ABSTRACT
BACKGROUND:
This study investigates the effects of hydroxychloroquine (HCQ) on a sepsis-induced acute respiratory distress syndrome (ARDS) model in rats, initiated by a fecal intraperitoneal injection procedure (FIP).
METHODS:
Three groups were established: control (n=8), FIP + saline (n=7), and FIP + HCQ (20 mg/kg/day) (n=9). Blood samples were collected for arterial blood gas and biochemical analyses, and bilateral pneumonectomy was performed for histopathologic examination.
RESULTS:
In the FIP + saline group, PaO2 decreased and PaCO2 increased, whereas these levels normalized in the FIP + HCQ group compared to the control (p<0.001 and p<0.05, respectively). Histopathological scores for alveolar congestion, perivascular/interstitial edema, hemorrhage in alveolar tissue, leukocyte infiltration or aggregation in air spaces/vascular walls, and alveolar wall/hyaline membrane thickness increased in the FIP + saline group compared to the control group (p<0.01). These scores decreased in the FIP + HCQ group compared to the FIP + saline group (p<0.01). HCQ reversed the sepsis-induced increase in malondialdehyde, tumor necrosis factor-alpha, interleukin-6, and lactic acid.
CONCLUSION:
HCQ may be an effective and safe option to mitigate the severe progression of ARDS.
Keywords: Acute respiratory distress syndrome, hydroxychloroquine, lung, sepsis
INTRODUCTION
Sepsis and septic shock present global challenges across all healthcare systems. The pathogenesis of sepsis involves an extreme response by inflammatory mediators to pathogens infecting the body. Patients admitted to critical care units (ICUs) exhibit a high death rate from septic shock.[1] A recent large-scale, multicenter study revealed high prevalence rates of sepsis, severe sepsis, and septic shock (10.9%, 17.3%, and 13.5%, respectively) in Turkish ICUs, along with unacceptably high mortality rates for severe sepsis and septic shock (55.7% and 70.4%, respectively).[1]
Acute respiratory distress syndrome (ARDS), a diverse phenomenon, can be a severe consequence of sepsis. It involves basic mechanisms characterized by endothelial dysfunction and inflammation.[2] The incidence of ARDS in adult sepsis patients ranges from 6-7% across various populations.[3,4] Severe sepsis is the most common etiology of ARDS, which often progresses rapidly in septic patients and is associated with a higher risk of in-hospital death.[3,4] Mortality rates for sepsis-induced ARDS were found to exceed those for ARDS caused by other factors.[5] Currently, there is no specific treatment for sepsis-induced ARDS. The common treatment strategies for ARDS, which rely on the sufficient delivery of oxygen to tissues through mechanical ventilation, are similar across patients with different etiologies.[2,6] Consequently, diagnosing and treating sepsis in patients at risk of developing ARDS should be prioritized in each healthcare system.
Hydroxychloroquine (HCQ) is one of several antirheumatic medications that modify disease processes and are widely used to treat various rheumatic diseases and autoimmune conditions. HCQ is employed in both the prophylaxis and treatment of malaria due to its strong immunomodulatory properties that prevent organ damage and inflammatory flare-ups.[7] Additionally, HCQ is considered to potentially exert an antiviral effect and may effectively prevent the severe progression of coronavirus disease 2019 (COVID-19) to acute respiratory distress syndrome by inhibiting a cytokine storm through the suppression of T cell activation.[8] Due to its safety, affordability, and broad availability, HCQ is extensively used in diverse populations, including children and pregnant women, often with little or no monitoring.[9] However, there is no data on the therapeutic effects of HCQ in sepsis-induced ARDS.
This research aims to determine how HCQ medication affects blood gas levels, lipid peroxidation, histopathogenesis, and inflammatory levels in rats with ARDS caused by sepsis.
