Abstract
Study objectives:
In April 2015, a multistate outbreak of illness linked to synthetic cannabinoid (SC) use was unprecedented in magnitude and severity. We identified Mississippi cases in near-real time, collected information on cases to characterize the outbreak, and identified the causative SC.
Methods:
A case was defined as any patient of a Mississippi healthcare facility who was suspected of SC use and presenting with ≥2 of the following symptoms: sweating, severe agitation, or psychosis during April 2–May 3, 2015. Clinicians reported cases to the Mississippi Poison Control Center (MPCC). We used MPCC data to identify cases at the University of Mississippi Medical Center (UMMC) to characterize in further detail, including demographics and clinical findings. Biologic samples were tested for known and unknown SCs by liquid chromatography quadrupole time-of-flight mass spectrometry (LC-QTOF/MS).
Results:
Clinicians reported 721 cases (11 deaths) statewide; 119 (17%) were UMMC patients with detailed data for further analysis. Twelve (10%) were admitted to an intensive care unit and 2 (2%) died. Aggression (32%), hypertension (33%), and tachycardia (42%) were common. SCs were identified in serum from 39/56 patients (70%); 33/39 patients (85%) tested positive for MAB-CHMINACA (N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide) or its metabolites. Compared to all patients tested for SCs, those positive for MAB-CHMINACA were more likely to have altered mental status on examination (OR = 3.3, p = .05).
Conclusion:
SC use can cause severe health effects. MAB-CHMINACA was the most commonly detected SC in this outbreak. As new SCs are created, new strategies to optimize surveillance and patient care are needed to address this evolving public health threat.
Keywords: Drugs of abuse, epidemiology, outbreak investigation, poison centers, synthetic cannabinoids
Introduction
Synthetic cannabinoids (SC) are a class of recreational drugs of abuse that continue to evolve with the development of new compounds. These frequently used illicit drugs are popularly known by various names such as “Spice” or “K2.” They are typically manufactured outside of the USA and are then purchased and shipped in bulk to the USA, where they are dissolved in solvents, sprayed on various types of leaves (such as marshmallow, damiana, and mullein), and packaged as herbal incense or potpourri [1]. SC products are typically smoked or vaped. While the overall prevalence of SC use is not known, annual surveys of U.S. high school seniors reveal annual rates of past year use from 2012 to 2015 ranging from 5 to 10% [2,3]. Prior studies have found that the reasons for their popularity include being undetectable in standard urine drug screening tests, perceived safety and legality, and anticipated cannabis-like effects [4]. Since the first reports of trafficking in the USA in 2008, a growing number of reports have included related adverse events, including outbreaks of severe illness [5–8]. Signs and symptoms can be psychiatric (psychosis, anxiety), neurologic (altered mental status, seizures), cardiovascular (tachycardia, arrhythmias), or gastrointestinal (nausea, vomiting); rhabdomyolysis and acute kidney injury are also reported [2,7–12]. Deaths related to SC use are rare [7,11].
During the spring of 2015, a large outbreak of acute toxicity from SC use occurred in multiple states in the USA. Poison Centers, which receive and track calls nationwide from clinicians and the public about suspected poisonings, received 1501 calls regarding SC exposures during April 2015, a 330% increase from January 2015 [13]. In Mississippi, the outbreak was first recognized on April 2, 2015, as described in a previously published field report [14]. The Mississippi State Department of Health (MSDH) initiated active surveillance to identify additional cases in collaboration with the Mississippi Poison Control Center (MPCC) and emergency departments across the state. The MSDH issued an alert through the MS Health Alert Network requesting healthcare providers to report possible cases of SC-associated illness to the MPCC for surveillance purposes. In addition to reporting any new cases, the MSDH instructed providers to retrospectively complete data collection forms on patients meeting the case definition since April 2, 2015. Staff from the MPCC entered clinical data from these case reports into their state’s poison center database. All US local and state poison center data are routinely uploaded in near-real time to the National Poison Data System (NPDS), a reporting database and electronic surveillance system used by poison centers nationwide.
On April 23, the MSDH formally requested technical assistance from the U.S. Centers for Disease Control and Prevention (CDC) to investigate and respond to the outbreak. Our objectives were to gather and analyze surveillance data in near-real time to inform the public health response, characterize the outbreak by describing the affected population and the typical clinical presentation and disease course, and identify the SC variants associated with the outbreak.
Methods
A case was defined as any individual with a history of known or suspected SC use presenting in MS during April 2–May 3, 2015, with at least two of the following symptoms documented by a healthcare provider: sweating, severe agitation, or psychosis (see Appendix 1 for the line list form). We identified cases using the MPCC database, including calls from healthcare providers and the general public. To contextualize the MS outbreak, we also queried NPDS to obtain the frequency of calls related to SC exposure in people nationwide. To better understand the evolution of the outbreak in near-real time, we performed daily queries of the MS state poison center data on NPDS and created a visual dashboard on Epi Info 7 (Epi Info version 7.1.3.10, CDC, 2012). This tool informed the team of the number of daily cases and allowed us to monitor for any changes in the demographic characteristics of the patients or the severity of illness over time. We performed descriptive analyses of all cases reported to the MPCC, considering day of presentation, demographics, and level of health care received. The data were relayed daily to the MSDH during the outbreak to guide the public health response.
