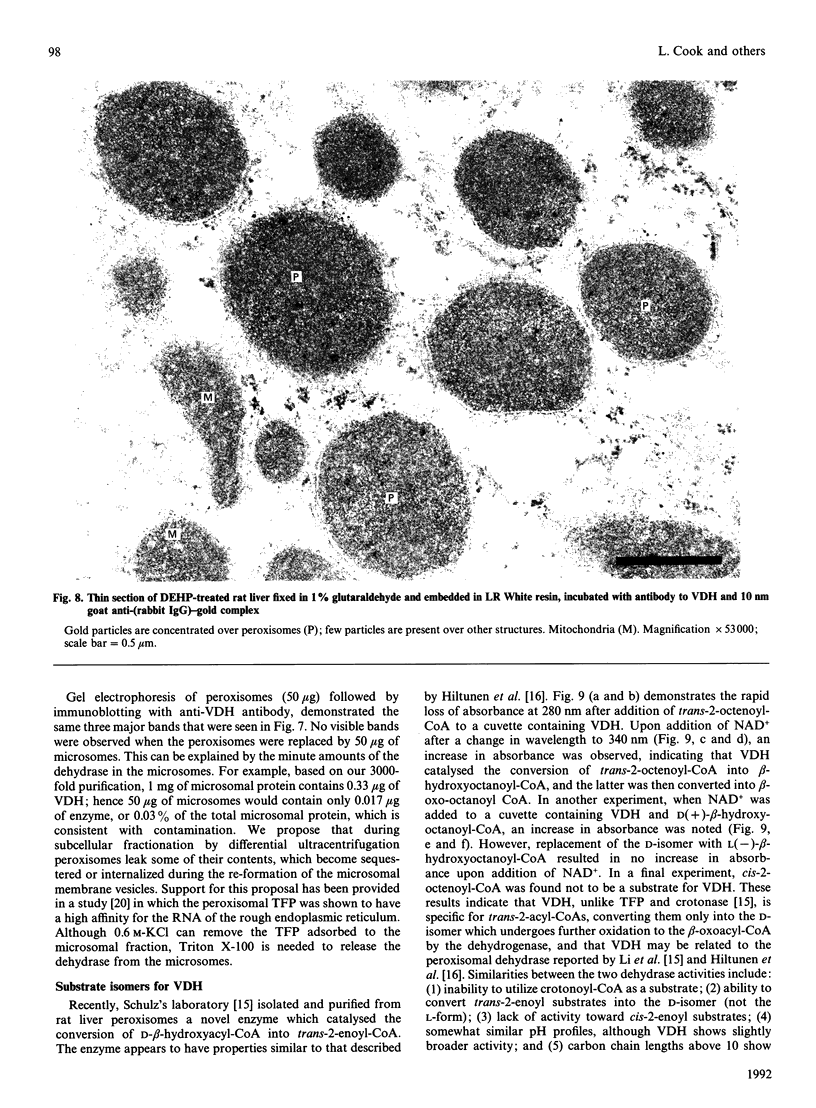
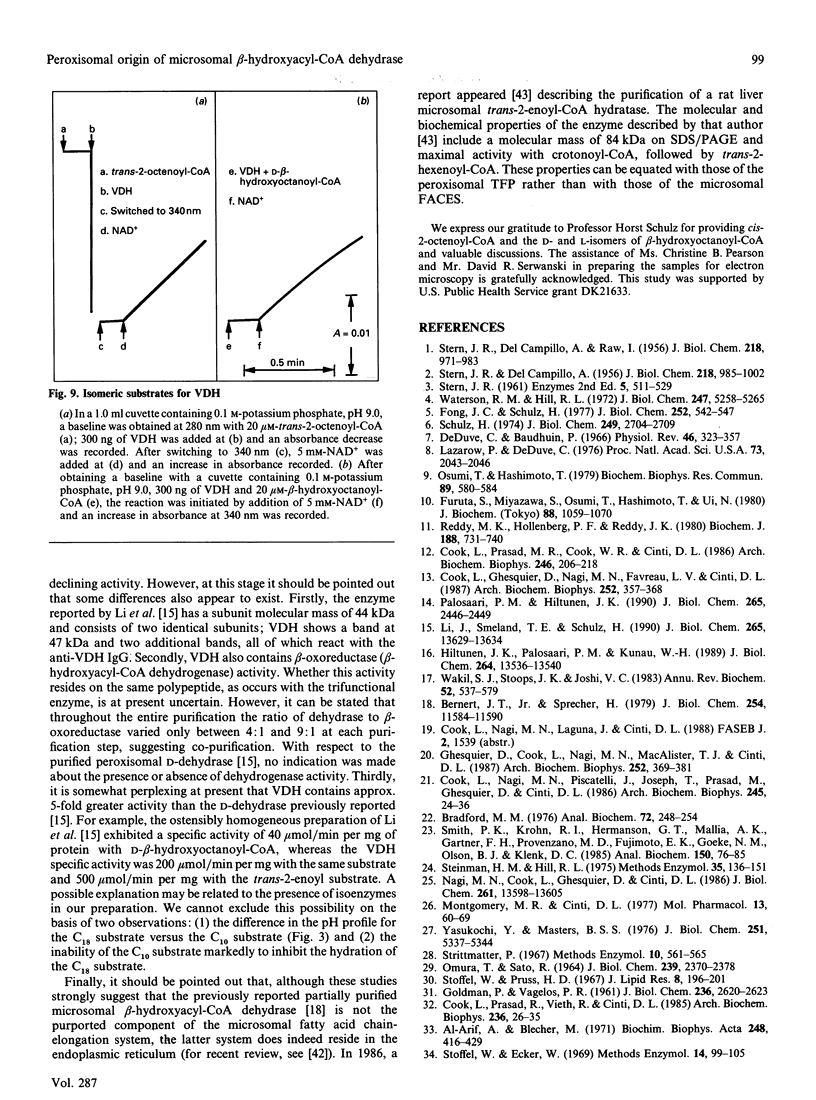
The present study provides strong evidence that the previously isolated hepatic microsomal beta-hydroxyacyl-CoA dehydrase (EC 4.2.1.17), believed to be a component of the fatty acid chain-elongation system, is derived, not from the endoplasmic reticulum, but rather from the peroxisomes. The isolated dehydrase was purified over 3000-fold and showed optimal enzymic activity toward beta-hydroxyacyl-CoAs or trans-2-enoyl-CoAs with carbon chain lengths of 8-10. The purified preparation (VDH) displayed a pH optimum at 7.5 with beta-hydroxydecanoyl-CoA, and at 6.0 with beta-hydroxystearoyl-CoA. Competitive-inhibition studies suggested that VDH contained dehydrase isoforms, and SDS/PAGE showed three major bands at 47, 71 and 78 kDa, all of which reacted to antibody raised to the purified preparation. Immunocytochemical studies with anti-rabbit IgG to VDH unequivocally demonstrated gold particles randomly distributed throughout the peroxisomal matrix of liver sections from both untreated and di-(2-ethylhexyl) phthalate-treated rats. No labelling was associated with endoplasmic reticulum or with the microsomal fraction. Substrate-specificity studies and the use of antibodies to VDH and to the peroxisomal trifunctional protein indicated that VDH and the latter are separate enzymes. On the other hand, the VDH possesses biochemical characteristics similar to those of the D-beta-hydroxyacyl-CoA dehydrase recently isolated from rat liver peroxisomes [Li, Smeland & Schulz (1990) J. Biol. Chem. 265, 13629-13634; Hiltunen, Palosaari & Kunau (1989) J. Biol. Chem. 264, 13536-13540]. Neither enzyme utilizes crotonoyl-CoA or cis-2-enoyl-CoA as substrates, but both enzymes convert trans-2-enoyl substrates into the