Abstract
The spliceosome performs two consecutive transesterification reactions using one catalytic center, thus requiring its rearrangement between the two catalytic steps of splicing. The Prp16 ATPase facilitates exit from the first-step conformation of the catalytic center by destabilizing some interactions important for catalysis. To better understand rearrangements within the Saccharomyces cerevisiae catalytic center, we characterize factors that modulate the function of Prp16: Cwc2, N-terminal domain of Prp8, and U6-41AACAAU46 region. Alleles of these factors were identified through genetic screens for mutants that correct cs defects of prp16-302 alleles. Several of the identified U6, cwc2, and prp8 alleles are located in close proximity of each other in cryo-EM structures of the spliceosomal catalytic conformations. Cwc2 and U6 interact with the intron sequences in the first step, but they do not seem to contribute to the stability of the second-step catalytic center. On the other hand, the N-terminal segment of Prp8 not only affects intron positioning for the first step, but it also makes important contacts in the proximity of the active site for both the first and second steps of splicing. By identifying interactions important for the stability of catalytic conformations, our genetic analyses indirectly inform us about features of the transition-state conformation of the spliceosome.
Keywords: spliceosomal catalytic center, transition-state conformation, Prp16, U6 snRNA, Prp8
INTRODUCTION
The spliceosome, a highly dynamic molecular machine composed of five small nuclear RNAs (U1, U2, U4, U5, and U6 snRNAs) and over 100 proteins (Burge et al. 1999; Wahl et al. 2009), is responsible for splicing of all pre-mRNAs. The spliceosome assembles on each intron de novo and requires extensive compositional and conformational remodeling of its small nuclear ribonucleoprotein (snRNP) subunits to create the functional catalytic center. Substrate recognition is facilitated by pairing interactions with snRNAs: The branch site (BS) is bound by U2 snRNA (Parker et al. 1987), whereas the 5′ splice site (5′SS) is initially recognized by U1 snRNA (Zhuang and Weiner 1986; Séraphin et al. 1988; Siliciano and Guthrie 1988), and subsequently, within the catalytic spliceosome, it interacts with U6-ACA within the conserved 47ACAGA51 motif (Kandels-Lewis and Séraphin 1993; Lesser and Guthrie 1993b). The cryo-EM structures capture the spliceosome at various stages of splicing and depict its catalytic center as a stable, unchanged structure (Galej et al. 2016; Yan et al. 2016, 2017; Bertram et al. 2017a, b; Fica et al. 2017; Plaschka et al. 2017; Bai et al. 2018; Wan et al. 2019). In contrast, genetic data indicate that the catalytic center must change between the two catalytic steps of splicing to allow for the repositioning of substrates for catalysis (Madhani and Guthrie 1994; Mefford and Staley 2009; Eysmont et al. 2019).
Consecutive rearrangements of the spliceosome are facilitated by dedicated ATPases/RNA helicases of the DExH/D-box family, e.g., Prp5, Prp28, Brr2, Prp2, Prp16, Prp22, and Prp43, acting at different stages of the splicing pathway, from assembly through catalytic reactions, to the release of products and spliceosome recycling (Staley and Guthrie 1998). Thus, monitoring defects caused by ATPase alleles and identifying suppressors of these defects allow us to study conformational changes of the spliceosome at the selected transition in the splicing process. For example, Prp2 remodels protein interactions around the BS, facilitating the entry of the spliceosome into the first catalytic conformation (Kim and Lin 1996; Warkocki et al. 2009; Lardelli et al. 2010). Thus, prp2 alleles inhibit entry into the first-step catalytic conformation. Prp16 facilitates the transition between the first and second catalytic steps (Schwer and Guthrie 1991, 1992; Burgess and Guthrie 1993; Query and Konarska 2004; Villa and Guthrie 2005). In Saccharomyces cerevisiae, a defective prp16-302 allele carrying two point mutations, R456K + G691R in the ATPase domain, can serve as a convenient stage-specific marker that stabilizes the first-step conformation of the spliceosome, resulting in improved first-step catalysis but reduced progression to the second step. Thus, the cold-sensitivity (cs) defects of prp16-302 reflect an inefficient exit from the first-step conformation. Suppressors of prp16-302 are expected to destabilize the first-step conformation and facilitate the transition from the first-to-second steps of splicing.
The RNA core of the catalytic center of the spliceosome involves sequences of U2, U6, and U5 snRNAs. Elements of U6 and U2 snRNAs form the catalytic triplex (Fica et al. 2014), which, together with the adjacent U6 intramolecular stem–loop (U6-ISL), coordinate metal ions for catalysis (Steitz and Steitz 1993) and through adjacent U2-34GUAGUA39 and U6-47ACAGA51 interactions, help to juxtapose the BS and 5′SS for catalysis. Despite the mostly unchanged structure of the RNA catalytic core seen in cryo-EM structures, multiple elements of U6 and U2 have been genetically identified to suppress the cs defects of prp16 alleles, indicating that they destabilize structures of the first-step catalytic conformation of the spliceosome (U6-41AACAAU46: [Madhani and Guthrie 1994]; U2 stem IIa/IIc: [Hilliker et al. 2007; Perriman and Ares 2007]; U2/U6 helix Ia: [Mefford and Staley 2009]; catalytic triplex: [Fica et al. 2014]; U6-lower ISL: [Fig. 1B; Eysmont et al. 2019]). Although snRNA mutants are thought to destabilize the catalytic conformation in all these cases, the detailed mechanisms responsible for the improved transition to the second-step conformation are unknown.
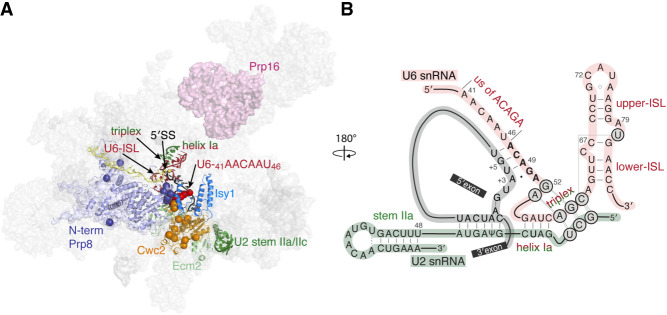
FIGURE 1.
Structural representation of spliceosomal components involved in the first-to-second steps transition. (A) Structure of S. cerevisiae spliceosome immediately after branching (5LJ5) (Galej et al. 2016). Spliceosome factors involved in the transition between the first and second steps are shown in color: Prp16 (pink), Isy1 (blue), Ecm2 (light green), U6–U2 helix Ia and the catalytic triplex (red/green), U2 stem IIa/IIc (green), U6-ISL and U6-41AACAAU46 (red), Cwc2 (orange), and Prp8 N-terminal domain (purple). Location of cwc2, U6, and prp8 alleles suppressing prp16-302 growth defects described in this paper are marked by spheres. (B) Schematic representation of U6 (red), U2 (green), and pre-mRNA (gray) at the catalytic center of the spliceosome. Base-pair interactions altered by Prp16 action are depicted.
Analysis of U6 alleles in the catalytic triplex and the adjacent ISL revealed the presence of two competing conformations that modulate the transition between the two catalytic steps (Eysmont et al. 2019). A cluster of U6 alleles in the region of 41AACAAU46 (Fig. 3B) has been previously identified to suppress the cs phenotype of prp16 alleles (Madhani and Guthrie 1994), thus improving transition to the second-step conformation. Because this cluster of alleles is adjacent to the U6-47ACAGA51 motif known to bind the 5′SS, we sought to understand its function better.
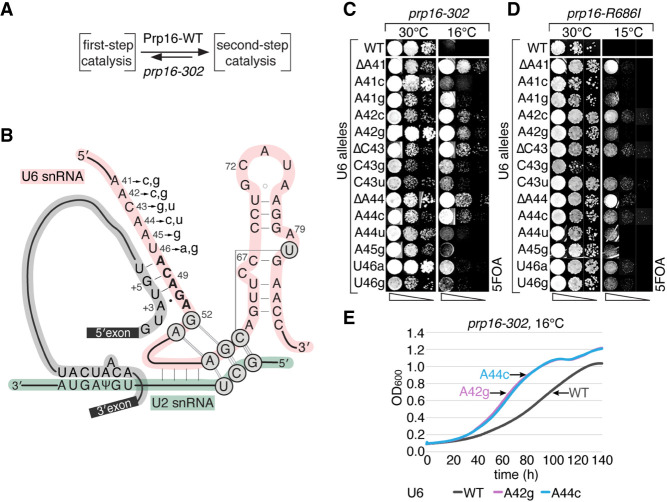
FIGURE 3.
U6 alleles upstream of ACAGA motif support transition from the first-to-second steps of splicing. (A) Prp16 promotes transition from the first to the second step, whereas prp16-302 allele inhibits this transition. (B) Schematic representation of U6 (red), U2 (green), and pre-mRNA (gray) at the catalytic center of the spliceosome. Mutations of U6 in the 41AACAAU46 region are marked. In prp16Δ U6Δ strain (yCQ166), prp16-302 (C) and prp16-R686I (D) alleles inhibit transition to the second step, exhibiting cs phenotypes suppressed by mutations within the U6 region 41AACAAU46, as shown by spotting on 5FOA plates. (E) Growth curves (OD600) of yeast strains harboring prp16-302 allele in combination with U6-A42g and A44c alleles exhibit improved growth, compared to U6-WT at 16°C.