We further characterized a subset of the cases in the MPCC database by analyzing those cases with medical records at the University of Mississippi Medical Center (UMMC). We used a standardized chart abstraction tool to collect data from emergency medical service and UMMC hospital electronic medical records (including any transfer hospital records, if applicable), as well as MPCC records. Variables collected included demographics, clinician-suspected or patient-reported SC use history, past medical history, clinical information (illness signs and symptoms, mental status, medical interventions performed, medications provided) documented during prehospital and hospital care, highest level of care received (discharged from the emergency department, left against medical advice, admitted to general inpatient service, admitted to an intensive care unit, or died), and hospital laboratory results.
To identify which SCs were associated with the outbreak, MSDH encouraged healthcare providers to submit blood and urine samples for laboratory testing at UMMC. To discover previously unreported SCs, the UMMC laboratory sent a subset of the clinical samples to the University of California San Francisco (UCSF) Clinical Toxicology and Environmental Biomonitoring Laboratory, which has the capability to test for known and novel compounds by liquid chromatography-quadrupole time-of-flight mass spectrometry (LC-QTOF/MS).
To analyze the epidemiologic, clinical, and laboratory data, the team entered the data into an Epi Info 7 database and analyzed it using Epi-Info and SAS 9.3 (SAS Institute, Cary, NC). We calculated frequencies and proportions for demographic and illness-related characteristics. We performed χ2-tests or Fisher’s exact tests for categorical variables and Wilcoxon rank sum tests for continuous variables, with statistical significance defined as p ≤ .05.
Institutional review board approval was not required for this investigation, which was classified as public health nonresearch.
Results
Preliminary data from this investigation were previously published in a field report [14]; final data and additional analyses are presented here. There were 721 cases identified from the MPCC database, accounting for 45% of the 1,587 SC-related exposure calls made to poison centers nationwide during this period. The ages of people affected in MS ranged from 12 to 69 years (median 29 years), and most (n = 589; 82%) were male (Table 1). Most counties in the state, including both predominantly rural and urban counties (n = 48; 59%), reported at least one case (Figure 1). The largest proportion of cases originated from the Jackson, MS Metropolitan Area (n = 169; 23%). The number of cases peaked during April 18–21 (Figure 2). Most patients were treated and released from the emergency department (n = 469; 66%), while 87 (12%) were admitted to a noncritical care unit, 72 (10%) were admitted to intensive care services, and 11 (2%) were admitted to psychiatric facilities; 73 (10%) were lost to follow-up. Eleven (2%) patients died. An example of the daily visual dashboard is shown in Figure 3; these dashboards were included in daily situation reports for MSDH, UMMC, and CDC.
Table 1.
Demographics for cases of synthetic cannabinoid-associated illness—state of Mississippi and university of Mississippi medical center (UMMC), April 2–May 3, 2015.
| Demographics | Statewide (n = 721) | UMMC (n = 119) |
|---|---|---|
|
| ||
| Sex (%) | ||
| Female | 132 (18) | 18 (15) |
| Male | 589 (82) | 101 (85) |
| Median age, years (range) | 29 (12–69) | 29 (14–62) |
| ≤18 (%) | 52 (7) | 8 (7) |
| 19–28 (%) | 289 (40) | 47 (40) |
| 29–38 (%) | 233 (32) | 40 (34) |
| 39–48 (%) | 92 (13) | 17 (14) |
| ≥49 (%) | 55 (8) | 7 (6) |
| County of residence (%) | ||
| Hinds | 159 (22) | 81 (68) |
| Other | 562 (78) | 35 (29) |
| Missing | 0 | 3 (3) |
Figure 1.

Map of synthetic cannabinoid-related exposure calls to the Mississippi Poison Control Center per 100,000 population, by county, April 2–May 3, 2015.
Figure 2.

Cases of synthetic cannabinoid associated illness reported to the Mississippi Poison Control Center database, by 4 day intervals, April 2–May 3, 2015 (N = 721).
Figure 3.