MATERIALS AND METHODS
Animals
Thirty-three adult male Wistar albino rats, weighing 200-250 g and aged 12 weeks, were used in this investigation. The study received approval from Demiroglu Science University’s animal ethics committee (approval number 212378/c, July 25, 2020). All animal experiments were conducted in compliance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines and the National Institute of Health’s guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). The rats were procured from Demiroglu Science University’s Experimental Animal Laboratory. They were housed in pairs in steel cages under a 12-hour light/dark cycle in a temperature-controlled environment (22 °C±2 °C) and had free access to food.
Experimental Procedures
Using the fecal intraperitoneal injection technique (FIP) model, sepsis was induced in 25 of the rats. This model was based on a technique previously reported by Shrum et al.[10] and Tyml et al.[11] The rats were randomly divided into two groups: 12 were assigned to the FIP and HCQ group, and 13 to the FIP and saline group. Each rat received an intraperitoneal injection of 1 g of feces per kg of body weight, with the feces suspended in saline. Nine rats were excluded from the trial after they died during the first 48 hours of the procedure—six from the FIP + saline group and three from the FIP + HCQ group.
The three study groups were established as follows: Group 1 served as the control group (non-operative and received oral feeding, n=8); Group 2 received FIP and an oral gavage dose of 0.9% NaCl (saline) (n=7); and Group 3 received FIP and an oral gavage dose of 20 mg/kg/day of HCQ (Plaquenil 200 mg, Sanofi Aventis) (n=9). To anesthetize the rats for the procedure, an intraperitoneal injection containing 80 mg/kg of ketamine hydrochloride and 40 mg/kg of xylazine hydrochloride was administered. Each treatment session lasted for two days.
In order to obtain blood samples for biochemical examination, bilateral pneumonectomy was performed for histological evaluation at the conclusion of the inquiry. Furthermore, the animals were euthanized using 150 mg/kg of ketamine hydrochloride and 100 mg/kg of xylazine hydrochloric acid.
Arterial Blood Gas Analysis
Twenty-four hours post-operatively, a blood gas analyzer was used to measure the levels of PaCO2 and PaO2 in 0.2 mL of carotid artery blood from each group of rats.
Histopathological Examination of the Lungs
For morphological examination, 200 mL of 4% formaldehyde in 0.1 M phosphate-buffered saline was perfused intraperitoneally to all animals to induce unconsciousness. Ketamine (40 mg/kg, Alfamine®, Alfasan International B.V., Holland) and xylazine (4 mg/kg, Alfazyne®) were also administered. Lung slices, 5 μm thick and fixed in formalin, were stained with hematoxylin and eosin. Photographs of each segment were taken using an Olympus C-5050 digital camera mounted on an Olympus BX51 microscope. The primary lung damage score was assessed based on the previously mentioned histopathology criteria.[12] The methods used to assess histological lung injury included grading the thickness of the alveolar wall and hyaline membrane formation (TA), alveolar congestion (AC), hemorrhage (H), perivascular/interstitial edema (PE), as well as the infiltration or aggregation of leukocytes in air spaces and vessel walls (AL). Each item’s severity was ranked as follows: one (0% to 25%), two (25% to 50%), three (50% to 75%), or four (75% to 100%).
Measurement of Lipid Peroxidation
Lipid peroxidation was assessed by quantifying malondialdehyde (MDA) levels as thiobarbituric acid reactive species (TBARS) in plasma samples. The plasma samples were mixed with TBARS and trichloroacetic acid reagent, incubated for at 100°C 60 minutes, and then mixed again. The samples were cooled on ice prior to centrifugation for 20 minutes at 3000 rpm. The absorbance of the supernatant was then measured at 535 nm. The unit of measurement for MDA levels was nM, with tetraethoxypropane used as the calibration standard.
Measurement of Plasma Levels of Inflammatory Markers and Lactic Acid
Commercial ELISA (Enzyme-Linked Immunosorbent Assay) kits (Biosciences, Abcam) were used to measure plasma levels of tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6). Following the manufacturer’s guide, mean values of TNF-α and IL-6 were calculated from triplicate samples after diluting the plasma 1:2. Lactic acid concentration was determined using a blood gas analyzer.
