Abstract
Background
The appearance of the new coronavirus, SARS-CoV-2, in Wuhan - China, in 2019 led to the declaration of a COVID-19 pandemic by the World Health Organization. Peru confirmed its first case on March 6, 2020, prompting a significant change in medical care.
Purpose
Our objective was to determine the impact of the COVID-19 pandemic on cancer treatment in Peru.
Methods
A retrospective analysis of hospital data from the National Institute of Neoplastic Diseases revealed substantial decreases in oncological treatments in 2020 compared to 2019.
Results
Oncological treatments involving bone marrow transplantation had a greater impact between the months of April and September, at -100% (p=0.003). However, treatments involving surgery in April (-95% [p≤0.001]), radiotherapy in May (-76% [p=0.002]) and chemotherapy in June (-71% [p≤0.001]) also showed significant impacts. Comparative analysis with international data revealed similar trends in cancer care interruptions in different countries. However, variations in the magnitude of the impact were observed, influenced by regional health policies and the severity of the pandemic.
Conclusions
The findings underscore the challenges cancer care providers face during public health crises, requiring adaptive strategies to ensure continued access to essential treatments. Addressing these challenges requires comprehensive public health responses to mitigate the impact of future crises on cancer care systems.
Keywords: COVID-19 impact, cancer patient, cancer treatment, public health, Latin America
Introduction
In December 2019, a new coronavirus that primarily caused pneumonia was discovered in Wuhan, China. Due to its swift global dissemination, the World Health Organization (WHO) classified COVID-19 as a “public health emergency of international concern”.1,2 Peru confirmed its first case of COVID-19 on March 6, 2020. During the early months of the pandemic, health care facilities prioritized crisis management, leading to the closure of primary and specialized care centers, which play a role in cancer detection. 3
In Latin American (LATAM) countries, ongoing challenges in cancer control efforts persist due to limited funding and uneven distribution of medical resources compared to those in high-income nations.2,4 The global COVID-19 pandemic has further exacerbated disruptions in cancer diagnosis and screening programs worldwide, disproportionately affecting middle- and low-income countries.5,6
These circumstances have significantly impacted cancer patients, resulting in reduced access to necessary treatments and services.7,8 Therefore, this study aimed to assess the impact of the COVID-19 pandemic on cancer treatment for patients at the National Institute of Neoplastic Diseases (INEN) in Peru.
Methods
Public Data Recovery
This was a single‐center based retrospective study that was carried out using de-identified Hospital indicator reports from the National Institute of Neoplastic Diseases (https://www.inen.sld.pe/). The data obtained consisted of the number of oncological treatments that involving chemotherapy, surgery, radiotherapy and bone marrow transplantation between January and December 2019 and 2020. The reporting of this study conforms to STROBE guidelines. 9
Estimating the Impact of the COVID-19 Pandemic
A descriptive month-by-month comparison was carried out for the years 2019 and 2020. Subsequently, the impact of COVID-19 was calculated for each month of both years and expressed as a percentage. It was calculated with the following formula:
Impact of COVID-19= ((#cases 2020 - #cases 2019)/#cases 2019) x100
Statistical Analysis
The data were summarized in box plots for the median and interquartile range with 25th, 50th and 75th percentiles. Comparisons were made using the Mann-Whitney U test. Statistical significance was calculated at the P < 0.05 level.
Results
Between January 1 and December 31, 2020, we observed significant changes in the number of oncology treatments compared to 2019. The total number of treatments increased from 34,569 to 37,355 in January and from 18,895 to 34,997 in February. However, there was a substantial decline starting in March, with numbers falling from 34,352 to 21,057, and continuing to decrease sharply through the year. Specifically, the number of treatments decreased to 8032 in April, 9196 in May, 11,875 in June, 17,997 in July, 19,680 in August, 22,240 in September, 25,084 in October, 25,524 in November, and 24,448 in December.