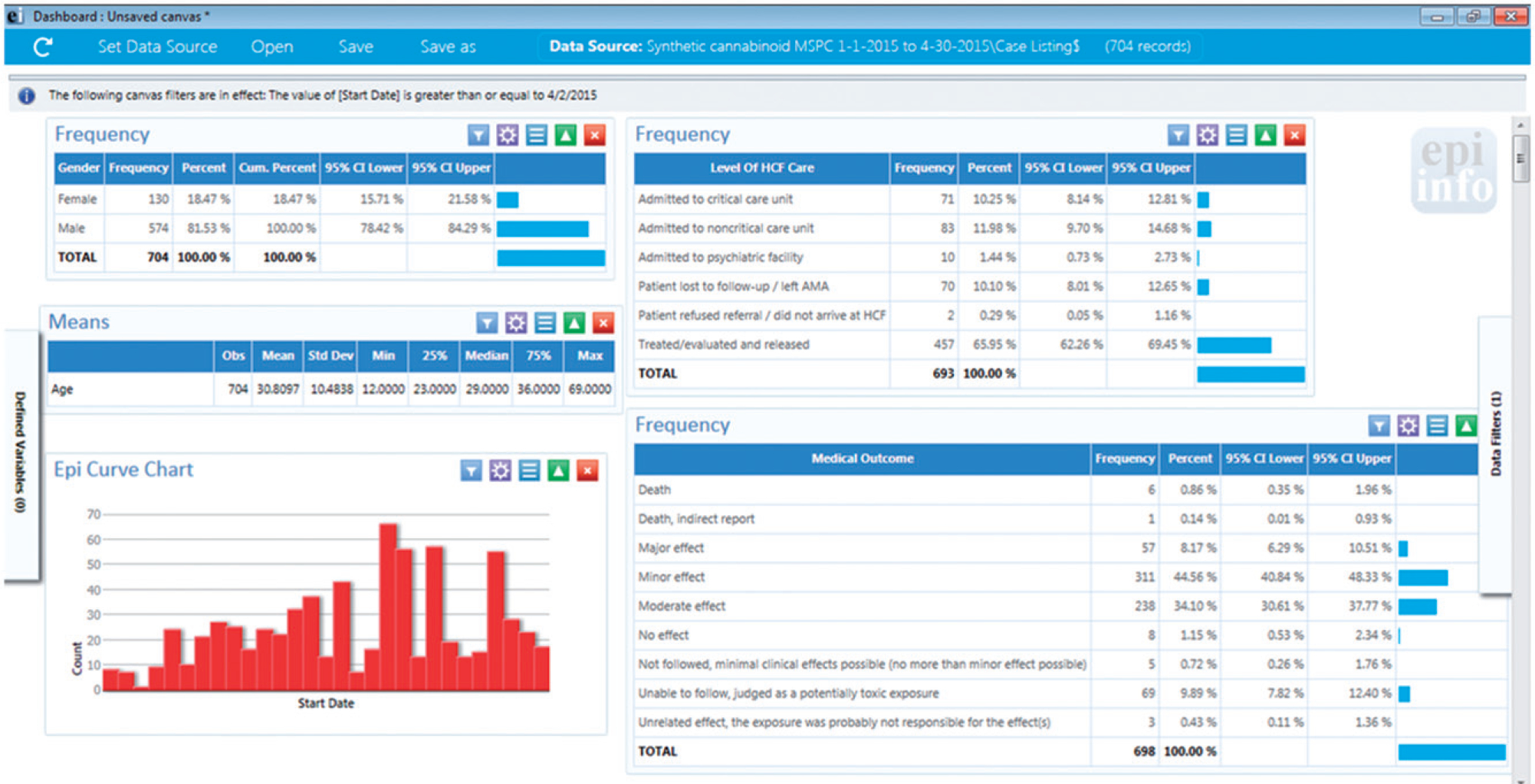
Screenshot of the Epi Info dashboard (April 30, 2015) displaying the cumulative number of reported cases of synthetic cannabinoid-associated illness and summary statistics of their demographics, level of care, and disposition.
We analyzed a subset of the 721 MS cases, composed of the 119 patients who presented to UMMC for care (17%). We abstracted all 119 UMMC medical charts (Table 2). No significant differences were found in sex (Odds ratio [OR] = 0.8, p = .4) and age (median non-UMMC = 29 years old versus UMMC = 30 years old, p = .4) of the UMMC patients compared to MS patients who did not go to UMMC for care (Table 1). Hospital data indicated a history of SC use for most patients (n = 108; 91%); 60 (77%) of these reported last use ≤24 h prior (Table 3). Before the administration of any sedatives, antidotes, or antipsychotic medications, 38 (32%) were reported to be aggressive or violent, 36 (30%) were agitated, 30 (25%) were confused, 24 (20%) were somnolent, and 19 (16%) were unresponsive (Table 4). Sixteen (13%) patients were reported to have a hyperactive (agitation, aggression, anxiety, psychosis, hallucinations, paranoia) mental state alternating with a hypoactive mental state (somnolence, unresponsiveness). According to emergency medical services (EMS) and emergency department (ED) reports, patients would fluctuate between these two extremes, at times rapidly.
Table 2.
Data sources used for analyzing 119 case patients with reported synthetic cannabinoid-associated illness—university of Mississippi medical center (UMMC), april 2–may 3, 2015.
| Data source | N (%)a |
|---|---|
|
| |
| Poison center database | 118 (99) |
| Emergency medical services recordsb | 61 (51) |
| Hospital recordsc | 119 (100) |
Multiple sources of data possible.