Statistical Analysis
Data analysis was conducted using SPSS (Statistical Package for the Social Sciences) version 15.0 for Windows. Results were presented as the mean plus the standard error of the mean. Initial data analysis utilized one-way analyses of variance (ANOVA). Post hoc comparisons between groups were conducted using the Tukey’s HSD (Honestly Significant Difference) test. Student’s t-test was employed to compare treatment values to control values, with a significance threshold set at a p-value greater than 0.05. The software program used was SPSS, developed by IBM Corporation, headquartered in Armonk, New York, USA.
RESULTS
Of the 33 rats initially used in the investigation, six from the FIP + saline group and three from the FIP + HCQ group perished within the first 48 hours post-procedure due to complications arising from FIP and were excluded from the study. The samples from the remaining 24 rats were analyzed as follows.
Microscopical Findings from Lung Tissue in Sepsis Treated with HCQ
The lung tissue of the control group exhibited typical interstitial and alveolar architecture, characterized by a smooth, pale pink surface, good expansion, a soft texture, and no visible anomalies (Figures 1a and 1b). In contrast, lung tissue from the FIP + saline group displayed typical signs of sepsis, such as a dark appearance, significant leukocyte infiltration in air spaces and vessel walls, localized alveolar hemorrhage, noticeable alveolar wall thickening, and perivascular or interstitial edema (Figures 1c and 1d). The rats in the FIP + HCQ group showed minor pulmonary histopathological changes, with reduced leukocyte infiltration and interstitial edema (Figures 1e and 1f).
Figure 1.
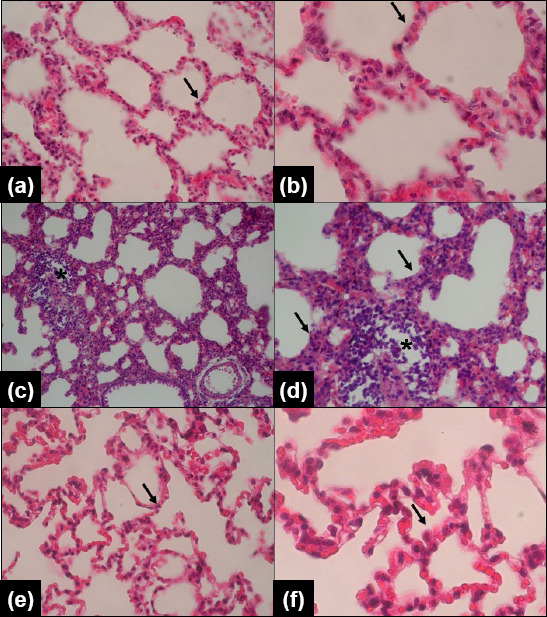
Microscopic images of lung tissue stained with Hematoxylin and Eosin (H&E, magnification: x20 and x40). a,b: Control group, displaying normal alveolar and interstitial structure (indicated by an arrow). c,d: FIP (fecal intraperitoneal injection procedure, sepsis induced) + saline group, showing leukocyte infiltration in air spaces and vessel walls (marked with*) and perivascular/interstitial edema (indicated by an arrow). e&f: FIP + 10 mg/kg hydroxychloroquine (HCQ) group, demonstrating reduced leukocyte infiltration and interstitial edema (indicated by an arrow).
Arterial Blood Gas Findings in Sepsis Treated with HCQ
Arterial blood gas analysis results are presented in Table 1. The mean PaO2 level was considerably lower in the FIP + saline group compared with the control group (p<0.001) and significantly higher in the FIP + HCQ group compared with the FIP + saline group (p<0.001). The mean PaCO2 level increased significantly in the FIP + saline group compared with the control group (p<0.05) and decreased significantly in the FIP + HCQ group compared with the FIP + saline group (p<0.05). These results demonstrate the effectiveness of HCQ in treating ARDS in the FIP model rats.
Table 1.
