ABSTRACT
BACKGROUND:
Traumatic liver injury is an acute event that triggers liver repair. The augmenter of liver regeneration (ALR) has been identified as a growth factor involved in this process. This study evaluates the impact of ALR on isolated liver blunt trauma and examines its relationship with various time intervals.
METHODS:
Forty healthy female Wistar albino rats were divided into five groups (n=8 each). Isolated blunt liver trauma was induced using a custom-designed trauma platform in all groups except for Group 1. The groups were categorized by the timing of euthanasia post-trauma: 2nd (15 minutes), 3rd (30 minutes), 4th (45 minutes), and 5th (60 minutes). Assessments included plasma ALR levels, liver tissue ALR levels (both intact and lacerated), biochemical indices, and liver histological analysis.
RESULTS:
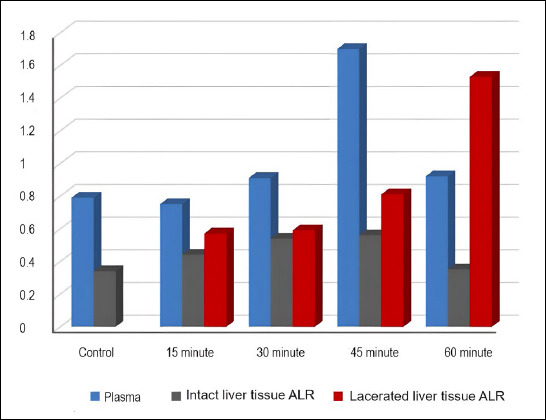
Plasma ALR levels in Group 4 were higher than in Groups 1 and 2 (p<0.01). Intact liver ALR levels in Groups 3 and 4 exceeded those in Group 1 (p<0.05, p<0.01, respectively). Intact liver tissue ALR levels in Group 5 were lower than in Groups 3 and 4 (p<0.05, p<0.01, respectively). Lacerated liver tissue ALR levels in Group 5 were higher than those in Groups 2 and 3. In Group 1, the plasma ALR level was higher than the intact liver tissue ALR level (p<0.05). In Group 2, plasma ALR levels exceeded those in intact liver tissue ALR levels (p<0.01). In Group 3, plasma ALR levels surpassed both lacerated and intact liver tissue ALR levels (p<0.05, p<0.001, respectively). In Group 4, the plasma ALR level was higher than the intact liver tissue ALR level (p<0.01), and the lacerated liver tissue level was higher than the intact liver ALR level (p<0.001). Additionally, inflammation scores were higher in Groups 3, 4, and 5 compared to Group 2 (p<0.05, p<0.01, p<0.01, respectively).
CONCLUSION:
This study is the first to explore the role of ALR in isolated blunt liver trauma. Following blunt liver trauma, both plasma and liver tissue ALR levels change within minutes.
Keywords: Augmenter of liver regeneration, blunt trauma, liver trauma
INTRODUCTION
Traumatic injury is one of the main causes of mortality worldwide. The liver is the organ most commonly affected by blunt abdominal trauma. When subjected to external impacts or crush injuries, the liver often sustains damage through severe acceleration followed by deceleration.[1,2]
In recent decades, there have been significant advances in the management of blunt hepatic trauma. The shift towards non-operative management has significantly improved survival rates following blunt liver injuries. This approach allows for the management of most severe cases without the need for surgical intervention.[3]
The process of liver regeneration, historically referenced in the Greek myth of Prometheus, comprises a complex array of mechanisms that are still only partially understood in contemporary scientific discourse.[4] Following liver trauma, hepatocyte death and compensatory proliferation occur in conjunction with inflammatory processes. These mechanisms facilitate the removal of dead hepatocytes and support the secretion of cytokines essential for tissue repair.[5] Several growth factors and cytokines play crucial roles in liver regeneration. These factors activate various intracellular signaling pathways that orchestrate this intricate process.[6]
The augmenter of liver regeneration (ALR) is a protein known for its antiapoptotic, antioxidative, and anti-inflammatory properties. While ALR is expressed in several organs including the liver, testes, kidneys, and brain, this underscores its diverse biological roles.[7] However, it is expressed more abundantly in the liver than in other organs and plays a pivotal role as a growth factor in hepatic repair and regeneration. Thus, the production and secretion of ALR by hepatocytes are critical processes in both the physiology and pathophysiology of the liver. ALR stimulates the synthesis of key inflammatory and regulatory molecules, including tumor necrosis factor-alpha (TNF-α), interleukin-6, and nitric oxide.[8]
We aimed to evaluate the impact of ALR on isolated liver blunt trauma and its relationship with time intervals in this study.