Twenty-three patients with reported emergency department arrival by ambulance do not have emergency medical service records available.
Includes emergency department, general inpatient, and intensive care records.
Table 3.
Reported synthetic cannabinoid use among 119 case patients presenting to the university of Mississippi medical center (UMMC), April 2–May 3, 2015.
| Reported synthetic cannabinoid use | N (%) |
|---|---|
|
| |
| Synthetic cannabinoid use recorded in hospital data | 108 (91) |
| Time since last use, hours (n = 78 responses) | |
| <24 | 60 (77) |
| 24–36 | 5 (6) |
| >36 | 13 (17) |
Table 4.
Demographic, clinical, and laboratory characteristics of 119 case patients with reported synthetic cannabinoid-associated illness by synthetic cannabinoid test results—University of Mississippi medical center (UMMC), April 2–May 3, 2015.
| Characteristic | Total (%) n = 119 | Total tested for SCs (%), n = 56 | SC positive (%), n = 39 | MAB positive (%), n = 33 |
|---|---|---|---|---|
|
| ||||
| Male sex | 101 (85) | 45 (80) | 30 (77) | 26 (79) |
| Median age, years (IQR) | 29 (23–36) | 29 (23.5–36.5) | 31 (23–38) | 25.5 (21–36) |
| Past medical history | ||||
| High blood pressure | 16 (13) | 8 (14) | 6 (15) | 5 (15) |
| Seizure disorder | 7 (6) | 4 (7) | 2 (5) | 2 (6) |
| Mental illness | 32 (27) | 18 (32) | 12 (31) | 9 (27) |
| Substance abuse | 37 (31) | 17 (30) | 12 (31) | 10 (30) |
| Mental status reported before medication administrationa | ||||
| Violent/aggressive behavior | 38 (32) | 18 (32) | 12 (31) | 12 (36) |
| Agitation | 36 (30) | 17 (31) | 11 (28) | 10 (30) |
| Confusion | 30 (25) | 13 (23) | 9 (23) | 8 (24) |
| Somnolence | 24 (20) | 8 (14) | 8 (21) | 8 (24) |
| Unresponsiveness | 19 (16) | 10 (18) | 8 (21) | 7 (21) |
| Anxiety | 16 (13) | 10 (18) | 7 (18) | 6 (18) |
| Hallucinations | 10 (8) | 4 (7) | 3 (8) | 1 (3) |
| Seizures | 8 (7) | 3 (5) | 3 (8) | 3 (9) |
| Psychosis | 6 (5) | 2 (4) | 1 (3) | 1 (3) |
| Paranoia | 2 (2) | 1 (2) | 0 | 0 |
| Initial vital signs | ||||
| Systolic blood pressure ≤90 mmHg | 9/115 (8) | 5/53 (9) | 5/37 (14) | 5/32 (16) |
| Systolic blood pressure ≥140 mmHg | 38/115 (33) | 19/53 (36) | 11/37 (30) | 9/32 (28) |
| Heart rate ≥100 beats per minute | 48/115 (42) | 16/54 (30) | 16/37 (43) | 13/32 (41) |
| Heart rate ≤60 beats per minute | 9/115 (8) | 5/54 (9) | 4/37 (11) | 4/32 (13) |
| Respiratory rate >20 breaths/minute | 25/113 (22) | 13/52 (25) | 11/37 (30) | 10/32 (31) |
| Oxygen saturation <90% | 5/112 (5) | 3/53 (6) | 2/36 (6) | 2/31 (6) |
| Initial physical examination findings | ||||
| Diaphoresis | 8 (7) | 3 (5) | 2 (5) | 1 (3) |
| Dilated pupils | 11 (9) | 6 (11) | 5 (13) | 5 (15) |
| Nonreactive, minimally reactive, or sluggish pupils | 13 (11) | 7 (13) | 6 (15) | 6 (18) |
| Tachycardia | 25 (21) | 11 (20) | 8 (21) | 8 (24) |
| Bradycardia | 3 (3) | 1 (2) | 1 (3) | 1 (3) |
| Altered mental statusb | 58 (49) | 25 (45) | 20 (51) | 19 (58) |
| Clinical laboratory result abnormalities (abnormal/tested) | ||||
| Hyponatremia (serum sodium <135 mEq/L) | 2/97 (2) | 9/49 (18) | 2/33 (6) | 2/31 (6) |
| Hypernatremia (serum sodium >145 mEq/L) | 5/97 (5) | 1/49 (2) | 1/33 (3) | 1/31 (3) |
| Hypokalemia (serum potassium <3.5mEq/L) | 16/97 (16) | 9/49 (18) | 6/33 (18) | 6/31 (19) |
| Hyperkalemia (serum potassium >5.0 mEq/L) | 5/97 (5) | 4/49 (8) | 4/33 (12) | 3/31 (10) |
| High serum creatinine (serum creatinine ≥1.5 mg/dL) | 13/97 (13) | 7/49 (14) | 5/33 (15) | 5/31 (16) |
| Hyperglycemia (glucose >140 mg/dL) | 17/95 (18) | 11/48 | 10/33 (30) | 10/31 (32) |
| Hypoglycemia (glucose <70 mg/dL) | 6/95 (6) | 1/48 (23) | 0 | 0 |
| Elevated serum creatinine kinase (CK) | ||||
| Creatinine kinase ≥150 units/L | 64/77 (83) | 32/37 (86) | 22/26 (85) | 21/25 (84) |