Comparison of histopathological scores among three groups
| Histopathological score | Control | FIP+Saline | FIP+HCQ |
|---|---|---|---|
| Alveolar congestion | 0.5±0.1 | 3.3±0.2** | 1.2±0.3# |
| Hemorrhage | 0.1±0.1 | 2.2±0.4* | 1.3±0.5# |
| Infiltration/aggregation of leukocytes in air spaces/vessel walls | 0.2±0.1 | 2.1±0.3* | 0.8±0.4## |
| Perivascular/interstitial edema | 0.5±0.2 | 3.2±0.3** | 1.4±0.5# |
| Thickness of the alveolar wall/hyaline membrane formation | 0.3±0.1 | 2.8±0.2* | 1.5±0.5# |
Results are presented as mean±standard error of the mean.
p<0.01;
p<0.0001 vs. Control group;
p<0.01;
p<0.001 vs. FIP+Saline group. FIP: Fecal intraperitoneal injection Group; HCQ: Hydroxychloroquine.
Histopathological Findings from the Lungs in Sepsis Treated with HCQ
Histopathological scores for lung damage are presented in Table 2. The mean scores for alveolar congestion and perivascular/interstitial edema in alveolar tissue considerably increased in the FIP + saline group compared with the control group (p<0.0001), while these scores decreased in the FIP + HCQ group compared with the FIP + saline group (p<0.01). The frequency of hemorrhages in alveolar tissue, the thickness of the alveolar wall/hyaline membrane, and the aggregation or infiltration of leukocytes in air spaces/vessel walls significantly increased in the FIP + saline group compared with the control group (p<0.01). These scores decreased in the FIP + HCQ group compared with the FIP + saline group (p<0.01). All these findings demonstrated the effectiveness of HCQ in the treatment of sepsis in FIP model rats.
Table 2.
Comparison of biochemical parameters among three groups
| Parameter | Control | FIP+Saline | FIP + HCQ |
|---|---|---|---|
| MDA (nM) | 68.2±4.2 | 205.2±14.5** | 91.1±10.6## |
| TNF-α (pg/ml) | 14.3±3.1 | 229.7±9.4** | 70.8±13.2## |
| IL-6 (pg/ml) | 20.9±5.7 | 81.9±8.8** | 29.7±5.6## |
| Lactic acid (mmol/L) | 1.5±0.5 | 6.8±0.8* | 3.9±0.4# |
Results are presented as mean±standard error of the mean.
p<0.001;
p<0.0001 vs. Control group.
p<0.01;
p<0.0001 vs. FIP+Saline group. FIP: Fecal intraperitoneal injection Group; HCQ: Hydroxychloroquine.
Lipid Peroxidation in Sepsis Treated with HCQ
Plasma MDA concentration reflects the level of lipid peroxidation in sepsis-induced ARDS. The mean concentration of MDA in the blood of the FIP + saline group was substantially higher than that of the control group (p<0.0001), while it was significantly lower in the FIP + HCQ group compared with the FIP + saline group (p<0.0001) (Table 3).
Plasma Levels of Inflammatory Markers and Acidosis in Sepsis Treated with HCQ
Plasma levels of TNF-α and IL-6 significantly increased in the FIP + saline group compared with the control group (p<0.0001), while these levels decreased in the FIP + HCQ group compared with the FIP + saline group (p<0.0001). The lactic acid concentration in the FIP + saline group was significantly higher than in the control group (p<0.001), and significantly lower in the FIP + HCQ group compared with the FIP + saline group (p<0.01) (Table 3). All these findings suggest that HCQ is effective in reducing lung damage in sepsis-induced ARDS.