D-isomer only. In addition, the VDH also contained beta-oxoacyl-CoA reductase (beta-hydroxyacyl-CoA dehydrogenase) activity, which co-purified with the dehydrase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Bendayan M., Roth J., Perrelet A., Orci L. Quantitative immunocytochemical localization of pancreatic secretory proteins in subcellular compartments of the rat acinar cell. J Histochem Cytochem. 1980 Feb;28(2):149–160. doi: 10.1177/28.2.7354212. [DOI] [PubMed] [Google Scholar]
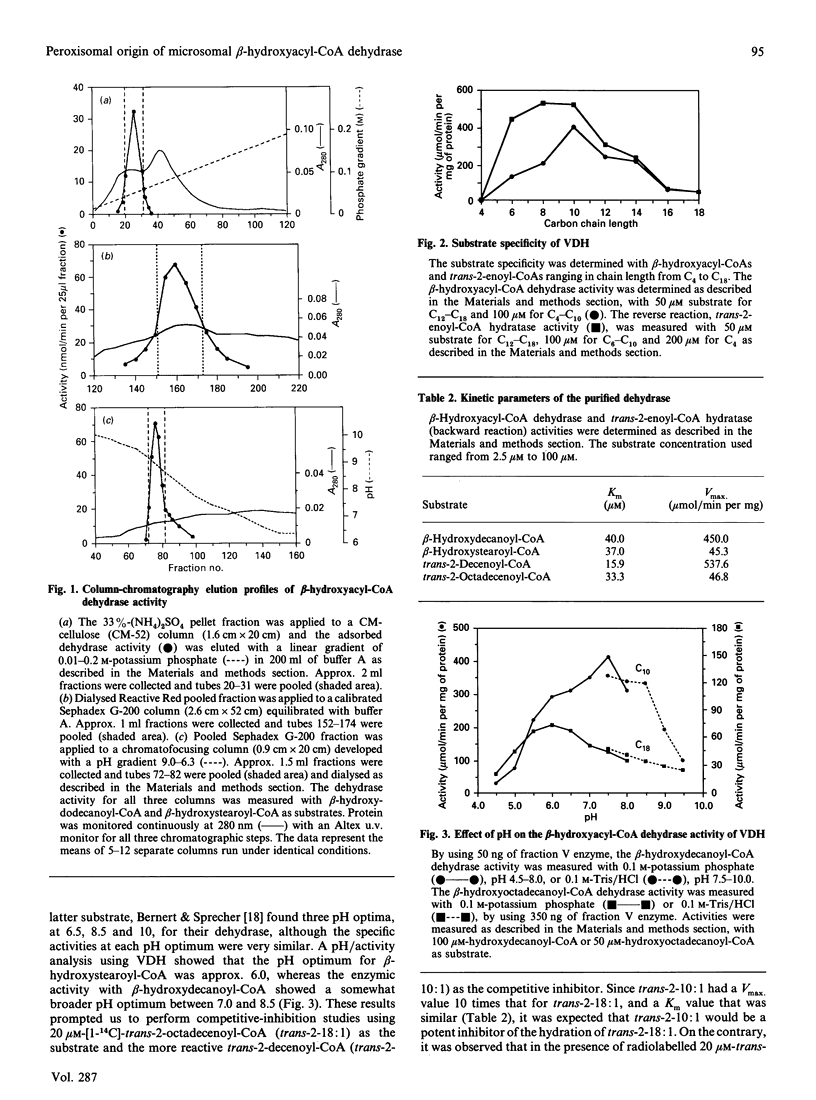
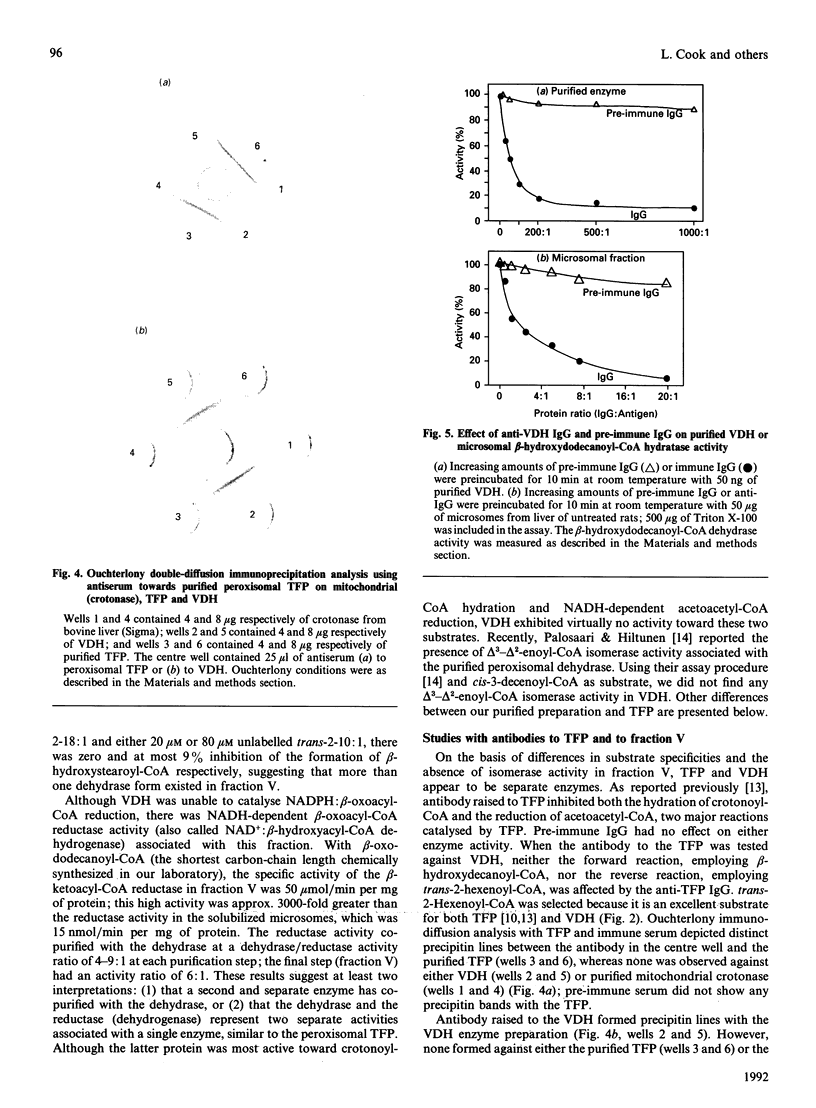
- Bernert J. T., Jr, Sprecher H. Solubilization and partial purification of an enzyme involved in rat liver microsomal fatty acid chain elongation: beta-hydroxyacyl-CoA dehydrase. J Biol Chem. 1979 Nov 25;254(22):11584–11590. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chiang C. F. Solubilization and purification of rat liver microsomal trans-2-enoyl-CoA hydratase. Prep Biochem. 1986;16(3):187–198. doi: 10.1080/00327488608062465. [DOI] [PubMed] [Google Scholar]
- Cinti D. L., Cook L., Nagi M. N., Suneja S. K. The fatty acid chain elongation system of mammalian endoplasmic reticulum. Prog Lipid Res. 1992;31(1):1–51. doi: 10.1016/0163-7827(92)90014-a. [DOI] [PubMed] [Google Scholar]
- Cinti D. L., Montgomery M. R. Pyridine nucleotide-dependent electron transport in kidney cortex microsomes: interaction with desaturase and other microsomal mixed-function oxidases. Mol Pharmacol. 1977 Jan;13(1):60–69. [PubMed] [Google Scholar]
- Cook L., Ghesquier D., Nagi M. N., Favreau L. V., Cinti D. L. Biochemical and immunological identity of the hepatic peroxisomal and microsomal trans-2-enoyl CoA hydratase bifunctional protein. Arch Biochem Biophys. 1987 Feb 1;252(2):357–368. doi: 10.1016/0003-9861(87)90042-7. [DOI] [PubMed] [Google Scholar]