| Creatinine kinase ≥1000 units/L | 14/77 (18) | 9/37 (24) | 4/26 (15) | 3/25 (12) |
| Clinical drug testing (% positive/tested) | ||||
| Benzodiazepines | 12/89 (13) | 6/43 (14) | 4/34 (12) | 4/32 (13) |
| Amphetamines | 6/89 (7) | 2/43 (5) | 1/34 (3) | 1/32 (3) |
| Cocaine | 19/89 (21) | 9/43 (21) | 7/34 (21) | 7/32 (22) |
| Cannabinoids | 60/89 (67) | 32/43 (74) | 25/34 (74) | 24/32 (75) |
| Ethanol | 11/49 (22) | 4/26 (15) | 3/20 (15) | 2/19 (11) |
| Any positive drug screen | 69/93 (74) | 35/43 (81) | 28/34 (82) | 27/32 (84) |
| Interventions | ||||
| Intubation | 12 (10) | 7 (13) | 7 (18) | 6 (18) |
| Cardiopulmonary resuscitation | 2 (2) | 2 (4) | 2 (5) | 1 (3) |
| Hemodialysis | 1 (1) | 1 (2) | 1 (3) | 1 (3) |
| Benzodiazepine administration | 33 (28) | 20 (36) | 14 (36) | 12 (36) |
| Antipsychotic administration | 23 (19) | 13 (23) | 8 (21) | 7 (21) |
| Naloxone administration | 8 (7) | 5 (9) | 3 (8) | 2 (6) |
| Highest level of care received and disposition | ||||
| Discharged from the emergency department | 88 (74) | 37 (66) | 26 (67) | 22 (67) |
| Left against medical advice | 9 (8) | 4 (7) | 0 | 0 |
| Admitted to general inpatient service | 13 (11) | 9 (16) | 6 (15) | 5 (15) |
| Admitted to intensive care unit | 12 (10) | 7 (13) | 7 (18) | 7 (21) |
| Death | 2 (2) | 1 (2) | 1 (3) | 0 |
CI: confidence interval; IQR: interquartile range; SC: synthetic cannabinoid; MAB-CHMINACA: (N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide.
The odds ratio of any reported pre-medication change in mental status between those testing positive for MAB-CHMINACA versus those who did not was 3.8 (95% confidence interval: 1.1–14.8, p = .02).
The odds ratio of documented altered mental status noted on physical exam between those testing positive for MAB-CHMINACA versus those who did not was 3.3, (95% confidence interval: 1.0–11.7, p = .05).
The most common abnormal laboratory finding was an elevated total serum creatinine kinase (CK ≥150 U/L), with 64/77 (83%) having CK levels consistent with rhabdomyolysis (median 499 units/L, maximum 100,000 units/L) (Table 4). Of the 97 patients whose serum creatinine levels were checked, 13 (13%) had a peak serum creatinine level ≥1.5 mg/dL. Of the 89 patients who had urine drug screens on arrival to the UMMC ED, 19 (21%) were positive for cocaine and 60 (67%) were positive by immunoassay for tetrahydrocannabinol, the main psychoactive component in cannabis (Table 4). The urine drug screen cannot detect SCs.
Treatments and outcomes of the UMMC patients are detailed in Table 4. No significant differences in patient outcomes were found between UMMC and non-UMMC patients. Twelve (10%) required intubation and mechanical ventilation. Only 1 (1%) UMMC patient required hemodialysis, after developing renal failure following cardiac arrest. Almost one-third (n = 33; 28%) received benzodiazepines. At UMMC, 12 (10%) patients were admitted to intensive care services, and 2 (2%) died. One of the decedents was asystolic upon arrival to UMMC, while the other developed pulseless electrical activity (PEA) arrest on hospital day 1.
Fifty-six patients (47%) had sufficient serum or plasma for SC testing at UCSF (Table 4). Over half (n = 39; 70%) tested positive for a SC. No differences in sex, past medical history, clinical presentation, outcomes, or results of urine drug screens were observed between patients who tested positive for SCs versus those that did not. Of the 39 patients who tested positive for a SC, most (n = 33; 85%) tested positive for N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide (MAB-CHMINACA), a recently recognized SC, or one of its metabolites. Other SCs associated with illness in this outbreak are listed in Table 5. Overall, 17 (44%) of those testing positive for SCs tested positive for multiple SC compounds.
Table 5.
