Abstract
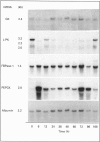
Levels of mRNA for glucokinase, L-pyruvate kinase, fructose-1,6-bisphosphatase and phosphoenolpyruvate carboxykinase were analysed during liver regeneration. Levels of mRNA for glycolytic enzymes (glucokinase and L-pyruvate kinase) decreased rapidly after partial hepatectomy. Glucokinase mRNA increased at 16-24 h, returning to normal values after this time. L-pyruvate kinase mRNA recovered control levels at 168 h. In contrast, phosphoenolpyruvate carboxykinase mRNA increased rapidly after liver resection and remained high during the regenerative process. However, the levels of fructose-1,6-bisphosphatase mRNA were not modified significantly. These results correlate with the reported increased rate of gluconeogenesis and changes in enzyme levels after partial hepatectomy. The effect of stress on the mRNA levels was also studied. All enzymes showed variations in their mRNA levels after the surgical stress. In general, the differences were more pronounced in regenerating liver than in sham-operated animals, being practically normalized at 24 h.
Full text
PDF
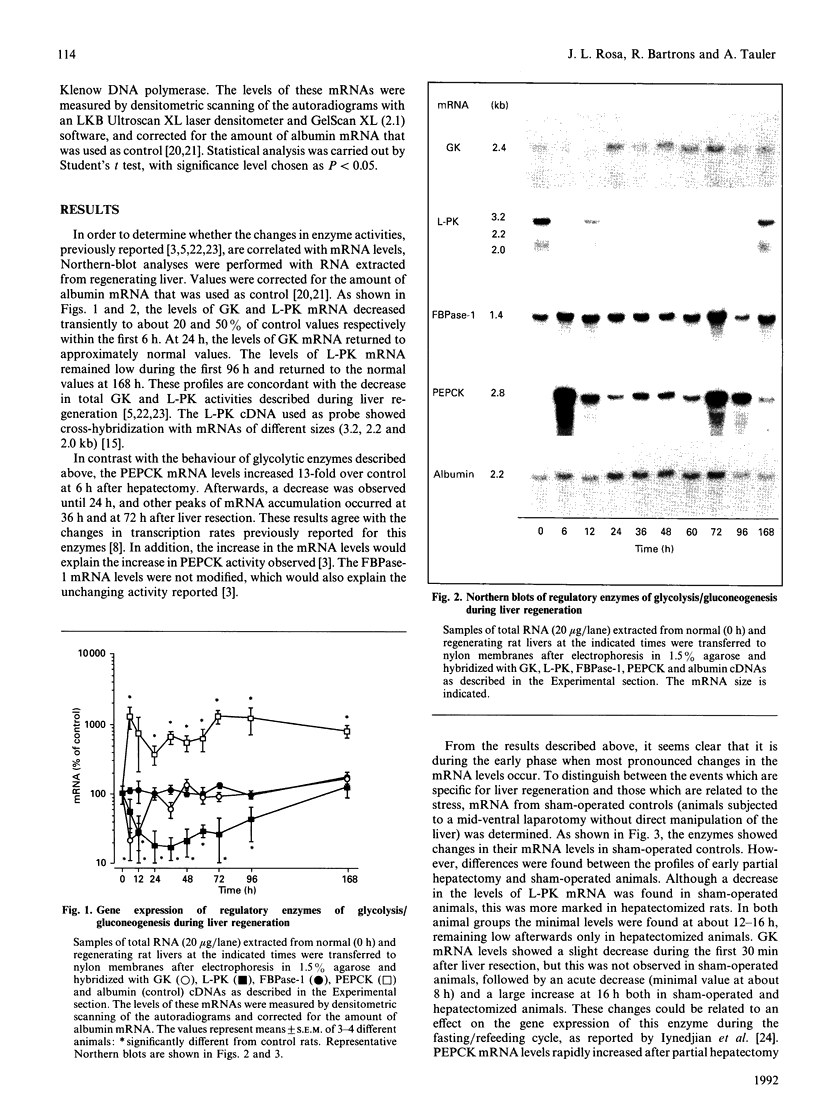


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreone T. L., Printz R. L., Pilkis S. J., Magnuson M. A., Granner D. K. The amino acid sequence of rat liver glucokinase deduced from cloned cDNA. J Biol Chem. 1989 Jan 5;264(1):363–369. [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Barbiroli B., Potter V. R. DNA synthesis and interaction between controlled feeding schedules and partial hepatectomy in rats. Science. 1971 May 14;172(3984):738–741. doi: 10.1126/science.172.3984.738. [DOI] [PubMed] [Google Scholar]
- Bonney R. J., Hopkins H. A., Walker P. R., Potter V. R. Glycolytic isoenzymes and glycogen metabolism in regenerating liver from rats on controlled feeding schedules. Biochem J. 1973 Sep;136(1):115–124. doi: 10.1042/bj1360115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher N. L., Weir G. C. Insulin, glucagon, liver regeneration, and DNA synthesis. Metabolism. 1976 Nov;25(11 Suppl 1):1423–1425. doi: 10.1016/s0026-0495(76)80156-4. [DOI] [PubMed] [Google Scholar]
- Fausto N., Mead J. E. Regulation of liver growth: protooncogenes and transforming growth factors. Lab Invest. 1989 Jan;60(1):4–13. [PubMed] [Google Scholar]
- Granner D., Pilkis S. The genes of hepatic glucose metabolism. J Biol Chem. 1990 Jun 25;265(18):10173–10176. [PubMed] [Google Scholar]
- Hers H. G., Hue L. Gluconeogenesis and related aspects of glycolysis. Annu Rev Biochem. 1983;52:617–653. doi: 10.1146/annurev.bi.52.070183.003153. [DOI] [PubMed] [Google Scholar]
- Iynedjian P. B., Pilot P. R., Nouspikel T., Milburn J. L., Quaade C., Hughes S., Ucla C., Newgard C. B. Differential expression and regulation of the glucokinase gene in liver and islets of Langerhans. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7838–7842. doi: 10.1073/pnas.86.20.7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz N. Correlation between rates and enzyme levels of increased gluconeogenesis in rat liver and kidney after partial hepatectomy. Eur J Biochem. 1979 Aug 1;98(2):535–542. doi: 10.1111/j.1432-1033.1979.tb13214.x. [DOI] [PubMed] [Google Scholar]
- Koide Y., Earp H. S., Ong S. H., Steiner A. L. Alterations in the intracellular distribution of cGMP and guanylate cyclase activity during rat liver regeneration. J Biol Chem. 1978 Jun 25;253(12):4439–4445. [PubMed] [Google Scholar]
- Leffert H. L., Koch K. S., Moran T., Rubalcava B. Hormonal control of rat liver regeneration. Gastroenterology. 1979 Jun;76(6):1470–1482. [PubMed] [Google Scholar]