In addition to U2 and U6 alleles, multiple changes in protein components of the spliceosome have been identified to suppress defects of prp16 alleles and are thus predicted to affect the conformational change between the catalytic steps (Fig. 1A). Deletion of isy1, a component of the NineTeen Complex (NTC), suppresses prp16-302 defects and has been shown to genetically interact with mutations in the U6 upstream of the ACAGA region (Villa and Guthrie 2005). Deletion of NTC-associated protein ecm2 suppresses prp16-R686I defects (van der Feltz et al. 2021). Additionally, biochemical analyses indicate that weakened binding of the first-step factor Yju2 bypasses the requirement of Prp16 for the transition to the second step (Chiang and Cheng 2013). Cwc2 protein, an NTC component (Ohi and Gould 2002), joins the spliceosome during the activation for the first step of catalysis, stabilizes intron:U6 interactions, and contacts the U6-41AACAAU46 region (McGrail et al. 2009; Rasche et al. 2012; Schmitzova et al. 2012; Hogg et al. 2014).
Prp8, a large, highly conserved spliceosomal protein, directly contacts all of the components of the catalytic center. Prp8 joins the spliceosome as a part of the U5·U4/U6 snRNP and remains in the spliceosome throughout the catalysis (for review, see Grainger and Beggs 2005). Previously, alleles within the Prp8 linker domain (1376–1649 aa), endonuclease domain (1653–1824 aa), and RNaseH-like domain (1839–2092 aa) that suppress the first- or second-step defects in splicing catalysis were described (Collins and Guthrie 1999; Liu et al. 2007). These alleles are thought to stabilize the first or the second catalytic conformations, although specific structural changes leading to this stabilization are not known. However, no prp8 alleles capable of suppressing prp16 alleles defects have been identified. The Prp8 N-terminal domain (1–884 aa) is the least well-studied part of this protein.
Manipulation of Prp16 function by using wt versus mutant alleles provides a sensitive tool for monitoring interactions at the catalytic center. Using the yeast S. cerevisiae system, we explore contributions of three different classes of suppressors of prp16-302 growth defects to altering stability of the catalytic center. Alleles in Cwc2, U6, and Prp8 correct prp16-302 defects by destabilizing the first-step catalytic conformation. All these alleles seem to act by destabilizing the 5′SS positioning for the first-step catalysis, whereas prp8 alleles are likely to additionally alter catalytic interactions at the active site affecting both catalytic conformations.
RESULTS
A genetic screen to identify cwc2 alleles that destabilize the first-step conformation
A pool of CWC2 sequences generated by error-prone PCR mutagenesis was introduced into a prp16Δ yeast strain carrying the prp16-302 allele (thus, in the presence of a wt genomic copy of CWC2) by homologous recombination (gap repair). To increase the yield of analyzed mutants, selection was carried out in parallel for two CWC2 regions, spanning the segments corresponding to 1–159 aa and 159–339 aa. The screen identified 13 cwc2 alleles that rescue the cs phenotype of prp16-302 allele at 16°C (Fig. 2D), including a previously described cwc2-W37A allele in the Torus domain (Hogg et al. 2014). For the strongest cwc2 alleles (W37A and S38P), suppression of prp16-302 defects was confirmed by measuring growth rates in liquid media (Fig. 2F). Nearly all of the identified alleles (cwc2-E17D, W37A, S38P, P55S, Q56H, D98V, K101T, C111R, F162I, and W225R) also suppress cs defects of prp16-R686I allele at 15°C, implying the generality of these effects (Fig. 2E).
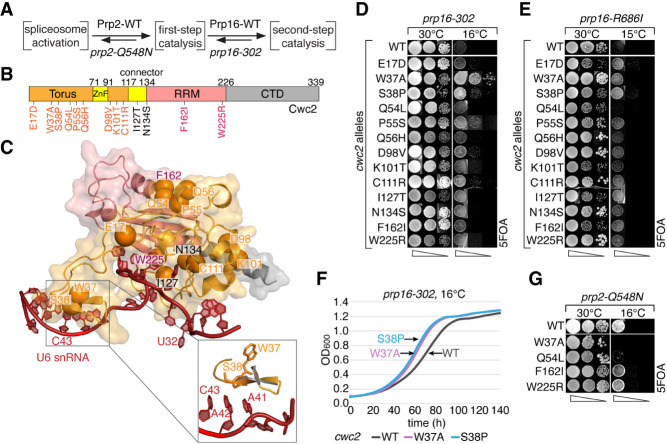
FIGURE 2.
cwc2 alleles identified in a genetic screen alter the first-step conformation, supporting transition to the second step. (A) Schematic of the catalytic phase of splicing. Conformations of the catalytic center are shown in brackets, and factors affecting transitions between these conformations are listed above (promoting) or below the arrows (inhibiting the transition). (B) Schematic of the yeast Cwc2 protein domain structure. Positions of alleles identified in the genetic screen are marked. (C) cryo-EM structure of Cwc2 (orange) and part of U6 snRNA (red) within the spliceosome complex C (5LJ5) (Galej et al. 2016). Positions of cwc2 alleles are marked as spheres. The inset shows two Cwc2 amino acids, W37 and S38, adjacent to U6 C43-G39. In prp16Δ strain (yMK36), the cs growth phenotype of (D) prp16-302 and (E) prp16-R686I alleles is suppressed by the identified cwc2 alleles, as shown by spotting on 5-fluoroorotic acid (5FOA) plates. (F) OD600 measurements of yeast strains harboring prp16-302 allele and a second copy of cwc2-W37A and S38P alleles show an improved growth as compared to Cwc2-WT at 16°C. (G) In prp2Δ strain (yAAH1915), cwc2 alleles W37A and Q54L exacerbate the prp2-Q548N cs phenotype, as shown by spotting on 5FOA plates.
The majority of identified cwc2 alleles are located in the Torus domain (cwc2-E17D, W37A, S38P, Q54L, P55S, Q56H, D98V, K101T, and C111R), two alleles are in the connector (I127T and N134S) and three are found in the RRM domain (Y138F, F162I, and W225R). The only Cwc2 region which did not yield any prp16 suppressors is its C-terminal domain (CTD) known to interact with the WD40 domain of Prp19 (Fig. 2B; Ohi and Gould 2002). Spliceosomal cryo-EM structures position cwc2-W37 and S38 in proximity of the U6-41AAC43 and the N-terminal domain of Prp8 (Figs. 2C and 4A) (5LJ5, Galej et al. 2016).
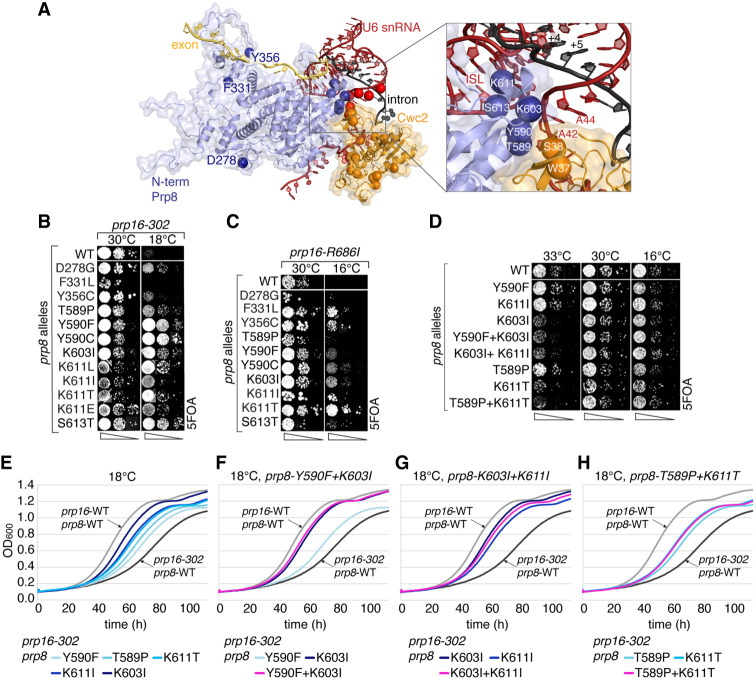
FIGURE 4.
prp8 alleles located in the N-terminal domain suppress prp16 defects. (A) Prp8 N-terminal domain (purple), U6 (red), Cwc2 (orange), and pre-mRNA (intron-black, 5′ exon-yellow) as seen in complex C (5LJ5) (Galej et al. 2016). Locations of prp8, cwc2, and U6 alleles are marked by spheres. The inset: a region of close interactions between Prp8, Cwc2, and U6. In prp16Δ prp8Δ strain (yCQ06), the cs phenotype of prp16-302 (B) and prp16-R686I (C) alleles is suppressed by mutations in the Prp8 N-terminal domain identified in the genetic screen. (D) Growth of combinations of prp8 alleles Y590F + K603I, K603I + K611I, and T589P + K611T is not affected compared to single mutations. Suppression effects in B–D were monitored by spotting of the analyzed strains on 5FOA plates. (E) Growth curves (OD600) of yeast strains harboring prp16-302 allele in combination with prp8-Y590F, T589P, K611T/I, and K603I alleles show improved growth compared to Prp8-WT at 18°C. Growth curves of combinations of prp8 alleles Y590F + K603I (F), K603I + K611I (G), and T589P + K611T (H) demonstrate no additive growth improvement of strains carrying prp16-302 allele at 18°C compared to single alleles.