Synthetic cannabinoids detected by liquid chromatography-quadrupole time-of-flight mass spectrometry (LC-QTOF/MS) in serum collected from patients with synthetic cannabinoid-associated illness—University of Mississippi medical center (UMMC), April 2–May 3, 2015.
| Synthetic cannabinoid detected | Number (% of total patients testing positive for synthetic cannabinoids, n = 39)a |
|---|---|
|
| |
| MAB-CHMINACA | 33 (85) |
| 5-fluoro-THJ | 8 (21) |
| AM 2201 N-(3-chloropentyl) isomer | 5 (13) |
| AB-PINACA | 4 (10) |
| AB-CHMINACA | 3 (8) |
| AM 1248 | 2 (5) |
| Other | 3 (8) |
17 (44%) patients tested positive for multiple synthetic cannabinoids. MAB-CHMINACA = N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(cyclohexyl-methyl)-1H-indazole-3-carboxamide.
5-fluoro-THJ =1-(5-fluoropentyl)-N-(quinolin-8-yl)-1H-indazole-3-carboxamide.
AM 2201 N-(3-chloropentyl) isomer = (1-(3-chloropentyl)-1H-indol-3-yl)(naphthalen-1-yl)methanone.
AB-PINACA = (S)-N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide.
AB-CHMINACA = N-[(1S)-1-(aminocarbonyl)-2-methylpropyl]-1-(cyclohexyl-methyl)-1H-indazole-3-carboxamide.
AM 1248 = 1-[(1-methyl-2-piperidinyl)methyl]-1H-indol-3-yl]tricyclo[3.3.1.13,7]-dec-1-yl-methanone.
Table 4 shows the clinical features of patients testing positive for MAB-CHMINACA exposure. No significant differences were found between the patients who tested positive for MAB-CHMINACA compared to those who did not in terms of demographics, past medical history, vital signs, clinical laboratory results, and interventions received. Patients testing positive for MAB-CHMINACA were more likely to have altered mental status documented by EMS or ED staff prior to medication administration (odds ratio [OR] = 3.8, p = .02) or have altered mental status specifically documented as a physical exam finding (OR = 3.3, p = .05). One of the two patients who died tested positive for SCs, but neither tested positive for MAB-CHMINACA. No significant differences were found in the need for inpatient admission (OR = 0.7, p = .9) or intensive care (OR = 5.8, p = .2) among patients testing positive for MAB-CHMINACA compared to those that did not. A higher proportion of patients admitted to the intensive care unit tested positive for MAB-CHMINACA compared to those testing positive for other SCs (21% versus 18%); however, this trend did not reach statistical significance.
Discussion
This investigation of an outbreak of SC-associated adverse health effects in MS was part of a large multi-state outbreak in the USA to date [13,14]. During April–May 2015, 2716 exposure calls related to SCs were made from 43 states, the District of Colombia, and Puerto Rico to U.S. poison centers, compared to 563 during April–May 2014 [15]. The predominant SC detected in Mississippi during this outbreak was MAB-CHMINACA. MAB-CHMINACA (also known as ADB-CHMINACA), an indazole-based SC with high affinity for the cannabinoid CB1 receptor [16], has been trafficked in the USA since October 2014. At least four deaths associated with MAB-CHMINACA use were reported worldwide prior to this outbreak, in Hungary, Japan, and the USA [7,17]. After an increase in ED visits in Baton Rouge, Louisiana associated with MAB-CHMINACA use, Louisiana state officials specifically banned it [18]. MS officials had legislated a general ban of SCs in 2013 [19]; hence, MAB-CHMINACA was illegal at the time of the outbreak. In February 2016, the U.S. Drug Enforcement Administration placed MAB-CHMINACA into temporary Schedule I, effectively banning the manufacture, sale, and possession of this specific compound in the USA [17].
During our field investigation, we used the MPCC database and visual dashboard as situational awareness tools to monitor the evolving outbreak. While dashboards have been used as a tool to quickly track and respond to infectious outbreaks, we demonstrated that they can also be used during non-infectious outbreaks. Using the integrated data from the dashboard, the MSDH was able to provide status updates of the outbreak to healthcare providers and the public through social media, newspaper and television stories, email alerts, and a MSDH “Spice” webpage. Conducting formal stakeholder evaluations and incorporating enhancements such as additional data sources, messaging platforms, links to hospitals and provider offices to request information and send updates, and mapping tools could further improve the usefulness of a dashboard system for future non-infectious outbreaks.
Compared with other SC-associated outbreaks, the affected population throughout the state was composed of a similar proportion of males; however, the affected population in MS was older (29 years versus early to mid-twenties) [20,21]. Like prior outbreaks, the affected population came from both urban and rural communities. The patients in this investigation presented with similar clinical effects as those described in published case series of people with MAB-CHMINACA poisoning: altered mental status, ranging from hyperactive and neuro-excitatory to hypoactive mental status [22,23]. In MS, we also noted that some patients oscillated between hyperactive and hypoactive mental statuses. Although acute effects could be severe, most affected individuals in our investigation recovered quickly.