- Cook L., Nagi M. N., Piscatelli J., Joseph T., Prasad M. R., Ghesquier D., Cinti D. L. Hepatic subcellular distribution of short-chain beta-ketoacyl coenzyme A reductase and trans-2-enoyl coenzyme A hydratase: 25- to 50-fold stimulation of microsomal activities by the peroxisome proliferator, di-(2-ethylhexyl)phthalate. Arch Biochem Biophys. 1986 Feb 15;245(1):24–36. doi: 10.1016/0003-9861(86)90186-4. [DOI] [PubMed] [Google Scholar]
- Cook L., Prasad M. R., Cook W. R., Cinti D. L. Isolation of rat liver microsomal short-chain beta-ketoacyl-coenzyme A reductase and trans-2-enoyl-coenzyme A hydratase: evidence for more than one hydratase. Arch Biochem Biophys. 1986 Apr;246(1):206–216. doi: 10.1016/0003-9861(86)90465-0. [DOI] [PubMed] [Google Scholar]
- Cook L., Prasad M. R., Vieth R., Cinti D. L. Hepatic microsomal short-chain beta-hydroxyacyl-CoA dehydrase distinct from the fatty acid elongation component: substrate specificity of the membrane-extracted enzyme. Arch Biochem Biophys. 1985 Jan;236(1):26–35. doi: 10.1016/0003-9861(85)90602-2. [DOI] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fong J. C., Schulz H. Purification and properties of pig heart crotonase and the presence of short chain and long chain enoyl coenzyme A hydratases in pig and guinea pig tissues. J Biol Chem. 1977 Jan 25;252(2):542–547. [PubMed] [Google Scholar]
- Furuta S., Miyazawa S., Osumi T., Hashimoto T., Ui N. Properties of mitochondria and peroxisomal enoyl-CoA hydratases from rat liver. J Biochem. 1980 Oct;88(4):1059–1070. doi: 10.1093/oxfordjournals.jbchem.a133057. [DOI] [PubMed] [Google Scholar]
- GOLDMAN P., VAGELOS P. R. The specificity of triglyceride synthesis from diglycerides in chicken adipose tissue. J Biol Chem. 1961 Oct;236:2620–2623. [PubMed] [Google Scholar]