Since cwc2 mutants correct prp16 defects by destabilizing first-step interactions, they also could inhibit entry into the first-step conformation, exacerbating defects of prp2 alleles (Fig. 2A). To investigate whether cwc2 alleles impact splicing at the spliceosome activation step, we looked for their genetic interactions with prp2-Q548N, a cs allele defective in the spliceosome activation (Fig. 2G; Wlodaver and Staley 2014). Both cwc2-W37A and Q54L exacerbate growth defects of prp2-Q548N at 16°C in the presence of a chromosomal copy of Cwc2, supporting the notion that they destabilize the first-step catalytic conformation.
Together, these data indicate that changes in Cwc2–U6 interactions destabilize interactions in the first-step catalytic center, inhibiting entry into and facilitating exit from the first-step spliceosomal conformation.
Multiple mutations in the U6-41AACAAU46 motif correct defects of prp16-302 allele, altering the transition between the first and second steps of splicing
Both biochemical and structural analyses indicate close contacts of Cwc2 with U6, including U6-ISL and the region upstream of ACAGA motif (McGrail et al. 2009; Rasche et al. 2012; Schmitzova et al. 2012; Hogg et al. 2014).
Therefore, to test if Cwc2–U6 interactions functionally affect the first-step spliceosomal conformation, we analyzed the known U6 alleles located upstream of the ACAGA motif (Fig. 3B) that suppress cs defects of prp16 alleles (Madhani and Guthrie 1994). To better understand the function of this U6 region, we prepared several single-point U6 alleles: ΔA41, A41c/g, A42c/g, ΔC43, C43g/u, ΔA44, A44c/u, A45g, and U46a/g, transformed them into a double-delete ΔU6 Δprp16 yeast strain, and confirmed their ability to suppress prp16-302 cs defects at 16°C (Fig. 3C). Sensitive growth curve analyses confirm these suppression effects at 16°C; prp16-302 strains carrying U6-A42g or A44c alleles grow faster than those carrying wt U6 (Fig. 3E). Similar suppression by U6 alleles was also observed for prp16-R686I allele at 15°C (except for U6-A41c and C43g alleles) (Fig. 3D). These findings confirm and extend earlier reports (Lesser and Guthrie 1993b; Madhani and Guthrie 1994), demonstrating that multiple mutations in the U6 region upstream of the ACAGA motif suppress growth defects of prp16 alleles by destabilizing interactions essential for the first-step catalytic conformation.
The N-terminal domain prp8 alleles suppress prp16-302 cs growth defects
To better understand interactions stabilizing the catalytic center, we next focused on Prp8, the largest component of the spliceosome that surrounds the RNA catalytic core. We carried out a genetic screen for prp8 alleles within the Prp8 N-terminal domain that suppress cs defects of prp16-302. A library of PCR-mutagenized prp8 segments corresponding to aa 4–838 was introduced by homologous recombination into PRP8 plasmids in a prp8Δ, prp16Δ yeast strain harboring prp16-302 allele. Resulting alleles were selected to improve strain growth at 18°C (Fig. 4B). This screen yielded multiple prp8 alleles located close to the catalytic center: prp8-T589P, Y590F/C, K603I, K611L/T/I/E, S613T, as well as a few more distally positioned alleles: D278G, F331L, and Y356C (Fig. 4A) (5LJ5, Galej et al. 2016). These prp8 alleles significantly suppress growth defects of prp16-302 allele (Fig. 4B), and most of them (prp8-F331L, Y356C, Y590C/F, K603I, K611I/T, and S613T) also correct growth defects of prp16-R686I, demonstrating the generality of these effects (Fig. 4C). Suppression of prp16-302 cs defects by selected prp8 alleles was also confirmed by growth curve measurements (Fig. 4E), with the strongest suppression phenotype observed for prp8-K603I. These results suggest that destabilization of N-terminal Prp8 domain contacts with the catalytic core facilitates exit from the first-step conformation. Based on their location, prp8-T589P, Y590F/C, K603I, K611L/T/I/E, and S613T alleles are expected to disrupt interactions with the catalytic center, suggesting that this part of Prp8, together with U6 and Cwc2, stabilize substrate positioning for the first-step catalysis (Fig. 4A). prp8 alleles located further away from the catalytic center may act by a different mechanism. Although prp8-F331L was isolated from the original screen for prp16-302 suppression, upon retesting, it was found to suppress prp16-R686I but not prp16-302. In contrast, prp8-Y356C suppresses prp16-302 but not prp16-R686I. Whereas the location of prp8-F331L suggests its possible interactions with Snu114 (Brenner and Guthrie 2005), the mechanism of action of prp8-D278G and Y356C alleles needs further investigation.
In general, additivity of effects of combined alleles is observed when they individually affect the same process but act at different points or follow distinct mechanisms. Furthermore, combination of alleles that individually confer partial effects, acting by the same mechanism and affecting the same point in the process, will either lead to additivity (if they act in the same direction) or partial cancellation of effects (if they act in opposing directions). In contrast, the action of so-called “null” alleles, which by themselves are sufficient to fully exert a particular function, is not affected by combination with other null or partial alleles that act in the same process in the same direction. To test if the isolated prp8 alleles located in the proximity of the catalytic center act through a single or different mechanisms, we prepared several double mutants and tested their effects on growth (Fig. 4D) and on suppression of prp16-302 defects (Fig. 4F–H). Importantly, double mutants of prp8 N-terminal domain Y590F + K603I, K603I + K611I, and T589P + K611T do not exert synthetic effects, as expected of alleles null for a function at a single event in the pathway (Fig. 4D). Each of the prp8 alleles suppresses prp16-302 defects (Fig. 4E) to a different extent, with K603I and K611I/T exerting the strongest suppression. Most of the tested combinations of mutant alleles, Y590F + K603I, K603I + K611I, and T589P + K611T, do not display additive effects consistent with their classification as null alleles (Fig. 4F–H). Only a combination of T589P + K611I alleles does not follow this pattern as they act additively both for suppression of prp16-302 defects (Supplemental Fig. S1A) and for ts growth phenotypes (Supplemental Fig. S1B), consistent with their partial allele status.
Together, we identified multiple alleles of cwc2, U6, and prp8 that suppress defects of prp16-302 and thus facilitate the first-to-second steps transition. Some of the cwc2 alleles are located in close proximity to the U6-41AACAAU46 motif, consistent with the described Cwc2–U6 interaction (Rasche et al. 2012). However, almost any disruption of the Cwc2 structure appears to correct prp16-302 cs defects, suggesting that overall stability/folding of Cwc2 is important for the function of the first-step catalytic center. Striking clustering of several prp8 N-terminal alleles close to U6/cwc2 mutants suggests their functional similarity.
Effects of the U6, cwc2, and prp8 alleles on splicing of suboptimal introns
To further characterize the identified prp16 suppressors, we analyzed their effects on splicing of suboptimal intron mutants defective in the first and/or second steps of splicing. Introns carrying an A–C mutation at the branch site (BS-c) are defective at both catalytic steps of splicing (Query and Konarska 2004), an A–C change at the intron position +3 (5′SS-A3c) inhibits transition to the second step (Konarska et al. 2006), and 3′SS-gAG/ mutation inhibits second-step catalysis (Fig. 5A; Collins and Guthrie 2001; Liu et al. 2007). Splicing of ACT1–CUP1 reporters (Lesser and Guthrie 1993a) produces functional metallothionein Cup1, allowing ΔCUP1 strains to tolerate the presence of copper proportionally to the efficiency of splicing.
FIGURE 5.