Fewer physical examination and laboratory abnormalities were seen in this outbreak compared to those reported in outbreaks in Georgia and Colorado in 2013 [5,6], which were linked to a different SC, ADB-PINACA. For example, hyperglycemia, hypokalemia, and vomiting were commonly reported in the 2013 Georgia outbreak, but were not common features in the 2015 MS outbreak. The diversity of clinical effects reported during SC-related outbreaks has been attributed to the perpetual variability in composition and concentration of chemicals within SC products. The toxicokinetics of individual SCs are not well understood, and the clinical effects may vary among individuals. Variations in drug-related factors such as dose and duration of exposure could impact health effects. Furthermore, we found that concurrent exposure to other psychoactive drugs (including multiple SCs) is common and may confound a clear clinical syndrome.
On May 1, 2015, authorities from the MS Bureau of Narcotics confiscated SCs along with empty packets printed with a variety of known brand names and heat sealing devices (Figure 4). Users, health care providers, health departments, and law enforcement should be aware that it is impossible to draw conclusions about the contents of a packet of SC product based on a brand name or label. Almost half of patients testing positive for SCs tested positive for multiple compounds, suggesting that either products on the market are mixtures of different SCs or patients used multiple products. Past reports have also found other psychoactive and toxic compounds within SC product mixtures, including cathinones and opioids [24].
Figure 4.

Packets of synthetic cannabinoid products confiscated by the Mississippi Bureau of Narcotics, May 1, 2015.
We decided to include the cases without laboratory confirmation of SC exposure in the analyses. Little is known about the metabolism and half-lives of most SCs, including MAB-CHMINACA. Excluding the SC-negative cases would likely exclude true SC-related illness, because laboratory testing by QToF-MS is limited to metabolites that have been predicted to occur based on the chemical structure of the parent compound. Also, the manufacturer who synthesized the predicted compounds was unable to synthesize all the predicted compounds for laboratory analysis.
Limitations
While using the preexisting MPCC and NPDS systems for outbreak surveillance allowed us to quickly quantify and respond to the outbreak, one potential limitation of using these data for outbreak surveillance is reporting lag. MSDH issued reporting instructions to all MS hospitals on April 13th. The dates recorded in the MPCC database are the dates that cases were entered in the database, not the exposure dates. The MPCC data show that the number of cases occurring at UMMC peaked the first week of April before the number of statewide cases, which peaked from mid-April to mid-May. The difference may represent a reporting lag artifact. While MSDH requested hospitals to submit daily reports, MPCC noted inconsistencies in reporting times between hospitals. We cannot discern if any non-UMMC cases occurring before this date were simply not reported, if they occurred earlier than the report date, or if the outbreak truly started in Jackson and did not reach other areas of the state until later in the month.
Another potential limitation to using poison center data for surveillance is the under-recognition of cases, particularly as an outbreak evolves. When providers become more comfortable with managing patients with a particular toxic exposure, they may be less likely to seek a poison center consultation [25]. The MSDH addressed this limitation by issuing explicit instructions to providers to use the MPCC as a reporting mechanism; however, underreporting, particularly of less severe cases, is a possibility. Conversely, over-reporting was also possible following the health action alert and increased media attention; as providers were reporting all agitated patients, those experiencing intoxications with other psychoactive substances or acute episodes of psychiatric illness not related to SCs were also likely included. We chose to maintain a broader, clinical case definition rather than a laboratory-based case definition, but we have likely included individuals who were not exposed.
Conclusions
The described outbreak of adverse health effects associated with SC use adds to the growing body of evidence that SCs can cause severe health outcomes, including death. The outbreak appeared to be associated primarily with one particular cannabinoid, MAB-CHMINACA, but not to a particular brand or label. Patients with detectable MAB-CHMINACA were more likely to have mental status changes compared to those who did not. During the outbreak, we were able to quickly leverage preexisting data systems to provide near real-time, case-based definition surveillance. The timeliness of poison center data allowed health officials to understand the magnitude and severity of the outbreak, conduct an epidemiologic investigation, and distribute targeted messaging to healthcare providers and the public while the outbreak was ongoing. However, investigations may be limited by underreporting of cases. As novel SCs continue to be developed and trafficked, health care providers, public health officials, laboratory scientists, and law enforcement should continue to work together to identify strategies to curb SC use, strengthen surveillance for adverse events, and optimize patient care.
Funding
This study was supported by the US Centers for Disease Control and Prevention.
APPENDIX 1. Mississippi poison control center line list

Footnotes
Disclosure statement
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the institutions with which the authors are affiliated.
References
- [1].Ciolino LA. Quantitation of synthetic cannabinoids in plant materials using high performance liquid chromatography with UV detection (validated method). J Forensic Sci. 2015;60:1171–1181. [DOI] [PubMed] [Google Scholar]
- [2].DrugFacts: high school and youth trends. National Institute on Drug Abuse website [cited 2016 Jul 20]. Available from: https://www.drugabuse.gov/publications/drugfacts/high-school-youth-trends.