DISCUSSION
Sepsis-induced ARDS is a primary cause of high mortality rates in ICUs. ARDS and sepsis share similar underlying mechanisms, although they differ in how they affect the lungs. Lung-protective mechanical ventilation is the principal strategy in the treatment of ARDS patients. Options for treating patients with refractory hypoxemia include invasive mechanical ventilation,[13] administration of a neuromuscular blocking agent,[14] and extracorporeal membrane oxygenation.[15] Other experimental and clinical studies have utilized anti-inflammatory therapies, including corticosteroids,[16] platelet inhibitors,[17] and statins.[18] However, no studies have yet explored the efficacy of HCQ in managing ARDS induced by sepsis. In this study, we have demonstrated the effectiveness of HCQ in managing ARDS induced by sepsis in rats, across microscopical, histopathological, and biochemical levels.
Assessment and treatment of acid-base status are often necessary, as severe sepsis can lead to shock associated with multisystem organ failure, acute renal injury, metabolic acidosis, and organ dysfunction.[19] Traditionally, arterial blood gas analysis, including measurements of PaO2 and PaCO2, is used to evaluate acid-base status.[20] In our study of sepsis-induced rats, HCQ treatment significantly increased the mean level of PaO2 and significantly decreased the mean level of PaCO2, further demonstrating the efficacy of HCQ in treating sepsis-induced ARDS.
ARDS involves increased permeability of alveolar epithelial cells and pulmonary capillary endothelial cells, making it a heterogeneous condition. Sepsis may indirectly cause lung injury. This indirect injury begins with hypoxemia-induced systemic endothelial damage that triggers a systemic inflammatory response. Additionally, the actions of free radicals and immune destruction lead to altered levels of inflammatory mediators.[21,22] In an in vitro study, Lu et al. evaluated the therapeutic potential of HCQ as an autophagy inhibitor in bacterial infection-induced sepsis. They noted that autophagy contributes to the progression of sepsis by exacerbating the cytokine storm and vascular leakage.[23] They found that HCQ effectively reduced cytokine production by human leukocytes in response to infection and decreased endothelial hyperpermeability. Furthermore, they demonstrated that while pretreatment with HCQ was ineffective, post-treatment with HCQ effectively protected mice from fatality caused by bacterial infections by simultaneously reducing vascular leakage, cytokine production, and enhancing bacterial clearance.[23] In this study, we also demonstrate that HCQ treatment significantly reduced the aggregation or infiltration of leukocytes in vessel walls or air spaces, hemorrhages in alveolar tissue, the thickness of the hyaline membrane or alveolar wall, and perivascular/interstitial edema in alveolar tissue, as well as alleviated alveolar congestion in FIP rats. The microscopical images of the lung tissues supported these outcomes. All these findings suggest that HCQ is an effective treatment for sepsis in the sepsis-induced ARDS model.
In a study conducted by Sarma et al.,[24] hydroxychloroquine showed potential in decreasing the progression of lung disease in COVID-19 patients, as observed through radiological progression. Its safety profile is similar to that of control/conventional treatment. However, further data are required to establish a definitive conclusion. Moreover, Zhao et al.[25] note that hydroxychloroquine has a limited impact on the regenerative capacity of epithelial stem/progenitor cells. This highlights the value of organoid technologies and lung injury repair mouse models in evaluating the medication’s effectiveness in COVID-19. In our study, although we could not observe radiological evidence of a healing effect, we can infer improvements from better histopathology after hydroxychloroquine use.
Tissue and serum concentrations of MDA are indicators of lipid peroxidation levels during sepsis. In rats subjected to lethal sepsis induced by intraperitoneal injection of multidrug-resistant (MDR) bacterial isolates, MDA concentrations in the spleen, liver, and aorta were noticeably higher compared to control animals.[26] These results paralleled the clinical outcomes of patients with ventilator-associated pneumonia caused by MDR bacteria, where increased serum MDA concentrations during the first seven days of the disease were associated with survival in sepsis-induced hepatic or cardiac failure.[27] In the present study, FIP notably elevated the mean concentration of MDA in serum compared to the control group, while HCQ treatment reduced this concentration, suggesting that HCQ successfully prevents lipid peroxidation in sepsis-induced ARDS.