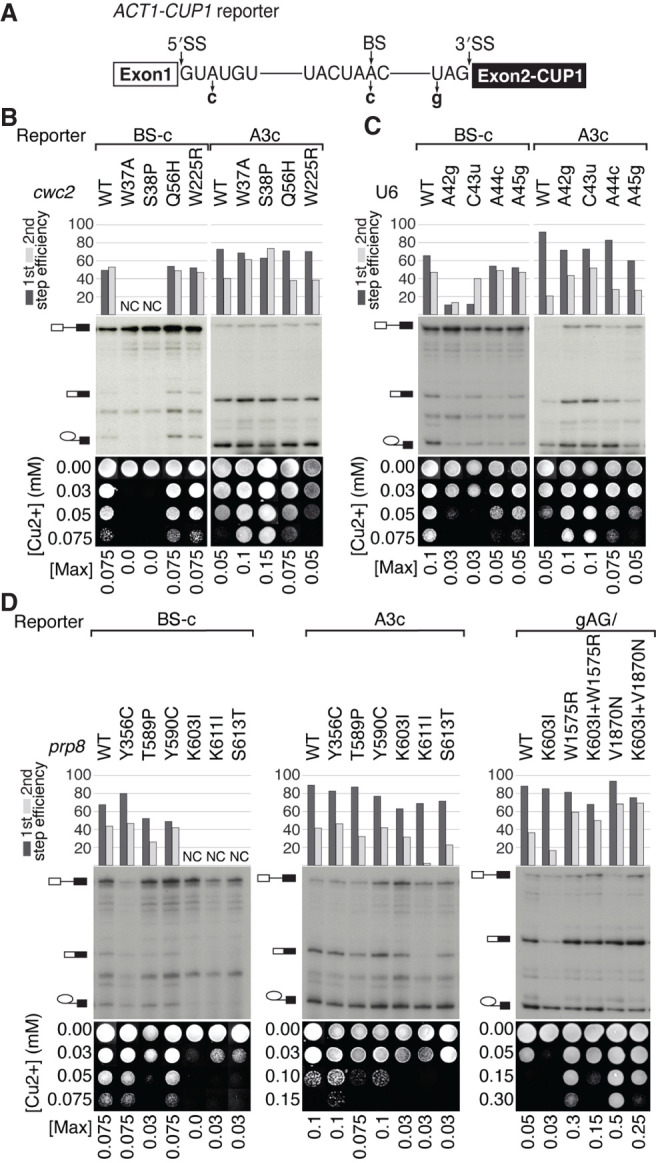
Effects of U6, cwc2, and prp8 alleles on splicing of suboptimal introns. (A) Schematic of ACT1–CUP1 reporter indicating the used 5′SS, BS, and 3′SS mutants. (B–D) Primer extension and copper growth assays for intron mutant reporters in strains carrying U6 (yMK20), cwc2 (yMK79), or prp8 (yJU75) alleles. (NC) Not calculated. (B) cwc2-W37A and S38P alleles exacerbate splicing defects of BS-c reporters (NC: bands corresponding to lariat-intermediate and spliced mRNA products are not detectable, preventing calculation of splicing efficiency) and improve the second step of splicing of A3c reporters. (C) U6-A42g and C43u alleles exacerbate the first step of splicing of the BS-c reporter while improving the second step of splicing of the A3c reporter (U6-A44c and A45g alleles display a similar, though very modest effect). (D) prp8-T589P, K603I, K611I, S613T alleles inhibit splicing of BS-c (for K603I, K611I, and S613T, alleles bands corresponding to lariat-intermediate and spliced mRNA products are not visible, preventing calculation of splicing efficiency) and A3c reporters. prp8-V1870N and W1575R second-step alleles improve splicing of gAG/reporters and prp8-K603I inhibits it. prp8-K603I allele opposes prp8 second-step W1575R allele, diminishing its improvement of gAG/intron splicing. Only the first step of gAG/intron splicing is inhibited by prp8-K603I + V1870N allele compared to prp8-V1870N allele.
To confirm the predicted role of Cwc2 stabilizing the first-step interactions, we analyzed splicing of intron reporters in yeast carrying various cwc2 alleles (Fig. 5B). Among all cwc2 alleles that suppress prp16-302 defects, only cwc2-W37A and S38P significantly inhibit splicing of BS-c introns, as evidenced by reduced copper tolerance and reduced accumulation of the first-step products in primer extension analysis (Fig. 5B). However, the same alleles improve splicing of A3c introns, as shown by increased copper tolerance and increased accumulation of the second-step products in the corresponding primer extension results (Fig. 5B). Furthermore, within the spliceosome structure, these Cwc2 residues map near U6-AC42,43 (Fig. 2C), suggesting that destabilization of Cwc2–U6 interactions downstream from the 5′SS improves progression to the second step of splicing. Nonetheless, cwc2 alleles do not significantly affect splicing of gAG/introns defective in the second catalytic step (Supplemental Fig. S2B).
All analyzed U6 suppressors of prp16-302 defects inhibit splicing of BS-c introns, with U6-A42g and C43u exhibiting the strongest effects as evidenced by copper tolerance and primer extension (Fig. 5C). In contrast, the second step of splicing of A3c introns is strongly improved by U6-A42g and C43u, with a slight improvement seen for A44c and A45g (Fig. 5C). By analogy to cwc2 effects, U6 upstream of ACAGA alleles do not significantly affect splicing of gAG/ reporters (Supplemental Fig. S2C). Thus, changes within U6-41AACAAU46 inhibit the first step of splicing, consistent with their prp16-302 suppressor effect, with U6-A42g and C43u alleles particularly strongly destabilizing positioning of the 5′SS.
Finally, we analyzed prp8 suppressor alleles for their effects on splicing. Four of them, T589P, K603I, K611I, and S613T, inhibit first and second steps of the splicing of BS-c reporters, as evidenced by reduced copper tolerance and reduced (T589P) or not detectable (K603I, K611I, and S613T) accumulation of splicing products in primer extension analysis (Fig. 5D). Additionally, prp8-Y590C slightly inhibits the first step of splicing of BS-c reporters, as seen in primer extension results, but this inhibition is not detectable by copper assay (Fig. 5D). The strongest inhibition was observed for K603I, K611I, and S613T alleles, located in proximity to the active site (Fig. 4A). In contrast to U6 and cwc2 alleles that improve the second step for A3c reporters, the tested prp8-T589P, K603I, K611I, and S613T alleles inhibit it, as evidenced by copper assays and primer extension analyses, with K611I exhibiting the strongest effect (Fig. 5D). We also analyzed the effect of prp8 N-terminal K603I allele on splicing of the 3′SS-gAG/reporter defective for the second step. Whereas prp8 second-step alleles V1870N and W1575R improve splicing of the 3′SS-gAG/introns (Liu et al. 2007), N-terminal K603I allele inhibits the second step and diminishes the second-step improvement of prp8-W1575R allele (K603I + W1575R) (Fig. 5D). The analogous combination of prp8-K603I with the strongest second-step allele, prp8-V1870N, only slightly inhibits the first step (K603I + V1870N).
Whereas the analyzed U6 and cwc2 alleles inhibit only the first step of splicing by destabilizing the positioning of the 5′SS in the first catalytic conformation, prp8 alleles appear to inhibit both the first and second steps by destabilizing the interactions within the catalytic core of the spliceosome.
How is the first-to-second-step transition affected by various alleles in U6 and Prp8?
To understand the effects of the isolated suppressor alleles on the catalytic center during the transition between the catalytic steps, we combined several prp8 N-terminal alleles with U6 alleles, shown to suppress or exacerbate prp16-302 defects (Eysmont et al. 2019). Combining different suppressor alleles can be used to determine their individual contributions to an analyzed process. Thus, to establish if the characterized prp8 alleles follow the same or distinct mechanisms from different classes of U6 alleles, we analyzed these alleles in pairwise combinations.
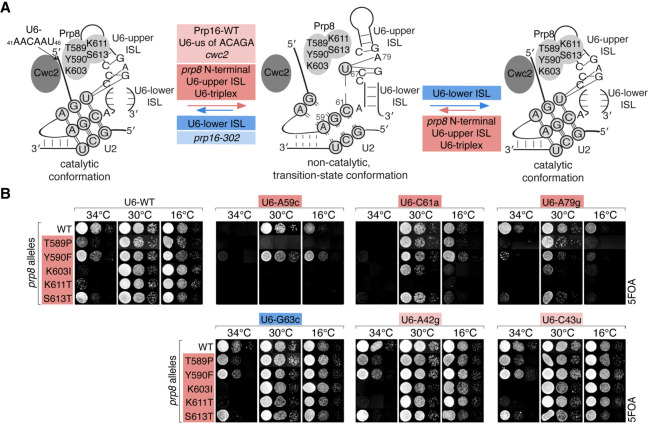
Individually, U6-A59c, C61a, and A79g alleles destabilize the catalytic triplex directly (A59 and C61 form the catalytic triplex) or indirectly (A79 is adjacent to the triplex-forming U80), and thus suppress prp16-302 defects by destabilizing the catalytic conformation (shown in red, Fig. 6A; Table 1; Eysmont et al. 2019). U6-A59c allele becomes synthetically lethal in combination with most of the prp8 N-terminal alleles T589P, K603I, K611T, and S613T (Fig. 6B). Similarly, U6-C61a or U6-A79g alleles become synthetically lethal with prp8-K611T allele or ts and/or cs in the presence of prp8-T589P, Y590F, K603I, and S613T alleles (Fig. 6B).
FIGURE 6.
Interactions between various classes of U6 and prp8 N-terminal alleles. (A) The model of rearrangements of the catalytic center between the two catalytic steps. Four classes of alleles are marked by colored boxes: those promoting exit from the first-step catalytic conformation and entry into the transition-state conformation (light red); those promoting entry into the transition-state conformation through destabilization of both catalytic steps (red); those inhibiting exit from the first-step catalytic conformation (light blue), and those promoting both catalytic conformations (blue). (B) Genetic interactions between prp8-T589P, Y590F, K603I, K611T, S613T and U6-A59c, C61a, A79g, G63c, A42g, C43u alleles in prp8Δ U6Δ strain (yCQ05) tested by growth in the presence of 5FOA. prp8-T589P, K603I, K611T, S613T alleles become synthetically lethal in combination with U6-A59c. U6-C61a and A79g become synthetically lethal with prp8-K611T allele or cs and/or ts in combination with prp8-T589P, Y590F, K603I, S613T alleles. prp8-T589P, Y590F, K603I, K611T alleles do not affect growth in combination with U6-G63c, A42g, C43u, compared to wt U6.