- [3].Palamar JJ, Acosta P. Synthetic cannabinoid use in a nationally representative sample of US high school seniors. Drug Alcohol Depend. 2015;149:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Every-Palmer S Synthetic cannabinoid JWH-018 and psychosis: an explorative study. Drug Alcohol Depend. 2011;117:152–157. [DOI] [PubMed] [Google Scholar]
- [5].Centers for Disease Control and Prevention. Notes from the field: severe illness associated with synthetic cannabinoid use—Brunswick, Georgia, 2013. MMWR Morb Mortal Wkly Rep. 2013;62:939. [PMC free article] [PubMed] [Google Scholar]
- [6].Centers for Disease Control and Prevention. Notes from the field: severe illness associated with reported use of synthetic marijuana — Colorado. MMWR Morb Mortal Wkly Rep. 2013;62:1016–1017. [PMC free article] [PubMed] [Google Scholar]
- [7].Trecki J, Gerona RR, Schwartz MD. Synthetic cannabinoid-related illnesses and deaths. N Engl J Med. 2015;373:103–107. [DOI] [PubMed] [Google Scholar]
- [8].Tyndall JA, Gerona R, De Portu G, et al. An outbreak of acute delirium from exposure to the synthetic cannabinoid AB-CHMINACA. Clin Toxicol (Phila). 2015;53:950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Centers for Disease Control and Prevention. Acute kidney injury associated with synthetic cannabinoid use—multiple states, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:93–98. [PMC free article] [PubMed] [Google Scholar]
- [10].Louh IK, Freeman WD. A ‘spicy’ encephalopathy: synthetic cannabinoids as cause of encephalopathy and seizure. Crit Care. 2014;18:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Castaneto MS, Gorelick DA, Desrosiers NA, et al. Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014;144:12–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lapoint J, James LP, Moran CL, et al. Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol. 2011;49:760–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Law R, Schier J, Martin C, et al. Increase in reported adverse health effects related to synthetic cannabinoid use—United States. MMWR Morb Mortal Wkly Rep. 2015;64:618–619. [PMC free article] [PubMed] [Google Scholar]
- [14].Kasper AM, Ridpath AD, Arnold JK, et al. Severe illness associated with reported use of synthetic cannabinoids—Mississippi, April 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1121–1122. [DOI] [PubMed] [Google Scholar]
- [15].Synthetic Cannabinoids [cited 2018 May 26]. Available from: The American Association of Poison Control Centers website. http://www.aapcc.org/alerts/synthetic-cannabinoids.
- [16].Banister SD, Longworth M, Kevin R, et al. Pharmacology of Valinate and tert-Leucinate synthetic cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and their analogues. ACS Chem Neurosci. 2016;7:1241–1254. [DOI] [PubMed] [Google Scholar]
- [17].Schedules of controlled substances: Temporary placement of the synthetic cannabinoid MAB-CHMINACA into Schedule I. Federal register 81 (24). Drug Enforcement Administration website; [cited 2016 Jul 20]. Available from: http://www.deadiversion.usdoj.gov/fed_regs/rules/2016/fr0205_2.htm. [PubMed] [Google Scholar]
- [18].DHH Bans Another Synthetic Drug. Baton Rouge, LA WAFB 9 News website. October 29, 2014. [cited 2015 May 11]. Available from: http://www.wafb.com/story/27157019/dhh-bans-another-synthetic-drug.
- [19].Schedule I of controlled substances, MS Code § 41–29-113 (2013). [Google Scholar]
- [20].Hoyte CO, Jacob J, Monte AA, et al. A characterization of synthetic cannabinoid exposures reported to the National Poison Data System in 2010. Ann Emerg Med. 2012;60:435–438. [DOI] [PubMed] [Google Scholar]
- [21].Wood KE. Exposure to bath salts and synthetic tetrahydrocannabinol from 2009 to 2012 in the United States. J Pediatr. 2013;163:213–216. [DOI] [PubMed] [Google Scholar]
- [22].Adamowicz P, Gieroń J. Acute intoxication of four individuals following use of the synthetic cannabinoid MAB-CHMINACA. Clin Toxicol. 2016;54:650–654. [DOI] [PubMed] [Google Scholar]
- [23].Katz KD, Leonetti AL, Bailey BC, et al. Case series of synthetic cannabinoid intoxication from one toxicology center. Westjem. 2016;17:290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dresen S, Ferreiros N, Putz M, et al. Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. J Mass Spectrom. 2010;45:1186–1194. [DOI] [PubMed] [Google Scholar]
- [25].Hoffman RS. Poison centers and poison epidemiology. In Hoffman R, Howland M, Lewin N, Nelson L, Goldfrank L, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York: McGraw Hill Education, 2015:1729–1730. [Google Scholar]


