Abstract
The major b-type cytochrome in microsomal membrane preparations from developing endosperm of castor bean (Ricinus communis) was cytochrome b5. Cytochrome P-450 was also present. The microsomal membranes had delta 12-hydroxylase activity and catalysed the NAD(P)H-dependent hydroxylation of oleate to yield ricinoleic acid. CO had no effect on the hydroxylase activity. Rabbit polyclonal antibodies were raised against the hydrophilic cytochrome b5 fragment purified from cauliflower (Brassica oleracea) floret microsomes. The anti-(cytochrome b5) IgG inhibited delta 12-hydroxylase, delta 12-desaturase and cytochrome c reductase activity in the microsomes. The results indicate that electrons from NAD(P)H were transferred to the site of hydroxylation via cytochrome b5 and that cytochrome P-450 was not involved.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
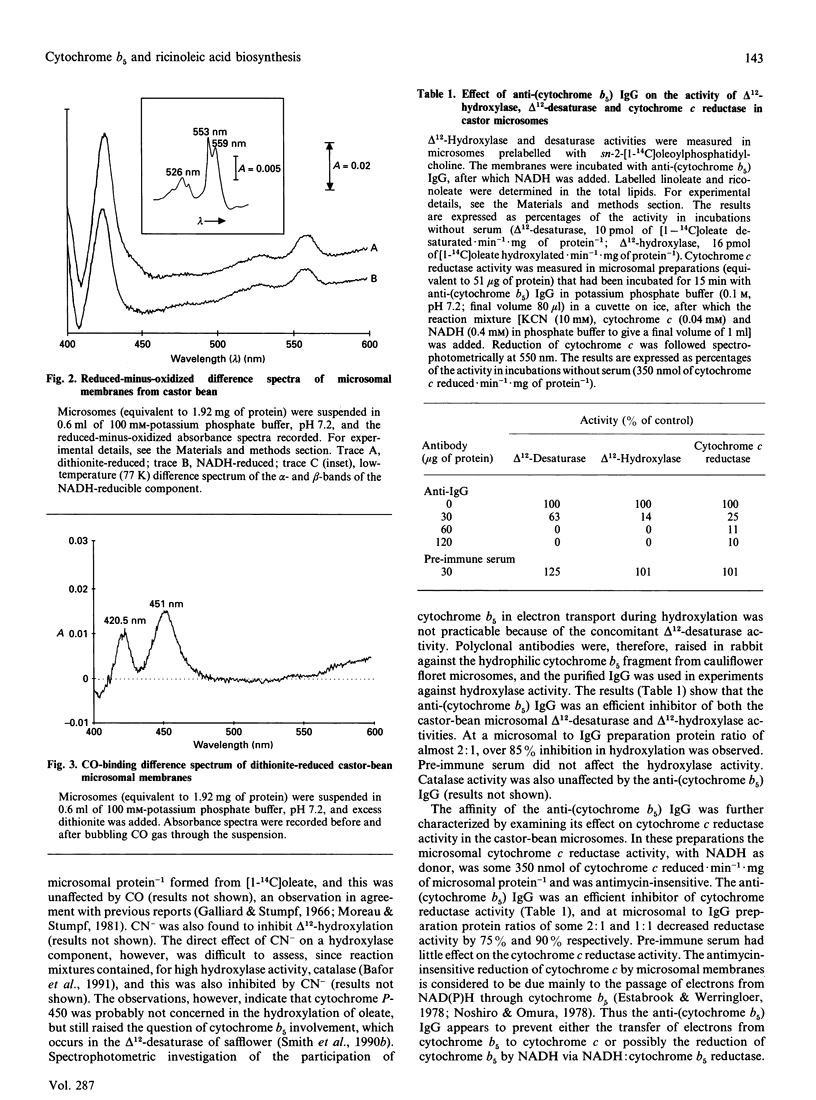
- Bafor M., Smith M. A., Jonsson L., Stobart K., Stymne S. Ricinoleic acid biosynthesis and triacylglycerol assembly in microsomal preparations from developing castor-bean (Ricinus communis) endosperm. Biochem J. 1991 Dec 1;280(Pt 2):507–514. doi: 10.1042/bj2800507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. P., Luster D. G. Multiple forms of plant cytochromes p-450. Plant Physiol. 1991 Jul;96(3):669–674. doi: 10.1104/pp.96.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch H. G., Catalá A., Strittmatter P. Mechanism of rat liver microsomal stearyl-CoA desaturase. Studies of the substrate specificity, enzyme-substrate interactions, and the function of lipid. J Biol Chem. 1976 Aug 25;251(16):5095–5103. [PubMed] [Google Scholar]
- Estabrook R. W., Werringloer J. The measurement of difference spectra: application to the cytochromes of microsomes. Methods Enzymol. 1978;52:212–220. doi: 10.1016/s0076-6879(78)52024-7. [DOI] [PubMed] [Google Scholar]
- Galliard T., Stumpf P. K. Fat metabolism in higher plants. 30. Enzymatic synthesis of ricinoleic acid by a microsomal preparation from developing Ricinus communis seeds. J Biol Chem. 1966 Dec 25;241(24):5806–5812. [PubMed] [Google Scholar]
- KATES M. SIMPLIFIED PROCEDURES FOR HYDROLYSIS OR METHANOLYSIS OF LIPIDS. J Lipid Res. 1964 Jan;5:132–135. [PubMed] [Google Scholar]
- Kearns E. V., Hugly S., Somerville C. R. The role of cytochrome b5 in delta 12 desaturation of oleic acid by microsomes of safflower (Carthamus tinctorius L.). Arch Biochem Biophys. 1991 Feb 1;284(2):431–436. doi: 10.1016/0003-9861(91)90319-e. [DOI] [PubMed] [Google Scholar]
- Moreau R. A., Stumpf P. K. Recent studies of the enzymic synthesis of ricinoleic Acid by developing castor beans. Plant Physiol. 1981 Apr;67(4):672–676. doi: 10.1104/pp.67.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L. J. The mechanism of ricinoleic acid biosynthesis in Ricinus communis seeds. Biochem Biophys Res Commun. 1967 Nov 17;29(3):311–315. doi: 10.1016/0006-291x(67)90454-8. [DOI] [PubMed] [Google Scholar]
- Noshiro M., Omura T. Immunochemical study on the electron pathway from NADH to cytochrome P-450 of liver microsomes. J Biochem. 1978 Jan;83(1):61–77. doi: 10.1093/oxfordjournals.jbchem.a131913. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Cross A. R., Jones O. T., Griffiths W. T., Stymne S., Stobart K. Electron-transport components of the 1-acyl-2-oleoyl-sn-glycero-3-phosphocholine delta 12-desaturase (delta 12-desaturase) in microsomal preparations from developing safflower (Carthamus tinctorius L.) cotyledons. Biochem J. 1990 Nov 15;272(1):23–29. doi: 10.1042/bj2720023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. E., Coon M. J. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]
- Wilson G. S. Determination of oxidation-reduction potentials. Methods Enzymol. 1978;54:396–410. doi: 10.1016/s0076-6879(78)54025-1. [DOI] [PubMed] [Google Scholar]