MATERIALS AND METHODS
Ethical clearance for this study was obtained from the SYLAB animal laboratory (Approval No. 18, Date: December 22). The study adhered to the ethical principles outlined in the Declaration of Helsinki regarding the care and use of laboratory animals.
Study Design
Forty healthy female Wistar albino rats (age: 8-10 weeks; weight: 250-300 g) were randomly assigned into five equal groups (Groups 1-5; n=8 each). All rats were individually housed in a controlled environment (temperature: 20°C - 23 °C; humidity: 60% - 70%; 12-hour light/dark cycle) with ad libitum access to standard food and water. Groups 2 to 5 underwent isolated blunt liver trauma using a custom-designed trauma platform. Group 1 served as the control group. Rats in Groups 2, 3, 4, and 5 were euthanized at 15, 30, 45, and 60 minutes after liver trauma, respectively. Levels of ALR in plasma and liver tissue (intact and lacerated), along with histopathological findings, were compared among the groups.
Modeling
Rats received an intraperitoneal injection of ketamine HCl (40 mg/kg, Ketalar®, Pfizer, Ortaköy, İstanbul, Türkiye) to induce anesthesia. Isolated blunt liver trauma was then induced using the custom-designed trauma platform (Fig. 1). This involved dropping a 50-gram cylindrical weight from a height of 100 cm onto the right upper abdominal quadrant of the rats, which were securely positioned on the platform. The kinetic energy of the impact, calculated using the formula E=(m)×(g)×(h), was determined to be 0.49 joules, considering the reduced weight (0.1 kg), gravity (9.8 m/s²), and drop height (50 cm).[9]
Figure 1.

Custom-designed trauma platform.
One rat in the 30-minute group died, necessitating its replacement. Before euthanasia, 2 mL of intracardiac blood was collected without mediastinal dissection. The abdomen was then accessed via a sterile 3 cm vertical median incision. Both abdominal and thoracic organs were examined for signs of injury by a researcher blinded to the group identity. Autopsy results consistently revealed Grade II hepatic lacerations according to the American Association for the Surgery of Trauma scale, characterized by specific hematoma and laceration features, with no concurrent damage to other organs.[10]
Liver weights in rodents, depending on species and strain, generally range from 4 to 5 grams (accounting for 2%-3% of body weight) in rats. After euthanasia, the liver was removed. Approximately one-third of the liver exhibited significant damage. Precise dissection of about 1 gram of the lacerated liver tissue was performed, specifically from the area adjacent to the mediastinal space. The surrounding undamaged liver tissue, making up about two-thirds of the total, was meticulously excised and removed using a scalpel.
Experimental Parameters
ELISA (Enzyme-Linked Immunosorbent Assay) Analysis
Blood samples were collected at 15, 30, 45, and 60 minutes after the injury and then placed into yellow-capped tubes for centrifugation. The resulting plasma was aliquoted and stored at -80°C. Liver tissue samples from both intact and lacerated regions were placed into Eppendorf tubes. The samples were homogenized in a volume of phosphate-buffered saline solution proportional to tissue size and subjected to sonication. The tissue homogenates were then centrifuged at 14,000 rpm for 15 minutes at 4°C.
Total protein in liver samples was quantified using the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific; Catalog number 23225). Adhering to the kit’s protocol, each sample was processed in duplicate for accurate measurement. The optical density was measured using a CLARIOstar microplate reader at a wavelength of 450 nm. This involved assessing the optical density of each well and comparing these values to a pre-established standard curve of ALR concentrations provided by the kit (range: 0.02-4.5 μg/L) to calculate the protein content in the samples.
Histopathological Evaluation
Liver tissue segments from both intact and lacerated regions were immediately immersed in a 10% buffered formalin solution for histopathological examination. After fixation, the samples underwent dehydration through a graded series of alcohol and were cleared in xylene for 20 minutes. The tissues were then embedded in paraffin for three hours. Subsequently, 4-µm thick sections were prepared and subjected to a dewaxing process in an oven set at 60°C overnight.