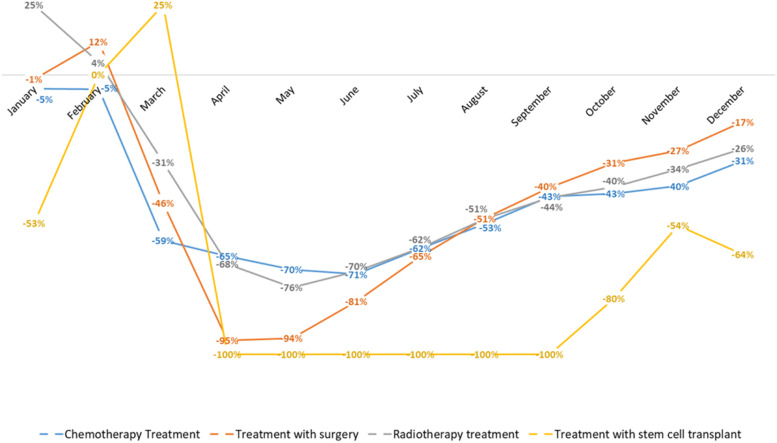
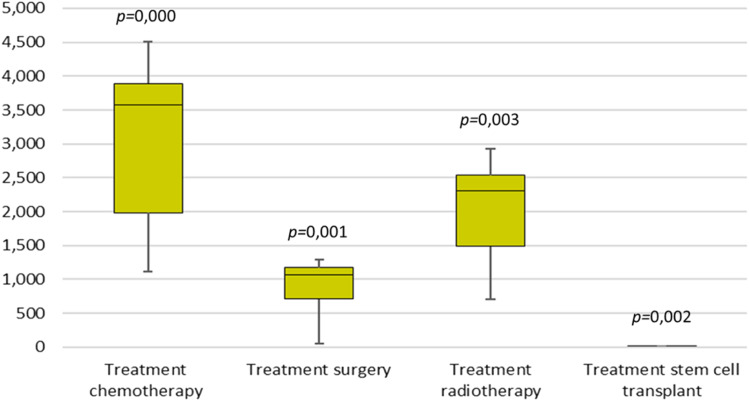
The impact of the COVID-19 pandemic on various types of oncology treatments in 2020 was notable. For chemotherapy treatments, there was a decrease of −5% in January, −5% in February, −59% in March, −65% in April, −70% in May, −71% in June, −62% in July, −53% in August, −43% in September, −43% in October, −40% in November, and −31% in December, with a significant P-value of <0.001. Surgical treatments experienced a different trend, with changes of −1% in January, 12% in February, −46% in March, −95% in April, −94% in May, −81% in June, −65% in July, −51% in August, −40% in September, −31% in October, −27% in November, and −17% in December, with a P-value of 0.001. Radiotherapy treatments showed fluctuations with a −25% decrease in January, 4% in February, −31% in March, −68% in April, −76% in May, −70% in June, −62% in July, −51% in August, −44% in September, −40% in October, −34% in November, and −26% in December, with a P-value of 0.003. Finally, bone marrow transplants exhibited a dramatic decline, with −53% in January, 0% in February, 25% in March, and a complete −100% reduction from April through September, followed by −80% in October, −54% in November, and −64% in December, with a P-value of 0.002 (Figures 1 and 2).
Figure 1.
Monthly percentage reduction in treatment types from January to December 2020.
Figure 2.
Comparative Analysis of Oncological Treatment Modalities (2019 vs 2020).
Discussion
The COVID-19 pandemic has created one of the most significant public health crises of this century. Unfortunately, several public health policies led to the limitation or cessation of oncological care during the first months of the pandemic.
We observed that the COVID-19 pandemic had an impact on chemotherapy treatment at the National Institute of Neoplastic Diseases (INEN) in 2020; we reported values of −59% in the month of March, −65% in April, −70% in May, and −71% in June, compared to secondary hospital management data from the National Institute of Neoplastic Diseases in 2019. However, a previous study by Vasquez-Rosas et al 10 reported −8.3% between March and April, −6.3% in May, and −6.7% in June at the Oswaldo Cruz Foundation in Brazil. In contrast, at the general hospital of México was reported −79% of cases between March and April, −83% in May, and −72% in June. On the other hand, data from the Cancer Institute of Mexico reported different percentages for the same period. They reported −27% between March and April, −34% in May, and −29% in June. In the case of the National Cancer Institute in Chile, it was reported a prevalence of −31% between March and April, −35% in May and −19% in June. In Uruguay, the study reported a prevalence of −11% between March and April, −22% in May, and −20% in June with data from the oncological radiotherapy unit of the University of Uruguay. Amador 11 reported a prevalence of −9.5% between March and June from an oncology hospital in Spain, and Ambroggi 12 reported a prevalence of 10.6% between the months of January and February 2019 and 2020. Likewise, Vallejos 13 reported an impact on treatment with −70% chemotherapy in June in Oncosalud, AUNA.