TABLE 1.
Alleles affecting transitions between the two catalytic conformations of the spliceosome
| Name | Allele | Source | |
|---|---|---|---|
| First-step catalytic conformation | prp16-302 | R456K and G691R | Madhani and Guthrie 1994 |
| prp16-R686l | R686l | Hotz and Schwer 1998 | |
| U6-lower ISL | G63c | Eysmont et al. 2019 | |
| Transition-state suppressors of prp16-302 | U6-upper ISL | A79g | Eysmont et al. 2019 |
| U6-catalytic triplex | A59c, C61a | Eysmont et al. 2019 | |
| U6-upstream of ACAGA | ΔA41, A41c, A41g, A42c, A42g, ΔC43, C43g, C43u, ΔA44, A44c, A44u, A45g, U46a, U46g | Madhani and Guthrie 1994; this study | |
| cwc2 | W37A | Hogg et al. 2014 | |
| E17D, S38P, Q54L, P55S, Q56H, D98V, K101T, C111R, l127T, N134S, F162l, W225R | This study | ||
| prp8 N-terminal | D278G, F331L, Y356C, T589P, Y590F, Y590C, K603l, K611L, K611l, K611T, K611E, S613T | This study | |
| Second-step catalytic conformation | U6-lower ISL | G63c | Eysmont et al. 2019 |
| prp8 second step | V1870N, W1575R | Liu et al. 2007 |
These results suggest that the analyzed U6-triplex mutants and prp8 alleles affect the catalytic center through distinct mechanisms, which, when combined, result in synthetic, synergistic effects. In contrast, the combination of prp8 N-terminal alleles that disrupt catalytic conformations and the U6-G63c lower ISL allele (Fig. 6B) that promotes catalytic conformations leads to cancellation of effects, yielding no significant growth phenotypes (red and blue, Fig. 6A; Table 1).
In contrast to U6-triplex mutants, U6-A42g and C43u alleles that destabilize the first-step conformation and suppress prp16-302 defects (Madhani and Guthrie 1994), do not affect cell growth when combined with prp8 N-terminal domain alleles (Fig. 6B), suggesting that these U6 alleles disrupt catalytic conformation by the same, or similar mechanism to that used by prp8 N-terminal alleles (light red and red, Fig. 6A). We note one observed exception to this rule: prp8-T589P in combination with U6-A42g + C43u exerts a stronger ts phenotype than either of the alleles by themselves (Supplemental Fig. S1C).
We conclude that the analyzed prp8 alleles favor the transition-state conformation by destabilizing both flanking catalytic conformations. These prp8 alleles may affect the first-step conformation acting similarly to U6 and cwc2 alleles, by destabilizing the 5′SS positioning. The same alleles also inhibit the second step by destabilizing different interactions at the catalytic center.
DISCUSSION
Although the catalytic center of the spliceosome has been extensively studied in a variety of systems and by diverse techniques, the mechanisms supporting its function are largely unknown. RNA-dependent ATPases/helicases facilitate important transitions in the spliceosome assembly, catalysis, release, and recycling. In particular, Prp16 modulates the transition between the two catalytic reactions of splicing, and its mutant alleles, e.g., prp16-302, serve as useful markers of this transition. Analysis of suppressor alleles that correct prp16-302 growth defects can therefore explain in part mechanisms involved in the exit from the first-step catalytic conformation and transition into the second step of splicing.
To better understand the nature of such changes at the transition between the two catalytic steps, we characterized three groups of suppressor alleles that correct prp16-302 growth defects: strong U6 suppressor alleles identified by Madhani and Guthrie (1994) (Fig. 3C), and two groups of suppressor alleles identified in genetic screens: in cwc2 (Fig. 2D) and prp8 (Fig. 4B). Mutant alleles of all these factors disrupt catalytic interactions and, by supporting Prp16 action, facilitate exit from the first-step catalytic conformation.
Characterization of U6, cwc2, and prp8 suppressor alleles
Previously, we identified prp16 suppressor alleles in the lower part of U6-ISL—these mutants destabilize the U6-ISL structure, allowing exit from the first-step catalytic conformation (Eysmont et al. 2019). Other suppressors of prp16 defects, e.g., cwc2 (Hogg et al. 2014), ecm2 (van der Feltz et al. 2021), U6 (Madhani and Guthrie 1994; Fica et al. 2014), isy1 (Villa and Guthrie 2005), and U2 alleles (Hilliker et al. 2007; Perriman and Ares 2007; Mefford and Staley 2009), have been identified; however, how they improve exit from the first-step conformation is not known.
Here, we focused on the U6-41AACAAU46 region bordering the U6-47ACAGA51 motif, where Madhani and Guthrie (1994) have found multiple alleles correcting prp16-302 cs growth defects. We confirmed and extended these findings by identifying a large cluster of U6 alleles that suppress prp16-302 defects, improving exit from the first-step catalytic conformation (Fig. 3). Next, based on biochemical data (Rasche et al. 2012; Schmitzova et al. 2012) and cryo-EM structures (5LJ5) (Galej et al. 2016), we analyzed Cwc2, a component of the NTC complex that interacts with U6-41AACAAU46 as well as with intron nucleotides downstream from the 5′SS. We identified multiple cwc2 alleles that, like U6-41AACAAU46 alleles, correct growth defects of prp16 mutants (Fig. 2). The strongest effects were observed for cwc2-W37A and S38P alleles located in proximity of U6-41AAC43, that likely disrupt Cwc2–U6 interactions and destabilize positioning of the 5′SS for the first step. The remaining cwc2 suppressors do not contact U6 directly and most likely act by generally destabilizing the protein, altering its interactions with other spliceosomal factors known to suppress prp16 defects, e.g., Ecm2 and Isy1 (Villa and Guthrie 2005; Hogg et al. 2014; van der Feltz et al. 2021).
Suppression of prp16 defects by cwc2 alleles places their action at the exit from the first-step conformation, facilitated by Prp16 ATPase. This notion is further supported by effects of these cwc2 alleles on prp2-Q548N, defective in promoting entry into the first-step conformation (Fig. 2A,G). Although wt Cwc2 is not required in vitro for Prp2-dependent remodeling of the activated spliceosome (Rasche et al. 2012), our results show that destabilization of the first-step interactions by cwc2 mutants inhibits Prp2 action (Fig. 2G). Both cwc2-W37A and Q54L alleles strongly exacerbate defects of the prp2-Q548N allele even in the presence of wt endogenous Cwc2. We conclude that Cwc2 promotes spliceosome activation mediated by Prp2 ATPase, which facilitates transition into the first-step conformation and activates the spliceosome for the first-step catalysis (Kim and Lin 1993, 1996; Bao et al. 2017). This confirms that cwc2 alleles affect the spliceosome conformation flanked by actions of Prp2 and Prp16 and thus destabilize the first-step spliceosomal conformation.
Independently, we analyzed Prp8, a protein forming extensive contacts with the entire catalytic core of the spliceosome, for alleles that suppress prp16-302 defects. The N-terminal domain of Prp8 directly contacts Cwc2 and U6 snRNA (5LJ5) (Galej et al. 2016), and several prp8 alleles located in proximity of U6 and cwc2 alleles (prp8-T589P, Y590C/F, and K603I), or the active site (K611L/T/I/E and S613T) (Fig. 4A), are likely to affect not only the 5′SS positioning but also U6-ISL and other catalytic interactions. The remaining prp8 alleles (D278G, F331L, and Y356C) may destabilize catalytic conformations by altering Prp8 interactions with other spliceosomal components, e.g., Snu114 (Brenner and Guthrie 2005).
prp8 alleles exhibiting the strongest splicing effects are clustered in proximity of the U6:Cwc2 interaction and U6-ISL (prp8-T589, Y590, K603, K611, and S613) (Fig. 5D). Interestingly, all of these amino acid positions (except for T589) are highly conserved in evolution (Supplemental Fig. S3). One of the Caenorhabditis elegans alleles altering the cryptic splice site selection, az43, corresponds to S. cerevisiae prp8-T607S, which is inviable in yeast (Mayerle et al. 2019). Prp8 residues T607, K611, and S613 lie within a short α-helix adjacent to U6-42ACA44 upstream of the 5′SS-binding site, and U6-ISL adjacent to the catalytic triplex, as well as intron position G1, suggesting close interactions of the corresponding alleles with important elements of the catalytic center.
Mechanisms of prp16-302 suppression by U6, cwc2, and prp8 alleles
Juxtaposition of U6 and cwc2 alleles immediately downstream from the 5′SS suggests that U6–Cwc2 interactions stabilize the first-step conformation by positioning 5′SS for catalysis. However, despite close proximity of some U6, cwc2, and prp8 alleles they exhibit some important differences, raising questions whether they act by the same or distinct mechanisms and/or affect the same or different events in the process. Analysis of several N-terminal prp8 alleles suggests that they display features of null alleles: Most of the tested combinations of these alleles exhibit a characteristic lack of additivity in suppression of prp16-302 defects (Fig. 4F–H) or exacerbation of ts growth phenotype (Fig. 4D). The notable exception is the combination of prp8-T589P and K611I mutants, which exhibits a ts phenotype (Supplemental Fig. S1B) and suppresses prp16-302 defects more strongly than either allele alone (Supplemental Fig. S1A), suggesting that they either represent partial function alleles or act through different mechanisms. Interestingly, the prp8-T589P + K611T combination does not additively suppress prp16-302 defects as compared to its individual alleles (Fig. 4H). This amino acid specificity of effects suggests that position K611 is involved in more than one functional interaction.