The sections were then stained with hematoxylin and eosin for histopathological examination. The degree of inflammation was graded on a scale ranging from 0 to 3: 0 (no inflammation), 1 (minimal inflammation), 2 (moderate inflammation), and 3 (intense inflammation). The scoring was conducted by an expert in histology and embryology who was blinded to the group identities.[11]
Biochemical Analysis
Biochemical assays were conducted using the Abbott Alinity C system (Catalog #0-AB-ALINITYC, USA). Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), gamma-glutamyl transferase (GGT), direct bilirubin, and indirect bilirubin were determined using commercial kits compatible with the Abbott Alinity C equipment. The enzyme levels were quantified in units per liter (U/L).
Statistical Analysis
Statistical analysis was performed using IBM SPSS software, version 20 (Chicago, IL, USA). Continuous variables were expressed as either mean ± standard deviation or median (range). Differences in ALR expression across different tissue types and biochemical indices between the groups were analyzed using the Kruskal-Wallis Variance Analysis. A multiple comparison test associated with the Kruskal-Wallis method was employed to assess differences between specific groups. Intragroup variations across different tissues were evaluated using the Friedman Test, with significant regional differences identified through the Friedman Multiple Comparison Test. Statistical significance was established at p<0.05.
RESULTS
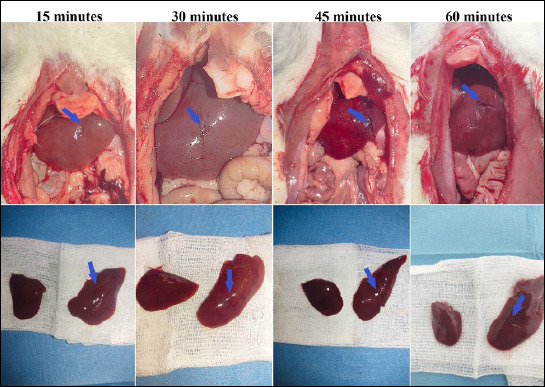
All experimental groups maintained eight model animals each, with no post-traumatic attrition in any of the groups (Fig. 2).
Figure 2.
Sequential images of lacerated livers at 15, 30, 45, and 60 minutes post-trauma (blue arrows).
Plasma ALR levels in Group 4 were significantly higher than those in Groups 1 and 2 (p<0.01). Similarly, ALR levels in intact liver tissues were higher in Groups 3 and 4 compared to Group 1 (p<0.05 and p<0.01, respectively). Conversely, intact liver tissue ALR levels in Group 5 were lower than those in Groups 3 and 4 (p<0.05 and p<0.01, respectively). Additionally, ALR levels in lacerated liver tissues of Group 5 were higher than those in Groups 2 and 3. In Group 1, plasma ALR levels were significantly higher than those in intact liver tissue (p<0.05) (Table 1).
Table 1.
Comparison of augmenter of liver regeneration levels in plasma, intact, and lacerated liver tissues across different groups
| ALR | Mean±SD; Median (min-max) | ||||
|---|---|---|---|---|---|
|
| |||||
| Plasma ALR (a) | Intact Liver Tissue ALR (b) | Lacerated Liver Tissue ALR (c) | p-value | Post Hoc Test (Interregional) | |
| Group 1 | 0.79±0.06 | 0.34±0.13 | |||
| 0.78 (0.72-0.93) | 0.30 (0.23-0.63) | - | 0.012* | ||
| - | |||||
| Group 2 | 0.75±0.08 | 0.44±0.11 | 0.57±0.21 | ||
| 0.76 (0.63-0.88) | 0.47 (0.21-0.58) | 0.56 (0.21-0.91) | 0.003 | a-b p=0.002** | |
| a-c p=0.182 | |||||
| b-c p=0.401 | |||||
| Group 3 | 0.91±0.09 | 0.54±0.09 | 0.59±0.16 | ||
| 0.94 (0.72-1.01) | 0.52 (0.44-0.78) | 0.56 (0.39-0.91) | 0.002 | a-b p=0.003** | |
| a-c p=0.018* | |||||
| b-c p=1.000 | |||||
| Group 4 | 1.70±0.35 | 0.56±0.05 | 0.81±0.15 | ||
| 1.72 (1.27-2.19) | 0.55 (0.50-0.66) | 0.85 (0.48-0.96) | 0.001 | a-b p=0.001** | |
| a-c p=0.073 | |||||
| b-c p=0.401 | |||||
| Group 5 | 0.92±0.04 | 0.35±0.05 | 1.53±0.45 | ||
| 0.90 (0.87-0.99) | 0.36 (0.21-0.42) | 1.56 (0.90-2.0) | <0.001 | a-b p=0.137 | |
| a-c p=0.137 | |||||
| b-c p<0.001*** | |||||
| p-value | <0.001 | <0.001 | <0.001 | ||
| Post Hoc Test | 1-2 p=1.000 | 1-2 p=1.000 | |||
| 1-3 p=0.390 | 1-3 p=0.046* | ||||
| 1-4 p<0.001*** | 1-4 p=0.005** | ||||
| 1-5 p=0.322 | 1-5 p=1.000 | ||||
| 2-3 p=0.148 | 2-3 p=1.000 | 2-3 p=1.000 | |||
| 2-4 p<0.001*** | 2-4 p=0.390 | 2-4 p=0.714 | |||
| 2-5 p=0.127 | 2-5 p=1.000 | 2-5 p=0.001** | |||
| 3-4 p=0.209 | 3-4 p=1.000 | 3-4 p=641 | |||
| 3-5 p=1.000 | 3-5 p=0.027* | 3-5 p=0.001** | |||
| 4-5 p=0.241 | 4-5 p=0.002** | 4-5 p=0.162 | |||
SD: Standard deviation; ALR: Augmenter of liver regeneration.