Similarly, we observed that there was an impact of the COVID-19 pandemic on surgical treatment at the INEN in 2020 of −46% in the month of March, −95% in the month of April, −94% in the month of May, and −81% in the month of June, compared to secondary hospital management data from the National Institute of Neoplastic Diseases in 2019. However, Vasquez-Rosas et al 10 reported an impact of −24.9% between the months of March and April, −31.7% in May, and −23.6% in June in Brazil. In the case of Mexico, they reported an impact of −57% between March and April, −75% in May, −100% in June for the general hospital of Mexico; and-24% between the months of March and April, −55% in May, −30% in June for the Cancer Institute of the same country. In Chile was reported a percentage of −34% between the months of March and April, −33% in May and −38% in June. Ranganathan 14 reported 52% between the months of January and May in a cancer center in India. Likewise, Vallejos 13 reported an impact of −21% on chemotherapy treatment between March and June 2020 in Oncosalud, AUNA.
In the case of radiotherapy treatment, we observed an impact of −31% in the month of March, −68% in April, −76% in May, and −70% in June, when compared to secondary hospital management data from the National Institute of Neoplastic Diseases in 2019. However, a multicentric study carried out in Latin America showed a prevalence of −8% between the months of March and April in Brazil. Likewise, data analyzed from the Colombian League against Cancer reported a −8.9% in May and −18.6% in June. Similarly, it was reported a prevalence of −31% between the months of March and April, −35% in May and −19% in June at the National Cancer Institute in Chile. In the case of Uruguay, it was reported a −0.35% in the month of March, −0.68% in April, −0.75% in May and −0.70% in June. 10 Morris et al 15 reported a significant impact of 44% between the months of January 2019 and October 2020. Likewise, Vallejos 13 reported an impact of −30% on chemotherapy treatment between March and June 2020 in Oncosalud-AUNA. Finally, we observed a greater impact of −100% between the months of April and September in 2020 for patients treated with bone marrow transplants (P = 0.003), compared to secondary hospital management data from the National Institute of Neoplastic Diseases 2019.
Several studies involving cancer patients who were infected with SARS-CoV-2 have already demonstrated that cancer is a serious risk factor for mortality and that it negatively impacts cancer patients’ recovery.16–22 Thus, Infection risk differs between cancer patients depending on their genetic predisposition, physical condition, ethnicity, nutritional status, age and sex. 23
During the pandemic, individuals with cancer faced a notably higher risk of contracting SARS-CoV-2, making COVID-19 a particularly serious threat for these patients. A comprehensive meta-meta-analysis conducted by Arayici et al. 24 incorporating data from 20 studies with a total of 1,031,783 participants, found that patients with COVID-19 had an elevated likelihood of developing severe symptoms. Specifically, cancer patients who contracted SARS-CoV-2 exhibited a higher mortality risk (OR = 2.02, 95% CI: 1.74-2.35, P < 0.001). Furthermore, there was a marked increase in ICU admissions among cancer patients with COVID-19 (OR = 1.84, 95% CI: 1.44-2.34, P < 0.001). Another meta-analysis, encompassing 58 studies with 709 908 participants, including 31 732 cancer patients, indicated significantly higher rates of both mortality and ICU admissions for cancer patients (RR = 2.26, 95% CI: 1.94-2.62, P < 0.001; RR = 1.45, 95% CI: 1.28-1.64, P < 0.001, respectively). 20 Nonetheless, it is important to recognize that several studies have found no substantial difference in COVID-19 mortality between cancer patients and those without cancer.25-27
This study has some limitations. Firstly, it cannot predict the effects of delays in new cancer diagnoses, treatments, and disease re-staging on cancer prognosis because it is a monocentric and retrospective study. This limitation restricts us from generalizing the findings to other populations or clinical settings. Secondly, the study does not evaluate patients’ demographic characteristics, which could introduce biases in interpreting the results, as factors such as age, gender, and socioeconomic conditions may influence the observed outcomes.