Sepsis involves two mostly concomitant inflammatory stages: a pro-inflammatory stage known as the Systemic Inflammatory Response Syndrome (SIRS) and an anti-inflammatory stage known as the Compensatory Anti-inflammatory Response Syndrome. Initial observations from animal models responding to lipopolysaccharide (LPS) or bacterial infections that mimicked sepsis led to the early belief that SIRS was the main cause of death in sepsis. Several therapeutic interventions have been developed to reduce the pro-inflammatory septic response mediated by pro-inflammatory cytokines such as interleukins and TNF-α, although some have been unsuccessful in treating sepsis.[28] In our sepsis-induced model, HCQ successfully reduced the plasma levels of TNF-α compared with the elevated levels observed in the untreated sepsis group. This suggests that HCQ is effective in mitigating lung damage in the sepsis-induced ARDS model.
Pro-inflammatory cytokines (IL-6, IL-18, IL-8, and TNF-α) and anti-inflammatory cytokines (IL-10) increase in sepsis in response to pathogen infection. IL-6 supports the host’s defense mechanisms against tissue damage and infections. Notably, lower levels of IL-6 are associated with a better prognosis. Conversely, elevated levels of IL-6 can trigger a “cytokine storm,” an acute, intense systemic inflammatory response often observed in sepsis patients. Additionally, high levels of IL-6 can activate the coagulation pathway and response in vascular endothelial cells.[29] A humanized anti-IL-6 receptor antibody, tocilizumab, has shown beneficial effects in patients with cytokine release syndrome complicated by T-cell engaged therapy.[30] In our study, we also demonstrated the beneficial effects of HCQ on sepsis-induced IL-6 synthesis in rats with FIP. HCQ’s ability to increase intracellular pH and inhibit lysosomal activity in antigen-presenting cells (APCs) may underlie these effects of HCQ, subsequently limiting antigen processing and major histocompatibility complex (MHC) class II-mediated autoantigen presentation to T cells.[31] This procedure reduces T cell activation, T cell and B cell differentiation, and the expression of cytokines such as IL-6 and TNF-α.[32] These mechanisms support the hypothesis that HCQ could provide early protection against the progression of ARDS, which is caused by an overactivation of the immune system triggered by sepsis. Previous studies using the same sepsis model treated with anti-inflammatory and antioxidant drugs like digitoxin,[33] octreotide,[34] beta blockers[35] gallic acid,[36] and folic acid[37] have shown similar results in sepsis-induced ARDS. However, advanced in vivo clinical studies are necessary to validate this hypothesis.
Since acidosis—particularly the onset of lactic acidosis—is a common complication of sepsis, assessing the patient’s acid-base state has become a standard component of diagnosing and treating sepsis. Given that sepsis patients often present with mixed acid-base disturbances and variable pH levels, plasma concentrations of lactic acid realistically offer useful data for clinical decision-making in sepsis.[20] Additionally, hyperlactatemia upon hospital admission is an early predictor of outcomes and is associated with increased mortality risk.[38] In the current study, the plasma concentration of lactic acid in sepsis-induced rats was significantly higher than in the control group, while it was notably lower in sepsis-induced rats treated with HCQ, suggesting that HCQ successfully reduced lung damage in the sepsis-induced ARDS model.
Study Limitations
A significant limitation of this experimental study is that the clinical relevance of animal study results can be challenging when adapting a medication for therapeutic use. Therefore, clinical trials involving patients hospitalized for sepsis-induced ARDS are needed to evaluate the effects of HCQ on the pathophysiology of the disease and to determine the clinical relevance of HCQ’s efficacy and safety in treating sepsis, septic shock, and ARDS.
CONCLUSION
Currently, there are no universal therapeutic strategies for sepsis and ARDS. Our findings indicate that chloroquine-based agents like HCQ can be effective and safe options to combat the severe progression of sepsis in ICUs. However, more advanced in vitro and in vivo studies are necessary to further validate the efficacy and safety of HCQ in treating sepsis, septic shock, and ARDS.