In Group 2, plasma ALR levels were significantly higher than the ALR levels in intact liver tissue following injury (p<0.01). In Group 3, plasma ALR levels exceeded those in both lacerated and intact liver tissues (p<0.05 and p<0.001, respectively). In Group 4, plasma ALR levels were higher than those in intact liver tissue (p<0.01). Additionally, ALR levels in the lacerated liver tissue of Group 4 were significantly greater than those in intact liver tissue (p<0.001) (Fig. 3).
Figure 3.
Comparative analysis of augmenter of liver regeneration (ALR) levels in plasma, intact liver, and lacerated liver tissues across different groups.
ALT levels in Groups 2 and 4 were significantly higher than those in Group 1 (p<0.001 for both). ALT levels in Group 3 were also higher than in Group 2 (p<0.001). AST levels in Groups 2 and 3 were significantly higher than those in Group 1 (p<0.001 for both), and AST levels in Group 2 were higher than in Group 5 (p<0.001). LDH levels in Groups 2 and 4 were significantly higher than those in Group 1 (p<0.001 for both), and LDH levels in Group 2 were also higher than those in Group 5 (p<0.001). Furthermore, GGT levels in Groups 3, 4, and 5 were significantly higher than those in Groups 1 and 2 (p<0.05 for all) (Table 2).
Table 2.
Comparison of alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, gamma-glutamyl transferase, direct bilirubin, and total bilirubin across different groups
| Alanine Aminotransferase | Aspartate Aminotransferase | Lactate Dehydrogenase | Gamma-Glutamyl Transferase | Direct Bilirubin | Total Bilirubin | |
|---|---|---|---|---|---|---|
| Group 1 | 90.50±1.60 | 88.62±2.97 | 367.75±1.03 | 1.5±0.5 | 0.15±0.05 | 0.15±0.05 |
| 90 (89-94) | 88.5 (83-93) | 358 (356-359) | 1.5 (1.0-2.0) | 0.15 (0.10-0.20) | 0.15 (0.10-0.20) | |
| Group 2 | 1055.37±4.37 | 887.50±1.41 | 4355.87±4.85 | 1.5±0.5 | 0.13±0.05 | 0.13±0.05 |
| 1057 (1048-1059) | 888 (885-889) | 4355 (4349-4365) | 1.5 (1.0-2.0) | 0.10 (0.10-0.20) | 0.10 (0.10-0.20) | |
| Group 3 | 2551.12±1.35 | 815.25±2.81 | 2084.00±1.30 | 2.2±0.7 | 0.15±0.05 | 0.13±0.05 |
| 2551.5 (2549-2553) | 815 (811-819) | 2084 (2082-2086) | 2.0 (1.0-3.0) | 0.15 (0.10-0.20) | 0.10 (0.10-0.20) | |
| Group 4 | 1996.37±6.27 | 660.12±4.29 | 2416.25±2.37 | 2.2±0.7 | 0.15±0.05 | 0.15±0.05 |
| 1998.5 (1981-2000) | 658 (657-667) | 2417 (2412-2419) | 2.0 (1.0-3.0) | 0.15 (0.10-0.20) | 0.15 (0.10-0.20) | |
| Group 5 | 1657.0±2653 | 644.62±2.06 | 1528.50±0.92 | 2.2±0.7 | 0.15±0.05 | 0.15±0.05 |