- Ghesquier D., Cook L., Nagi M. N., MacAlister T. J., Cinti D. L. Source of the hepatic microsomal trans-2-enoyl CoA hydratase bifunctional protein: endoplasmic reticulum or peroxisomes. Arch Biochem Biophys. 1987 Feb 1;252(2):369–381. doi: 10.1016/0003-9861(87)90043-9. [DOI] [PubMed] [Google Scholar]
- Hiltunen J. K., Palosaari P. M., Kunau W. H. Epimerization of 3-hydroxyacyl-CoA esters in rat liver. Involvement of two 2-enoyl-CoA hydratases. J Biol Chem. 1989 Aug 15;264(23):13536–13540. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. X., Smeland T. E., Schulz H. D-3-hydroxyacyl coenzyme A dehydratase from rat liver peroxisomes. Purification and characterization of a novel enzyme necessary for the epimerization of 3-hydroxyacyl-CoA thioesters. J Biol Chem. 1990 Aug 15;265(23):13629–13634. [PubMed] [Google Scholar]
- Nagi M. N., Cook L., Ghesquier D., Cinti D. L. Site of inhibition of rat liver microsomal fatty acid chain elongation system by dec-2-ynoyl coenzyme A. Possible mechanism of inhibition. J Biol Chem. 1986 Oct 15;261(29):13598–13605. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Osumi T., Hashimoto T. Peroxisomal beta oxidation system of rat liver. Copurification of enoyl-CoA hydratase and 3-hydroxyacyl-CoA dehydrogenase. Biochem Biophys Res Commun. 1979 Jul 27;89(2):580–584. doi: 10.1016/0006-291x(79)90669-7. [DOI] [PubMed] [Google Scholar]
- Palosaari P. M., Hiltunen J. K. Peroxisomal bifunctional protein from rat liver is a trifunctional enzyme possessing 2-enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and delta 3, delta 2-enoyl-CoA isomerase activities. J Biol Chem. 1990 Feb 15;265(5):2446–2449. [PubMed] [Google Scholar]