- Lone Y. C., Simon M. P., Kahn A., Marie J. Complete nucleotide and deduced amino acid sequences of rat L-type pyruvate kinase. FEBS Lett. 1986 Jan 20;195(1-2):97–100. doi: 10.1016/0014-5793(86)80138-7. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G. K. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990 Feb 1;4(2):176–187. [PubMed] [Google Scholar]
- Milland J., Schreiber G. Transcriptional activity of the phosphoenolpyruvate carboxykinase gene decreases in regenerating rat liver. FEBS Lett. 1991 Feb 25;279(2):184–186. doi: 10.1016/0014-5793(91)80144-r. [DOI] [PubMed] [Google Scholar]
- Mohn K. L., Laz T. M., Melby A. E., Taub R. Immediate-early gene expression differs between regenerating liver, insulin-stimulated H-35 cells, and mitogen-stimulated Balb/c 3T3 cells. Liver-specific induction patterns of gene 33, phosphoenolpyruvate carboxykinase, and the jun, fos, and egr families. J Biol Chem. 1990 Dec 15;265(35):21914–21921. [PubMed] [Google Scholar]
- Petenusci S. O., Freitas T. C., Roselino E. S., Migliorini R. H. Glucose homeostasis during the early stages of liver regeneration in fasted rats. Can J Physiol Pharmacol. 1983 Mar;61(3):222–228. doi: 10.1139/y83-034. [DOI] [PubMed] [Google Scholar]
- Petropoulos C., Andrews G., Tamaoki T., Fausto N. alpha-Fetoprotein and albumin mRNA levels in liver regeneration and carcinogenesis. J Biol Chem. 1983 Apr 25;258(8):4901–4906. [PubMed] [Google Scholar]
- Pilkis S. J., el-Maghrabi M. R., Claus T. H. Fructose-2,6-bisphosphate in control of hepatic gluconeogenesis. From metabolites to molecular genetics. Diabetes Care. 1990 Jun;13(6):582–599. doi: 10.2337/diacare.13.6.582. [DOI] [PubMed] [Google Scholar]
- Pilkis S. J., el-Maghrabi M. R., Claus T. H. Hormonal regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Biochem. 1988;57:755–783. doi: 10.1146/annurev.bi.57.070188.003543. [DOI] [PubMed] [Google Scholar]
- Rosa J. L., Tauler A., Lange A. J., Pilkis S. J., Bartrons R. Transcriptional and posttranscriptional regulation of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase during liver regeneration. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3746–3750. doi: 10.1073/pnas.89.9.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa J. L., Ventura F., Carreras J., Bartrons R. Fructose 2,6-bisphosphate and 6-phosphofructo-2-kinase during liver regeneration. Biochem J. 1990 Sep 15;270(3):645–649. doi: 10.1042/bj2700645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield P. S., Kerbey A. L., Sugden M. C. Hepatic pyruvate metabolism during liver regeneration after partial hepatectomy in the rat. Int J Biochem. 1986;18(5):453–458. doi: 10.1016/0020-711x(86)90188-6. [DOI] [PubMed] [Google Scholar]
- Sobczak J., Tournier M. F., Lotti A. M., Duguet M. Gene expression in regenerating liver in relation to cell proliferation and stress. Eur J Biochem. 1989 Mar 1;180(1):49–53. doi: 10.1111/j.1432-1033.1989.tb14613.x. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E. Fructose 2,6-bisphosphate. Adv Enzymol Relat Areas Mol Biol. 1987;59:315–395. doi: 10.1002/9780470123058.ch7. [DOI] [PubMed] [Google Scholar]
- Yoo-Warren H., Monahan J. E., Short J., Short H., Bruzel A., Wynshaw-Boris A., Meisner H. M., Samols D., Hanson R. W. Isolation and characterization of the gene coding for cytosolic phosphoenolpyruvate carboxykinase (GTP) from the rat. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3656–3660. doi: 10.1073/pnas.80.12.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Maghrabi M. R., Pilkis J., Marker A. J., Colosia A. D., D'Angelo G., Fraser B. A., Pilkis S. J. cDNA sequence of rat liver fructose-1,6-bisphosphatase and evidence for down-regulation of its mRNA by insulin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8430–8434. doi: 10.1073/pnas.85.22.8430. [DOI] [PMC free article] [PubMed] [Google Scholar]