| 1653.5 (1629-1689) | 644.5 (642-648) | 1528.5 (1527-1530) | 2.0 (1.0-3.0) | 0.15 (0.10-0.20) | 0.15 (0.10-0.20) | |
| p value | p<0.001 | p<0.001 | p<0.001 | p=0.032 | p=0.983 | p=0.964 |
| Post Hoc Test | 1-2 p=1.000 | 1-2 p<0.001*** | 1-2 p=0.001** | 1-2 p=1.000 | ||
| 1-3 p<0.001*** | 1-3 p<0.001*** | 1-3 p=0.061 | 1-3 p=0.036* | |||
| 1-4 p<0.001*** | 1-4 p=0.062 | 1-4 p<0.001*** | 1-4 p=0.036* | |||
| 1-5 p=0.062 | 1-5 p=1.000 | 1-5 p=1.000 | 1-5 p=0.036* | |||
| 2-3 p<0.001*** | 2-3 p=1.000 | 2-3 p=0.061 | 2-3 p=0.036* | |||
| 2-4 p=0.062 | 2-4 p=0.062 | 2-4 p=1.000 | 2-4 p=0.036* | |||
| 2-5 p=1.000 | 2-5 p<0.001*** | 2-5 p<0.001*** | 2-5 p=0.036* | |||
| 3-4 p=1.000 | 3-4 p=1.000 | 3-4 p=0.924 | 3-4 p=1.000 | |||
| 3-5 p=0.062 | 3-5 p=0.062 | 3-5 p=1.000 | 3-5 p=1.000 | |||
| 4-5 p=1.000 | 4-5 p=1.000 | 4-5 p=0.061 | 4-5 p=1.000 |
p<0.05;
p<0.01;
p<0.001.
There were significant between-group differences in inflammation scores (p<0.01). Inflammation scores in Groups 3, 4, and 5 were significantly higher than those in Group 2 (p<0.05, p<0.01, p<0.01, respectively) (Table 3, Fig. 4).
Table 3.
Comparison of inflammation scores among Groups 2-5
| Inflammation Score | ||||
|---|---|---|---|---|
|
| ||||
| Mean±SD | Median (min-max) | p-value | Post Hoc Test | |
| Group 2 | 0.3±0.51 | 0 (0-1) | 0.001 | 2-3 p=0.022* |
| Group 3 | 2.0±0.9 | 2 (1-3) | 2-4 p=0.001** | |
| Group 4 | 3.3±0.7 | 2.5 (1-3) | 2-5 p=0.004** | |
| Group 5 | 2.2±0.4 | 2 (2-3) | 3-4 p=1.000 | |
| 3-5 p=1.000 | ||||
| 4-5 p=1.000 | ||||
SD: Standard Deviation.
Figure 4.
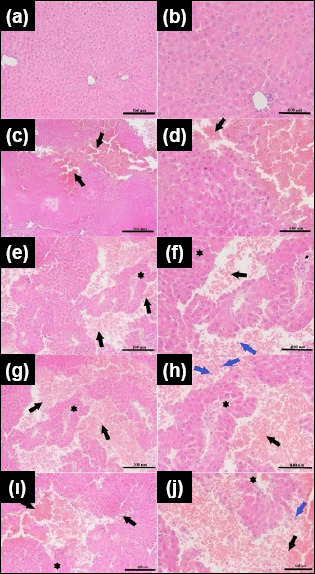
Histopathological assessment of lacerated liver tissues. a,b: 200x and 400x magnification for Group 1; c,d: 200x and 400x magnification for Group 2; e,f: 200x and 400x magnification for Group 3; g,h: 200x and 400x magnification for Group 4; ı,j: 200x and 400x magnification for the 60-minute post-trauma liver in Group 5. Black arrows mark the laceration regions with erythrocytes covering these areas; blue arrows point to neutrophils, and star symbols indicate scar tissue.
DISCUSSION
The process of liver regeneration involves various factors and signaling pathways. ALR has multiple intracellular and extracellular functions and plays a key role in maintaining liver homeostasis and promoting hepatic regeneration after injury.[7] To the best of our knowledge, this is the first study to evaluate the dynamic changes in ALR levels in liver tissues and plasma at different time points following isolated blunt liver trauma.