Based on our analyses, we conclude that at least prp8-K603I and K611T fulfill the criteria of null alleles. This allowed us to analyze combinations of prp8 and U6 alleles that individually suppress prp16-302 defects to establish if they act by the same or distinct mechanisms. In combination with U6 mutants of the catalytic triplex, all of the tested prp8 alleles showed additive effects, consistent with the notion that U6 catalytic triplex mutants destabilize catalytic conformation differently from N-terminal prp8 mutants. In contrast, combinations of prp8 alleles with U6 mutants upstream of ACAGA motif showed no detectable additivity. prp8-T589P is the only prp8 allele that exhibits modest additivity of ts phenotype in combination with U6-A42g + C43u double mutant (Supplemental Fig. S1C), consistent with its status of a partial function allele. Thus, U6 mutants upstream of ACAGA and prp8 alleles likely share the same mechanism to destabilize the first-step interactions. However, because these U6 alleles do not display any growth phenotypes on their own, this conclusion cannot be firmly established.
Hyperstable pairing of the 5′SS-A3c to U6-G50 (Konarska et al. 2006) interferes with the first-to-second-step transition, suggesting that transient destabilization of the 5′SS-U6 contacts is needed to exit the first step. Indeed, destabilization of U6–Cwc2–intron interactions facilitates splicing of 5′SS-A3c introns (Fig. 5B,C), specifically affecting the first, but not the second-step conformation (Supplemental Fig. S2). In contrast, N-terminal prp8 alleles inhibit both steps of splicing, perhaps by affecting not only positioning of the 5′SS but also contacting U6-ISL and thus interfering with overall rearrangements at the catalytic center. These results support the available cryo-EM structural information (Galej et al. 2016; Fica et al. 2017), suggesting that U6–Cwc2–5′SS interactions during the two catalytic conformations are unchanged, but argue that they do not significantly affect the stability of the second-step conformation.
The previously identified second-step prp8 alleles (Collins and Guthrie 1999; Query and Konarska 2004; Liu et al. 2007) also facilitate the transition from the first-to-second steps and could thus be considered to act similarly to the N-terminal domain alleles. However, none of these second-step alleles suppress prp16-302 defects. Furthermore, whereas second-step alleles improve the second step, the N-terminal alleles inhibit both catalytic steps, indicating that they act by different mechanisms.
Alleles in three spliceosomal factors: U6, Cwc2, and Prp8, that suppress defects of the prp16-302 allele, identified important catalytic interactions stabilizing the first-step conformation but destabilized by the Prp16 ATPase at the transition to the second step. Our results suggest that in the first step, the cluster of closely positioned U6, cwc2, and prp8 alleles appears to act jointly to destabilize binding of the 5′SS at the catalytic center. However, despite the similarity of the overall action, cwc2 and U6 alleles destabilize just the first-step catalytic conformation, whereas the identified prp8 alleles destabilize both the first and second catalytic steps. Thus, if Cwc2 and U6 interactions are present in the second-step conformation (as indicated by cryo-EM structures) (Fica et al. 2017), these contacts are unlikely to contribute to the stability of the second-step catalytic center.
In addition to altering the 5′SS positioning, prp8 alleles seem to also influence contacts with U6-ISL, interfering with overall rearrangements at the catalytic center at both the first and second steps of splicing. The identified prp8 alleles act to destabilize both catalytic conformations of the spliceosome, indirectly informing us about the features of the transition-state conformation that needs to form between the two catalytic steps (Fig. 6A). The existence of this conformation is deduced from genetic experiments and supported by biochemical studies (Bao et al. 2017), but to date are not confirmed by cryo-EM structural analyses. Our genetic analysis of prp16 suppressor alleles may help to understand the transition-state conformation, possibly leading to its future structural analysis.
MATERIALS AND METHODS
S. cerevisiae strains used in this study are described in Supplemental Table S1.
Plasmid-shuffle growth assays
Cell cultures were adjusted to OD600 = 1 and 10-fold serial dilutions were spotted on plates containing 5FOA, grown at indicated temperatures, and photographed after 3–6 days.
Copper growth assays
yMK20, yMK79, or JU75 strains carrying indicated alleles were transformed with LEU-marked ACT1–CUP1 reporter plasmids (Lesser and Guthrie 1993a). Cultures were grown in –LEU medium diluted to A600 = 1.0, and equal volumes were spotted on –LEU plates containing 0–1.0 mM CuSO4 and grown at 30°C for 4 days. Data for each experiment, cropped and aligned for clarity, originate from a single plate.
Primer extension assays
Total RNA was isolated from strains carrying ACT1–CUP1 reporter plasmids using glass beads and a standard phenol:chloroform protocol. Primer complementary to the second exon of ACT1 (5′-GGCACTCATGACCTTC-3′) was kinased at the 5′ end using T4 PNK and γ-32P ATP (Hartmann, SRP-501). Annealing (containing 3 μg of total yeast RNA and 32P-labeled primer) and extension reactions were carried out as described by Eysmont et al. (2019).
Quantitation of splicing efficiency
To calculate first and second steps splicing efficiency, images of radiography films were analyzed using the ImageJ tool, resulting in the intensity of each primer extension product. First-step efficiency was calculated as the intensity of the first-step products (lariat intermediates + mRNA)/total RNA (lariat intermediates + mRNA + pre-mRNA). Second-step efficiency was calculated as the intensity of the second-step product (mRNA)/first-step products (lariat intermediates + mRNA).
Bioscreen C growth measurements
Cell cultures were adjusted to OD600 = 0.1 and transferred in 300 μL triplicates to a 100-well Honeycomb 2 Microplate. Cultures were grown at 16°C or 18°C with continuous, double-orbital shaking in the Bioscreen C Pro system. OD600 measurements were taken at regular intervals of 15 min over 140 h. Growth curves were generated using the average of three repeats.
Screen for cwc2 suppressors of prp16-302
To increase the analyzed pool of mutants, we carried out the selection in parallel for two regions of Cwc2 (corresponding to aa 1–159, flanked by SnaBI and EcoRI sites, and aa 159–339, flanked by EcoRI and PflMI). In vivo gap repair selection for improvement of prp16-302 cs growth defects was carried out at 16°C. Error-prone PCR was performed using a buffer containing 0.5 mM MnCl2, 5.5 mM MgCl2, and 0.05 U/µL of Taq DNA polymerase.
Screen for prp8 N-terminal domain suppressors of prp16-302
To increase the number of tested mutants, mutagenesis of the N-terminal Prp8 domain was carried out in four regions using NheI, BamHI, SalI, SexAI, and SacI restriction sites, covering nucleotides +1 to +477 or +477 to +1017 of PRP8. In vivo gap repair selection for improvement of prp16-302 cs growth defects was carried out as for cwc2 suppressors.
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
ACKNOWLEDGMENTS
This work was supported by the 2020/39/B/NZ1/03273 and 2013/08/A/NZ1/00167 grants from the National Science Centre, Poland, to M.M.K. and the “Regenerative Mechanisms for Health—ReMedy” project MAB/2017/2, carried out within the International Research Agendas Program of the Foundation for Polish Science, cofinanced by the European Union under the European Regional Development Fund. We are grateful to Maja Cieplak-Rotowska and Marcin Magnus for helpful discussions and critical readings of the manuscript.
Author contributions: J.M., K.E., and K.M-K. performed all experiments. All authors analyzed the data, interpreted the results, and wrote the manuscript. M.M.K. conceived and supervised the project.
Footnotes
Article is online at http://www.rnajournal.org/cgi/doi/10.1261/rna.079886.123.
Freely available online through the RNA Open Access option.
MEET THE FIRST AUTHORS
Jadwiga Meissner.

Katarzyna Eysmont.

Meet the First Author(s) is an editorial feature within RNA, in which the first author(s) of research-based papers in each issue have the opportunity to introduce themselves and their work to readers of RNA and the RNA research community. Jadwiga Meissner and Katarzyna Eysmont are co-first authors of this paper, “Characterization of Cwc2, U6 snRNA, and Prp8 interactions destabilized by Prp16 ATPase at the transition between the first and second steps of splicing.” Jadzia is a graduate student and Kasia is a postdoctoral researcher at the Konarska Laboratory of RNA Biology at IMol, a new international institute of the Polish Academy of Sciences. Their major research focuses on understanding the mechanism of splicing.
What are the major results described in your paper and how do they impact this branch of the field?
Our studies focus on understanding the mechanisms by which the spliceosome, a molecular machine responsible for pre-mRNA splicing, transitions between its catalytic steps.
We identify multiple alleles in Cwc2, U6 snRNA, and Prp8 that suppress the defects of the prp16-302 allele in Saccharomyces cerevisiae. These alleles were found to destabilize the first-step catalytic conformation, facilitating the transition to the second step of splicing. These findings significantly contribute to the field of RNA splicing by elucidating the mechanisms of spliceosome rearrangements and identifying key factors that modulate transition-state conformation of the spliceosome.