- Reddy M. K., Hollenberg P. F., Reddy J. K. Partial purification and immunoreactivity of an 80 000-molecular-weight polypeptide associated with peroxisome proliferation in rat liver. Biochem J. 1980 Jun 15;188(3):731–740. doi: 10.1042/bj1880731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- STERN J. R., DEL CAMPILLO A. Enzymes of fatty acid metabolism. II. Properties of crystalline crotonase. J Biol Chem. 1956 Feb;218(2):985–1002. [PubMed] [Google Scholar]
- STERN J. R., DEL CAMPILLO A., RAW I. Enzymes of fatty acid metabolism. I. General introduction; crystalline crotonase. J Biol Chem. 1956 Feb;218(2):971–983. [PubMed] [Google Scholar]
- Schulz H. Long chain enoyl coenzyme A hydratase from pig heart. J Biol Chem. 1974 May 10;249(9):2704–2709. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Steinman H. M., Hill R. L. Bovine liver crotonase (enoyl coenzyme A hydratase). EC 4.2.1.17 L-3-hydroxyacyl-CoA hydrolyase. Methods Enzymol. 1975;35:136–151. doi: 10.1016/0076-6879(75)35149-5. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Pruss H. D. Synthesis of eicosa-2-trans-8,11,14-all cis-tetraenoic acid-3-14C and DL-3-hydroxy eicosa-8,11,14-all cis-trienoic acid-3-14C. J Lipid Res. 1967 May;8(3):196–201. [PubMed] [Google Scholar]
- Wakil S. J., Stoops J. K., Joshi V. C. Fatty acid synthesis and its regulation. Annu Rev Biochem. 1983;52:537–579. doi: 10.1146/annurev.bi.52.070183.002541. [DOI] [PubMed] [Google Scholar]
- Waterson R. M., Hill R. L. Enoyl coenzyme A hydratase (crotonase). Catalytic properties of crotonase and its possible regulatory role in fatty acid oxidation. J Biol Chem. 1972 Aug 25;247(16):5258–5265. [PubMed] [Google Scholar]
- Yasukochi Y., Masters B. S. Some properties of a detergent-solubilized NADPH-cytochrome c(cytochrome P-450) reductase purified by biospecific affinity chromatography. J Biol Chem. 1976 Sep 10;251(17):5337–5344. [PubMed] [Google Scholar]