ALR has different isoforms; the long-form of ALR is located in the mitochondrial intermembrane space.[7] ALR is involved in iron homeostasis and the maturation of cytosolic proteins, which are essential for maintaining the structural and functional integrity of mitochondria.[12] Additionally, ALR participates in the oxidative folding of mitochondrial proteins through the Mia40/ALR disulfide relay system, a process vital for hepatocyte survival and overall liver health.[8] These functions help prevent rapid declines in adenosine triphosphate (ATP) production and maintain cellular viability under stress, particularly relevant in liver injury and repair scenarios.[8] The short form of ALR, predominantly located in the cytosol, is secreted by hepatocytes.[7]
Liver injury triggers an inflammatory response characterized by the release of proinflammatory cytokines. ALR actively modulates this response by stimulating the synthesis of key proinflammatory cytokines, such as TNF-α and interleukin-6 (IL-6), in Kupffer cells through a G-protein coupled receptor mechanism.[8] Additionally, ALR is recognized as a hepatotropic growth factor that enhances hepatocyte proliferation and supports liver regeneration, which is crucial for recovery post-liver trauma.[14] Moreover, ALR has anti-apoptotic properties and protects hepatocytes against ischemia-reperfusion injury, a common consequence of liver trauma. This protective effect is mediated by the induction of autophagy and activation of the PINK1/Parkin pathway, essential for mitochondrial quality control and cellular survival.[15]
Hepatocytes are the primary source of ALR in plasma, highlighting their critical role in liver physiology and systemic health.[12,16] This underscores the importance of hepatocytes in maintaining circulatory ALR levels, further emphasizing ALR’s significance in energy balance and cellular viability, especially in liver cells where it is prominently active.[16] In Group 1 (control group), the ALR level in plasma was higher than that in the intact liver tissue, indicating that the release of ALR within the liver continues in a healthy state.
Wang et al. observed elevated levels of plasma ALR in patients with Hepatitis B Virus-related (HBV-related) hepatitis and cirrhosis compared to healthy controls.[17] They suggested that plasma ALR levels could be a sensitive and specific diagnostic marker for assessing liver-related conditions. Notably, plasma ALR levels may be useful in the early detection and monitoring of hepatic diseases.[17] Hongbo et al. found that in cases of acute-on-chronic liver failure, plasma ALR levels were significantly higher in patients who survived compared to those who did not, particularly in the early stages of the condition. This indicates that plasma ALR levels may potentially serve as a prognostic biomarker in acute-on-chronic liver failure.[18] Polimeno et al. reported a significant increase in serum ALR levels following partial hepatectomy,[19] indicating that ALR not only circulates to different regions of the liver but also reaches distant tissues. This suggests that ALR can influence cellular activities beyond the immediate liver environment. This interaction may have significant implications for cells in distant tissues, especially those involved in systemic regeneration and repair processes. Additionally, serum ALR levels showed a negative correlation with the activity of natural killer cells derived from peripheral blood, indicating a systemic immunomodulatory role for ALR.[8] Elevated serum ALR levels were also associated with improved clinical outcomes in cases of hepatic failure, highlighting its potential as both a biomarker and a therapeutic target.[7] In our study, Group 4, assessed at 45 minutes post-trauma, exhibited a significant increase in plasma ALR levels compared to the Control Group and the early post-trauma Group 2. This significant rise indicates an acute systemic response to liver injury, characterized by a rapid surge in ALR, which plays a crucial role in the liver’s immediate repair mechanisms.
In a sepsis rat model, there was a rapid and significant increase in plasma ALR levels within two hours; the levels peaked at around eight hours and remained elevated at 24 hours. In addition, the increase in ALR levels paralleled the elevation of inflammatory cytokines.[20] In this study, the peak plasma ALR levels observed in Group 4 at 45 minutes post-trauma potentially reflect the culmination of the inflammatory phase following the injury or the onset of liver regenerative processes. The role of ALR in enhancing liver regeneration and mitigating cell death is pivotal in preserving liver functionality and may influence the levels of liver enzymes. In the study by Li et al., rats overexpressing ALR incurred less liver damage compared to their wild-type counterparts, as evidenced by lower serum levels of ALT and AST, along with fewer histological lesions in the livers of the ALR-transfected mice.[21]
Our biochemical findings corroborate this, showing marked increases in ALT and AST in the initial stages post-trauma, indicative of acute hepatic injury. These enzymatic elevations, particularly notable at mid-time points, are consistent with the liver’s rapid inflammatory response to injury. LDH levels, peaking early, suggest immediate cellular injury, while the increase in GGT levels at later stages indicates continued liver repair and biliary regeneration.