Footnotes
Ethics Committee Approval: This study was approved by the Demiroğlu Science University Faculty of Medicine Ethics Committee (Date: 25.07.2020, Decision No: 212378/c).
Peer-review: Externally peer-reviewed.
Authorship Contributions: oncept: O.S.Ç., O.E.; Design: O.E., G.E.; Supervision: O.S.Ç., G.E.; Resource: O.S.Ç.; Materials: R.K.; Data collection and/or processing: E.S.B.; Analysis and/or interpretation: G.E.; Literature search: O.E.; Writing: E.S.B., R.K.; Critical review: R.K., O.E.
Conflict of Interest: None declared.
Use of AI for Writing Assistance: Not declared.
Financial Disclosure: The author declared that this study has received no financial support.
REFERENCES
- 1.Baykara N, Akalın H, Arslantaş MK, Hancı V, Çağlayan Ç, Kahveci F, et al. Sepsis Study Group. Epidemiology of sepsis in intensive care units in Turkey:a multicenter, point-prevalence study. Crit Care. 2018;22:93. doi: 10.1186/s13054-018-2013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim WY, Hong SB. Sepsis and acute respiratory distress syndrome:recent update. Tuberc Respir Dis (Seoul) 2016;79:53–7. doi: 10.4046/trd.2016.79.2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, et al. U. S. Critical Illness and Injury Trials Group:Lung Injury Prevention Study Investigators (USCIITG-LIPS) Early identification of patients at risk of acute lung injury:evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183:462–70. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mikkelsen ME, Shah CV, Meyer NJ, Gaieski DF, Lyon S, Miltiades AN, et al. The epidemiology of acute respiratory distress syndrome in patients presenting to the emergency department with severe sepsis. Shock. 2013;40:375–81. doi: 10.1097/SHK.0b013e3182a64682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest. 2005;128:525–32. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 6.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus:a systematic review. Ann Rheum Dis. 2010;69:20–8. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 8.Zhou D, Dai SM, Tong Q. COVID-19:a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020;75:1667–70. doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brufsky A. Hyperglycemia, hydroxychloroquine, and the COVID-19 pandemic. J Med Virol. 2020;92:770–5. doi: 10.1002/jmv.25887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrum B, Anantha RV, Xu SX, Donnelly M, Haeryfar SM, McCormick JK, et al. A robust scoring system to evaluate sepsis severity in an animal model. BMC Res Notes. 2014;7:233. doi: 10.1186/1756-0500-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tyml K, Swarbreck S, Pape C, Secor D, Koropatnick J, Feng Q, et al. Voluntary running exercise protects against sepsis-induced early inflammatory and pro-coagulant responses in aged mice. Crit Care. 2017;21:210. doi: 10.1186/s13054-017-1783-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon WY, Suh GJ, Kim KS, Kwak YH. Niacin attenuates lung inflammation and improves survival during sepsis by downregulating the nuclear factor-[kappa)B pathway. Crit Care Med. 2011;39:328–34. doi: 10.1097/CCM.0b013e3181feeae4. [DOI] [PubMed] [Google Scholar]
- 13.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–55. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 14.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–16. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 15.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR):a multicentre randomised controlled trial. Lancet. 2009;374:1351–63. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–84. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 17.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest. 2009;119:3450–61. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Truwit JD, Bernard GR, Steingrub J, Matthay MA, Liu KD, Albertson TE, et al. BT National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370:2191–200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States:analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 20.White HD, Vazquez-Sandoval A, Quiroga PF, Song J, Jones SF, Arroliga AC. Utility of venous blood gases in severe sepsis and septic shock. Proc (Bayl Univ Med Cent) 2018;31:269–75. doi: 10.1080/08998280.2018.1460133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menezes SL, Bozza PT, Neto HC, Laranjeira AP, Negri EM, Capelozzi VL, et al. Pulmonary and extrapulmonary acute lung injury:inflammatory and ultrastructural analyses. J Appl Physiol (1985) 2005;98:1777–83. doi: 10.1152/japplphysiol.01182.2004. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Liu XY, Ma MM, Qi ZJ, Zhang XQ, Li Z, et al. Changes in intestinal microflora in rats with acute respiratory distress syndrome. World J Gastroenterol. 2014;20:5849–58. doi: 10.3748/wjg.v20.i19.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu LH, Chao CH, Yeh TM. Inhibition of autophagy protects against sepsis by concurrently attenuating the cytokine storm and vascular leakage. J Infect. 2019;78:178–86. doi: 10.1016/j.jinf.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Sarma P, Kaur H, Kumar H, Mahendru D, Avti P, Bhattacharyya A, et al. Virological and clinical cure in COVID-19 patients treated with hydroxychloroquine:A systematic review and meta-analysis. J Medical Virology. 2020;92:776–85. doi: 10.1002/jmv.25898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao F, Wang J, Wang Q, Hou Z, Zhang Y, Li X, et al. Organoid technology and lung injury mouse models evaluating effects of hydroxychloroquine on lung epithelial regeneration. Experimental Animals. 2022;71:316–28. doi: 10.1538/expanim.21-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toufekoula C, Papadakis V, Tsaganos T, Routsi C, Orfanos SE, Kotanidou A, et al. Compartmentalization of lipid peroxidation in sepsis by multidrug-resistant gram-negative bacteria:experimental and clinical evidence. Crit Care. 2013;17:R6. doi: 10.1186/cc11930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, et al. National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. Antimicrobial-resistant pathogens associated with healthcare-associated infections:summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013;34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 28.Shubin NJ, Monaghan SF, Ayala A. Anti-inflammatory mechanisms of sepsis. Contrib Microbiol. 2011;17:108–24. doi: 10.1159/000324024. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhry H, Zhou J, Zhong Y, Ali MM, McGuire F, Nagarkatti PS, et al. Role of cytokines as a double-edged sword in sepsis. In Vivo. 2013;27:669–84. [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959–70. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 31.Lotteau V, Teyton L, Peleraux A, Nilsson T, Karlsson L, Schmid SL, et al. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990;348:600–5. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- 32.Van den Borne BE, Dijkmans BA, de Rooij HH, le Cessie S, Verweij CL. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J Rheumatol. 1997;24:55–60. [PubMed] [Google Scholar]
- 33.Bora ES, Erdoğan MA, Özkul B, Sever IH, Söğüt I, Hürdağ C, et al. Short term protective effect of digitoxin in sepsis-induced acute lung injury. BIOCELL. 2022;46:433–39. [Google Scholar]
- 34.Bora SE, Erdoğan A, Erdoğan MA, Yiğitturk G, Çakır A, Erbaş O. Short-term protective effect of octreotide on the lungs of rats with experimentally induced sepsis. Ulus Travma Acil Cerrahi Derg. 2022;28:8–14. doi: 10.14744/tjtes.2020.02589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rezan K, Ejder SB, Hüseyin A, Yigit U, Ibrahim HS, et al. Exploring beta blockers'efficacy in sepsis-induced acute lung injury and HMGB1-sRAGE interaction. Int J Pharmacology. 2023;19:296–304. [Google Scholar]
- 36.Kardaş S, Çınaroğlu OS, Bora ES, Erbaş O. Gallic acid protects from sepsis-induced acute lung injury. Curr Issues Mol Biol. 2023;46:1–10. doi: 10.3390/cimb46010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yurtsever G, Bora Es, Eroğlu E, Uyanikgil Y, Erdoğan Ma, Erbaş O. Effect of folic acid in sepsis-induced lung damage in rats. med records. Ekim. 2023;5:87–92. [Google Scholar]
- 38.Samaraweera SA, Gibbons B, Gour A, Sedgwick P. Arterial versus venous lactate:a measure of sepsis in children. Eur J Pediatr. 2017;176:1055–60. doi: 10.1007/s00431-017-2925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