What led you to study RNA or this aspect of RNA science?
KE: My journey into studying RNA began during my undergraduate studies at Warsaw University, where I was introduced to the RNA World by studying the RNA exosome in Professor Joanna Kufel's laboratory. There, I started to understand the importance and versatility of RNA in biological systems, its multifunctional nature, and its immense potential for scientific and medical advancements.
JM: My interest in RNA science began during my college years, when I studied molecular biology and became fascinated by how cellular processes are dictated by the genome. This naturally led to my interest in posttranscriptional modifications of RNA and the intricate process of splicing. At that time, I was fortunate to learn about these topics from my mentor, Magda Konarska, a pioneering figure in the splicing field with numerous contributions to the RNA community. What drives my interest in splicing mechanisms is the opportunity to dive into the molecular world and discover the workings of such a complex machine as the spliceosome.
During the course of these experiments, were there any surprising results or particular difficulties that altered your thinking and subsequent focus?
The input from reviewers of our paper provided invaluable insights that pushed our analysis of the study's alleles to a significantly higher level. Their feedback motivated us to explore the underlying mechanisms of these alleles more thoroughly, enhancing our knowledge of their impact on the catalytic center of the spliceosome. A particularly surprising finding was the difference between U6 and cwc2 alleles as compared to prp8 alleles. While all these factors interact to stabilize the first-step catalytic conformation, only prp8 alleles impact the second-step conformation. This discovery significantly clarified our concepts concerning rearrangements within the catalytic core of the spliceosome.
What are some of the landmark moments that provoked your interest in science or your development as a scientist?
KE: My initial interest in science was sparked by a high school biology class in Warsaw, where I first learned about the central dogma of molecular biology (DNA → RNA → Protein). The idea that genetic information flows in such a precise and regulated manner fascinated me and ignited my curiosity about molecular processes. A few years later, my internships at Aarhus University in Denmark and at Rockefeller University in New York City were among the most formative experiences in my scientific journey. Being surrounded by dedicated and passionate researchers was incredibly inspiring and deepened my commitment to pursuing a career in scientific research.
JM: One of the landmark moments that sparked my interest in science, particularly in the field of splicing, was the publication of the first cryo-EM spliceosome structures. It was incredible to see the detailed structure that aligned so well with years of biochemical and genetic analyses. Witnessing how accurately the splicing community had predicted the intricate workings of this complex machinery without previously seeing its structure was inspiring. This milestone motivated me to learn from these structures and to explore how we can go beyond the structural data to uncover even more about the inner workings of the spliceosome.
If you were able to give one piece of advice to your younger self, what would that be?
KE: Looking back, I would urge my younger self not to be afraid to ask questions, no matter how simple or complex they may seem. I would further say, stay persistent, keep an open mind, and always seek out mentors and collaborators who can guide and inspire you.
JM: Decisions are not permanent. Don't be afraid to make a choice, start something new, or take a path you're unsure about. You can always change direction if the initial choice doesn't work out. Embrace the journey and be open to course changes along the way.
Are there specific individuals or groups who have influenced your philosophy or approach to science?
KE: Two women, Professors Joanna Kufel and Magda Konarska, profoundly shaped my approach to science. They emphasized the importance of curiosity, persistence, and continual learning. I am deeply grateful for their influence in shaping me both as a scientist and as an individual.
JM: My scientific philosophy and approach have been profoundly shaped by Magda Konarska, whose lab I joined early in my bachelor's studies. She introduced me to the world of science, sharing her lifelong fascination with splicing. Her passion for uncovering the mechanistic details of the spliceosome continues to inspire me daily and has shaped my own dedication to seeking the mechanisms behind biological processes.
What are your subsequent near- or long-term career plans?
KE: My near-term career plans include continuing my research in RNA splicing, focusing on publishing my findings and expanding my expertise. In the long term, I aim to find my own niche within the RNA biology field and establish a research program that will significantly impact our understanding of RNA mechanisms and their applications.
JM: As I approach the defense of my PhD, I am considering the important decisions that lie ahead for my career. I am currently weighing whether to stay in academia to continue unraveling the mechanistic intricacies of life, or to transition into the industry, where I can apply my expertise to more practical, translational projects.
What were the strongest aspects of your collaboration as co-first authors?
When Jadzia first joined the lab, Kasia guided her through the intricacies of lab work and experimental techniques. This mentorship laid the foundation for a strong relationship both in and out of the lab. Our collaboration was seamless due to our complementary strengths: Kasia's focus, organization, and problem-solving skills, combined with Jadzia's exploratory approach to scientific questions. Throughout the project, we provided each other with continuous support and encouragement. This emotional and intellectual backing was crucial for maintaining our morale and enthusiasm, especially during challenging phases of the project. We tackled technically difficult tasks, like primer-extension analysis, by alternating turns, which made managing these challenges more effective.
How did you decide to work together as co-first authors?
The project was originally started by Kasia, who conducted most of the experiments. She then took a maternity leave break, during which Jadzia continued the project with great dedication. After navigating many pivotal moments and setbacks, Jadzia successfully completed the project and drafted the manuscript. At a crucial moment, following the first review, we decided to work together to address all the suggestions. This was an intense period, marked by technical problems with the experiments, but we combined our individual strengths and insights, ultimately enhancing the quality of the research and accelerating the process. It was natural for us to be co-first authors, without any doubt, and we are certain that without this collaboration, it would not have been possible.
REFERENCES
- Bai R, Wan R, Yan C, Lei J, Shi Y. 2018. Structures of the fully assembled Saccharomyces cerevisiae spliceosome before activation. Science 360: 1423–1429. 10.1126/science.aau0325 [DOI] [PubMed] [Google Scholar]
- Bao P, Höbartner C, Hartmuth K, Lührmann R. 2017. Yeast Prp2 liberates the 5′ splice site and the branch site adenosine for catalysis of pre-mRNA splicing. RNA 23: 1770–1779. 10.1261/rna.063115.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram K, Agafonov DE, Dybkov O, Haselbach D, Leelaram MN, Will CL, Urlaub H, Kastner B, Lührmann R, Stark H. 2017a. Cryo-EM structure of a pre-catalytic human spliceosome primed for activation. Cell 170: 701–713. 10.1016/j.cell.2017.07.011 [DOI] [PubMed] [Google Scholar]
- Bertram K, Agafonov DE, Liu WT, Dybkov O, Will CL, Hartmuth K, Urlaub H, Kastner B, Stark H, Lührmann R. 2017b. Cryo-EM structure of a human spliceosome activated for step 2 of splicing. Nature 542: 318–323. 10.1038/nature21079 [DOI] [PubMed] [Google Scholar]
- Brenner TJ, Guthrie C. 2005. Genetic analysis reveals a role for the C terminus of the Saccharomyces cerevisiae GTPase Snu114 during spliceosome activation. Genetics 170: 1063–1080. 10.1534/genetics.105.042044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge CB, Tuschl TH, Sharp PA. 1999. Splicing of precursors to mRNAs by the spliceosomes. In The RNA world, 2nd ed. (ed. Gesteland RF, et al.), pp. 525–560. Cold Spring Harbor Laboratory Press, New York. [Google Scholar]
- Burgess SM, Guthrie C. 1993. A mechanism to enhance mRNA splicing fidelity: the RNA-dependent ATPase Prp16 governs usage of a discard pathway for aberrant lariat intermediates. Cell 73: 1377–1391. 10.1016/0092-8674(93)90363-U [DOI] [PubMed] [Google Scholar]
- Chiang TW, Cheng SC. 2013. A weak spliceosome-binding domain of Yju2 functions in the first step and bypasses Prp16 in the second step of splicing. Mol Cell Biol 33: 1746–1755. 10.1128/MCB.00035-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Guthrie C. 1999. Allele-specific genetic interactions between Prp8 and RNA active site residues suggest a function for Prp8 at the catalytic core of the spliceosome. Genes Dev 13: 1970–1982. 10.1101/gad.13.15.1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Guthrie C. 2001. Genetic interactions between the 5′ and 3′ splice site consensus sequences and U6 snRNA during the second catalytic step of pre-mRNA splicing. RNA 7: 1845–1854. [PMC free article] [PubMed] [Google Scholar]