Parallel trends were observed in the analysis of ALR levels within intact liver tissues, where Groups 3 and 4 (30 and 45 minutes post-trauma, respectively) demonstrated significantly higher ALR levels compared to the Control Group. These data suggest pronounced regenerative activity within the hepatic environment, mirroring the systemic augmentation of ALR.
Interestingly, intact liver tissue from Group 5, assessed at 60 minutes post-trauma, exhibited a reduction in ALR levels relative to other post-trauma groups. This reduction may reflect the transition into a resolution phase of liver regeneration, where the proliferative activity begins to diminish.
Examination of ALR levels in lacerated liver tissue revealed significantly elevated ALR concentrations in Group 5 compared to Groups 2 and 3. This indicates a sustained regenerative response that persists well into the later stages of recovery. The disparity in ALR expression between lacerated and intact tissues within this group indicates a localized upregulation of ALR.
The evaluation of inflammation scores at various time points post-trauma provided further insights into the progression of the inflammatory response in the liver. Groups 3, 4, and 5 exhibited incrementally higher inflammation scores, statistically significant when compared with Group 2. This progressive increase in inflammation scores correlated with the observed patterns in ALR levels, suggesting that ALR may play a role in mediating the inflammatory and regenerative responses to hepatic trauma.
The predominant expression of ALR in the liver and testes justifies the use of female rats in this study, effectively minimizing the confounding effects of testicular ALR expression.[14] However, a limitation of the study is the absence of an analysis of other inflammatory markers. Additionally, in blunt trauma scenarios, other organs such as the spleen might also sustain injuries, which were not accounted for in this study. This aspect is crucial for a comprehensive understanding of the impact of trauma.
CONCLUSION
This study examined the role of ALR in isolated blunt liver trauma, thus bridging a crucial gap in understanding the post-traumatic inflammatory, reparative, and regenerative processes in the liver. The findings suggest ALR’s potential as a biomarker, reflected in the alterations and upward trends in its levels following liver injury. However, further in-depth cellular-level research is essential to unravel the underlying mechanisms of the observed ALR elevation in plasma and liver. These insights may provide the basis for future research to reduce secondary liver damage and enhance post-traumatic recovery.
Footnotes
Ethics Committee Approval: This study was approved by the Saki Yenilli Experimental Products Production and Application Laboratory Ethics Committee (Date: 22.12.2022, Decision No: 18).
Peer-review: Externally peer-reviewed.
Authorship Contributions: Concept: N.U., G.A., N.B.; Design: N.U., A.A.S., İ.G.; Supervision: N.U., G.A., N.B., R.P.K.; Resource: N.U., G.A., N.B., R.P.K., İ.G., A.A.S.; Materials: N.U., A.A.S., İ.G.; Data collection and/or processing: N.U., G.A., N.B., R.P.K.; Analysis and/or interpretation: N.U., R.P.K.; Literature search: G.A., N.B., A.A.S., İ.G.; Writing: N.U., G.A., N.B., A.A.S.; Critical review: İ.G., G.A., N.B., R.P.K.
Conflict of Interest: None declared.
Use of AI for Writing Assistance: Not declared.
Financial Disclosure: The author declared that this study has received no financial support.
REFERENCES
- 1.Nemzek-Hamlin JA, Hwang H, Hampel JA, Yu B, Raghavendran K. Development of a murine model of blunt hepatic trauma. Comparative Med. 2013;63:398–408. [PMC free article] [PubMed] [Google Scholar]
- 2.Jin W, Deng L, Lv H, Zhang Q, Zhu J. Mechanisms of blunt liver trauma patterns:An analysis of 53 cases. Experimental and Therapeutic Med. 2013;5:395–8. doi: 10.3892/etm.2012.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García IC, Villalba JS, Iovino D, Franchi C, Iori V, Pettinato G, et al. Liver trauma:until when we have to delay surgery?A review. Life (Basel) 2022;12:694. doi: 10.3390/life12050694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiseleva YV, Antonyan SZ, Zharikova TS, Tupikin KA, Kalinin DV, Zharikov YO. Molecular pathways of liver regeneration:A comprehensive review. World J Hepatology. 2021;13:270–90. doi: 10.4254/wjh.v13.i3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodimova S, Mozherov A, Elagin V, Karabut M, Shchechkin I, Kozlov D, et al. Effect of hepatic pathology on liver regeneration:the main metabolic mechanisms causing impaired hepatic regeneration. Int J Molecular Scie. 2023;24:9112. doi: 10.3390/ijms24119112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang C, Takahashi K, Furuya K, Ohkohchi N, Oda T. Dualistic role of platelets in living donor liver transplantation:Are they harmful? World J Gastroenterology. 2022;28:897–908. doi: 10.3748/wjg.v28.i9.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibrahim S, Weiss TS. Augmenter of liver regeneration:Essential for growth and beyond. Cytokine Growth Factor Rev. 2019;45:65–80. doi: 10.1016/j.cytogfr.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi CR. Augmenter of liver regeneration. Fibrogenesis &Tissue Repair. 2012;5:10. doi: 10.1186/1755-1536-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox JM, Kalns JE. Development and characterization of a rat model of nonpenetrating liver trauma. Comparative Med. 2010;60:218–24. [PMC free article] [PubMed] [Google Scholar]
- 10.Kozar RA, Crandall M, Shanmuganathan K, Zarzaur BL, Coburn M, Cribari C, et al. Organ injury scaling 2018 update:Spleen, liver, and kidney. J Trauma and Acute Care Surg. 2018;85:1119–22. doi: 10.1097/TA.0000000000002058. [DOI] [PubMed] [Google Scholar]
- 11.Ayten R, Aygen E, Cerrahoglu YZ, Camci C, Ilhan YS, Girgin M, et al. Effects of Copper, Zinc, and Vitamin Complex (Cernevit®) on hepatic healing in rats experimentally subjected to blunt hepatic trauma. Indian J Surg. 2015;77:1045–9. doi: 10.1007/s12262-014-1128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma AK, Sharma A, Subramaniyam N, Gandhi CR. Augmenter of liver regeneration:Mitochondrial function and steatohepatitis. J Hepatology. 2022;77:1410–21. doi: 10.1016/j.jhep.2022.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Dong Y, Zhang Y, Feng Y, An W. The protective roles of augmenter of liver regeneration in hepatocytes in the non-alcoholic fatty liver disease. Front Pharmacol. 2022;13:928606. doi: 10.3389/fphar.2022.928606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Farooq M, Sheng D, Chandramouli C, Lan T, Mahajan NK, et al. Augmenter of liver regeneration (alr) promotes liver outgrowth during zebrafish hepatogenesis. PLoS One. 2012;7:e30835. doi: 10.1371/journal.pone.0030835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong WN, Li W, Bai C, Dong Y, Wu Y, An W. Augmenter of liver regeneration-mediated mitophagy protects against hepatic ischemia/reperfusion injury. Am J Transplant. 2022;22:130–43. doi: 10.1111/ajt.16757. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, Verma AK, Rani R, Sharma A, Wang J, Shah SA, et al. Hepatic deficiency of augmenter of liver regeneration predisposes to nonalcoholic steatohepatitis and fibrosis. Hepatology (Baltimore, Md) 2020;72:1586–604. doi: 10.1002/hep.31167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang N, Sun H, Tang L, Deng J, Luo Y, Guo H, et al. Establishment and primary clinical application of competitive inhibition for measurement of augmenter of liver regeneration. Experimental and Therapeutic Med. 2014;7:93–6. doi: 10.3892/etm.2013.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hongbo S, Yu C, Ming K, Honglin S, Ping HY, Ping DZ. Augmenter of liver regeneration may be a candidate for prognosis of HBV related acute-on-chronic liver failure as a regenerative marker. Hepato-Gastroenterology. 2012;59:1933–8. doi: 10.5754/hge11679. [DOI] [PubMed] [Google Scholar]
- 19.Polimeno L, Pesetti B, Annoscia E, Giorgio F, Francavilla R, Lisowsky T, et al. Alrp, a survival factor that controls the apoptotic process of regenerating liver after partial hepatectomy in rats. Free Radic Res. 2011;45:534–49. doi: 10.3109/10715762.2011.555482. [DOI] [PubMed] [Google Scholar]
- 20.Vodovotz Y, Prelich J, Lagoa C, Barclay D, Zamora R, Murase N, et al. Augmenter of liver regeneration (ALR) is a novel biomarker of hepatocellular stress/inflammation:in vitro, in vivo and in silico studies. Molecular Med (Cambridge, Mass) 2013;18:1421–9. doi: 10.2119/molmed.2012.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Xie P, Li W, Wu Y, An W. Augmenter of liver regeneration protects against ethanol-ınduced acute liver ınjury by promoting autophagy. Am J Pathol. 2019;189:552–67. doi: 10.1016/j.ajpath.2018.11.006. [DOI] [PubMed] [Google Scholar]