- Eysmont K, Matylla-Kulińska K, Jaskulska A, Magnus M, Konarska MM. 2019. Rearrangements within the U6 snRNA core during the transition between the two catalytic steps of splicing. Mol Cell 75: 538–548. 10.1016/j.molcel.2019.05.018 [DOI] [PubMed] [Google Scholar]
- Fica SM, Mefford MA, Piccirilli JA, Staley JP. 2014. Evidence for a group II intron-like catalytic triplex in the spliceosome. Nat Struct Mol Biol 21: 464–471. 10.1038/nsmb.2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fica SM, Oubridge C, Galej WP, Wilkinson ME, Bai XC, Newman AJ, Nagai K. 2017. Structure of a spliceosome remodelled for exon ligation. Nature 542: 377–380. 10.1038/nature21078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galej WP, Wilkinson ME, Fica SM, Oubridge C, Newman AJ, Nagai K. 2016. Cryo-EM structure of the spliceosome immediately after branching. Nature 537: 197–201. 10.1038/nature19316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger RJ, Beggs JD. 2005. Prp8 protein: at the heart of the spliceosome. RNA 11: 533–557. 10.1261/rna.2220705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliker AK, Mefford MA, Staley JP. 2007. U2 toggles iteratively between the stem IIa and stem IIc conformations to promote pre-mRNA splicing. Genes Dev 21: 821–834. 10.1101/gad.1536107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg R, de Almeida RA, Ruckshanthi JP, O'Keefe RT. 2014. Remodeling of U2-U6 snRNA helix I during pre-mRNA splicing by Prp16 and the NineTeen complex protein Cwc2. Nucleic Acids Res 42: 8008–8023. 10.1093/nar/gku431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotz HR, Schwer B. 1998. Mutational analysis of the yeast DEAH-box splicing factor Prp16. Genetics 149: 807–815. 10.1093/genetics/149.2.807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandels-Lewis S, Séraphin B. 1993. Involvement of U6 snRNA in 5′ splice site selection. Science 262: 2035–2039. 10.1126/science.8266100 [DOI] [PubMed] [Google Scholar]
- Kim SH, Lin RJ. 1993. Pre-mRNA splicing within an assembled yeast spliceosome requires an RNA-dependent ATPase and ATP hydrolysis. Proc Natl Acad Sci 90: 888–892. 10.1073/pnas.90.3.888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lin RJ. 1996. Spliceosome activation by PRP2 ATPase prior to the first transesterification reaction of pre-mRNA splicing. Mol Cell Biol 16: 6810–6819. 10.1128/MCB.16.12.6810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska MM, Vilardell J, Query CC. 2006. Repositioning of the reaction intermediate within the catalytic center of the spliceosome. Mol Cell 21: 543–553. 10.1016/j.molcel.2006.01.017 [DOI] [PubMed] [Google Scholar]
- Lardelli RM, Thompson JX, Yates JR, Stevens SW. 2010. Release of SF3 from the intron branchpoint activates the first step of pre-mRNA splicing. RNA 16: 516–528. 10.1261/rna.2030510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser CF, Guthrie C. 1993a. Mutational analysis of pre-mRNA splicing in Saccharomyces cerevisiae using a sensitive new reporter gene, CUP1. Genetics 133: 851–863. 10.1093/genetics/133.4.851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser CF, Guthrie C. 1993b. Mutations in U6 snRNA that alter splice site specificity: implications for the active site. Science 262: 1982–1988. 10.1126/science.8266093 [DOI] [PubMed] [Google Scholar]
- Liu L, Query CC, Konarska MM. 2007. Opposing classes of prp8 alleles modulate the transition between the catalytic steps of pre-mRNA splicing. Nat Struct Mol Biol 14: 519–526. 10.1038/nsmb1240 [DOI] [PubMed] [Google Scholar]
- Madhani HD, Guthrie C. 1994. Genetic interactions between the yeast RNA helicase homolog Prp16 and spliceosomal snRNAs identify candidate ligands for the Prp16 RNA-dependent ATPase. Genetics 137: 677–687. 10.1093/genetics/137.3.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerle M, Yitiz S, Soulette C, Rogel LE, Ramirez A, Ragle JM, Katzman S, Guthrie C, Zahler AM. 2019. Prp8 impacts cryptic but not alternative splicing frequency. Proc Natl Acad Sci 116: 2193–2199. 10.1073/pnas.1819020116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrail JC, Krause A, O'Keefe RT. 2009. The RNA binding protein Cwc2 interacts directly with the U6 snRNA to link the nineteen complex to the spliceosome during pre-mRNA splicing. Nucleic Acids Res 37: 4205–4217. 10.1093/nar/gkp341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mefford MA, Staley JP. 2009. Evidence that U2/U6 helix I promotes both catalytic steps of pre-mRNA splicing and rearranges in between these steps. RNA 15: 1386–1397. 10.1261/rna.1582609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi MD, Gould KL. 2002. Characterization of interactions among the Cef1p-Prp19p-associated splicing complex. RNA 8: 798–815. 10.1017/S1355838202025050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R, Siliciano PG, Guthrie C. 1987. Recognition of the TACTAAC box during mRNA splicing in yeast involves base pairing to the U2-like snRNA. Cell 49: 229–239. 10.1016/0092-8674(87)90564-2 [DOI] [PubMed] [Google Scholar]
- Perriman RJ, Ares M. 2007. Rearrangement of competing U2 RNA helices within the spliceosome promotes multiple steps in splicing. Genes Dev 21: 811–820. 10.1101/gad.1524307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaschka C, Lin PC, Nagai K. 2017. Structure of a pre-catalytic spliceosome. Nature 546: 617–621. 10.1038/nature22799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Query CC, Konarska MM. 2004. Suppression of multiple substrate mutations by spliceosomal prp8 alleles suggests functional correlations with ribosomal ambiguity mutants. Mol Cell 14: 343–354. 10.1016/S1097-2765(04)00217-5 [DOI] [PubMed] [Google Scholar]
- Rasche N, Dybkov O, Schmitzová J, Akyildiz B, Fabrizio P, Lührmann R. 2012. Cwc2 and its human homologue RBM22 promote an active conformation of the spliceosome catalytic centre. EMBO J 31: 1591–1604. 10.1038/emboj.2011.502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitzová J, Rasche N, Dybkov O, Kramer K, Fabrizio P, Urlaub H, Lührmann R, Pena V. 2012. Crystal structure of Cwc2 reveals a novel architecture of a multipartite RNA-binding protein. EMBO J 31: 2222–2234. 10.1038/emboj.2012.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B, Guthrie C. 1991. PRP16 is an RNA-dependent ATPase that interacts transiently with the spliceosome. Nature 349: 494–499. 10.1038/349494a0 [DOI] [PubMed] [Google Scholar]
- Schwer B, Guthrie C. 1992. A conformational rearrangement in the spliceosome is dependent on PRP16 and ATP hydrolysis. EMBO J 11: 5033–5039. 10.1002/j.1460-2075.1992.tb05610.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séraphin B, Kretzner L, Rosbash M. 1988. A U1 snRNA:pre-mRNA base pairing interaction is required early in yeast spliceosome assembly but does not uniquely define the 5′ cleavage site. EMBO J 7: 2533–2538. 10.1002/j.1460-2075.1988.tb03101.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano PG, Guthrie C. 1988. 5′ splice site selection in yeast: genetic alterations in base-pairing with U1 reveal additional requirements. Genes Dev 2: 1258–1267. 10.1101/gad.2.10.1258 [DOI] [PubMed] [Google Scholar]
- Staley JP, Guthrie C. 1998. Mechanical devices of the spliceosome: motors, clocks, springs, and things. Cell 92: 315–326. 10.1016/S0092-8674(00)80925-3 [DOI] [PubMed] [Google Scholar]
- Steitz TA, Steitz JA. 1993. A general two-metal-ion mechanism for catalytic RNA. Proc Natl Acad Sci 90: 6498–6502. 10.1073/pnas.90.14.6498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Feltz C, Nikolai B, Schneider C, Paulson JC, Fu X, Hoskins AA. 2021. Saccharomyces cerevisiae Ecm2 modulates the catalytic steps of pre-mRNA splicing. RNA 27: 591–603. 10.1261/rna.077727.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa T, Guthrie C. 2005. The Isy1p component of the NineTeen complex interacts with the ATPase Prp16p to regulate the fidelity of pre-mRNA splicing. Genes Dev 19: 1894–1904. 10.1101/gad.1336305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl MC, Will CL, Lührmann R. 2009. The spliceosome: design principles of a dynamic RNP machine. Cell 136: 701–718. 10.1016/j.cell.2009.02.009 [DOI] [PubMed] [Google Scholar]
- Wan R, Bai R, Yan C, Lei J, Shi Y. 2019. Structures of the catalytically activated yeast spliceosome reveal the mechanism of branching. Cell 177: 339–351. 10.1016/j.cell.2019.02.006 [DOI] [PubMed] [Google Scholar]
- Warkocki Z, Odenwälder P, Schmitzová J, Platzmann F, Stark H, Urlaub H, Ficner R, Fabrizio P, Lührmann R. 2009. Reconstitution of both steps of Saccharomyces cerevisiae splicing with purified spliceosomal components. Nat Struct Mol Biol 16: 1237–1243. 10.1038/nsmb.1729 [DOI] [PubMed] [Google Scholar]
- Wlodaver AM, Staley JP. 2014. The DExD/H-box ATPase Prp2p destabilizes and proofreads the catalytic RNA core of the spliceosome. RNA 20: 282–294. 10.1261/rna.042598.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Wan R, Bai R, Huang G, Shi Y. 2016. Structure of a yeast activated spliceosome at 3.5 Å resolution. Science 353: 904–911. 10.1126/science.aag0291 [DOI] [PubMed] [Google Scholar]
- Yan C, Wan R, Bai R, Huang G, Shi Y. 2017. Structure of a yeast step II catalytically activated spliceosome. Science 355: 149–155. 10.1126/science.aak9979 [DOI] [PubMed] [Google Scholar]
- Zhuang Y, Weiner AM. 1986. A compensatory base change in U1 snRNA suppresses a 5′ splice site mutation. Cell 46: 827–835. 10.1016/0092-8674(86)90064-4 [DOI] [PubMed] [Google Scholar]