Conclusion
The COVID-19 pandemic has disrupted the delivery of cancer care across Latin American (LATAM) countries in various ways, including significant setbacks in cancer screening and diagnosis programs, and delays or cancelations in treatments of cancer. These disruptions have resulted into a higher proportion of cancer cases being diagnosed at more advanced stages and have contributed to excess mortality in specific cancer types.
Before the pandemic, LATAM countries were already facing health care system limitations and barriers to accessing medical resources. This research underscores the need to prioritize strategies for safeguarding and reinforcing cancer care delivery. Furthermore, addressing the vulnerabilities highlighted by the COVID-19 pandemic, will be crucial for mitigating adverse effects during future global health crises.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Statement
Due to its retrospective design, which included analysis of secondary sources, the present study was exempted from the requirement of ethics approval by the Ethics Committee of Universidad Privada San Juan Bautista (registration code: 1320-2024-CIEI-UPSJB).
ORCID iD
César H. Saravia https://orcid.org/0000-0002-4734-3145
Data Availability Statement
The datasets generated and analyzed during this study are available in the Dryad plataform https://datadryad.org/stash/share/bA2M_lfrZo8bLXLqVzd77qM5-phck7yDTe3GSuv4ZxU
References
- 1.Aapro M, Lyman GH, Bokemeyer C, et al. Supportive care in patients with cancer during the COVID-19 pandemic. ESMO open. 2021;6(1):100038. doi: 10.1016/j.esmoop.2020.100038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alagoz O, Lowry KP, Kurian AW, et al. Impact of the COVID-19 pandemic on breast cancer mortality in the US: estimates from collaborative simulation modeling. J Natl Cancer Inst. 2021;113(11):1484-1494. doi: 10.1093/jnci/djab097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delamare Fauvel A, Bischof JJ, Reinbolt RE. et al. Diagnosis of cancer in the Emergency Department: a scoping review. Cancer Med. 2023;12(7):8710-8728. doi: 10.1002/cam4.5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrios CH, Werutsky G, Mohar A. et al. Cancer control in Latin America and the Caribbean: recent advances and opportunities to move forward. Lancet Oncol. 2021;22(11):e474-e487. doi: 10.1016/S1470-2045(21)00492-7 [DOI] [PubMed] [Google Scholar]
- 5.Pinto JA, Pinillos L, Villarreal-Garza C. et al. Barriers in Latin America for the management of locally advanced breast cancer. Ecancermedicalscience. 2019;13:897. doi: 10.3332/ecancer.2019.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rentería ER, Céspedes P, Cerna K. et al. Epidemiologic patterns of COVID-19 incidence in the province of Lima. Ann Epidemiol. 2021;54:27-28. doi: 10.1016/j.annepidem.2020.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teglia F, Angelini M, Astolfi L, Casolari G, Boffetta P. Global association of COVID-19 pandemic measures with cancer screening: a systematic review and meta-analysis. JAMA Oncol. 2022;8(9):1287-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucas E, Murillo R, Arrossi S. et al. Quantification of impact of COVID-19 pandemic on cancer screening programmes – a case study from Argentina, Bangladesh, Colombia, Morocco, Sri Lanka, and Thailand. eLife. 2023;12:e86527. https://elifesciences.org/articles/86527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573-577. [DOI] [PubMed] [Google Scholar]
- 10.Vázquez Rosas T, Cazap E, Delgado L. et al. Social distancing and economic crisis during COVID-19 pandemic reduced cancer control in Latin America and will result in increased late-stage diagnoses and expense. JCO Glob Oncol. 2021;7:694-703. doi: 10.1200/GO.21.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amador M, Matias-Guiu X, Sancho-Pardo G. et al. Impact of the COVID-19 pandemic on the care of cancer patients in Spain. ESMO open. 2021;6(3):100157. doi: 10.1016/j.esmoop.2021.100157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambroggi M, Citterio C, Vecchia S, Riva A, Mordenti P, Cavanna L. Impact of the COVID-19 pandemic on the oncologic activities (diagnosis, treatment, clinical trials enrollment) of a general hospital in a district with high prevalence of SARS-COV-2 in Italy. Support Care Cancer. 2022;30(4):3225-3231. doi: 10.1007/s00520-021-06667-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallejos C. (12 de julio del 2022) Oncosalud. Retrieved from: https://bit.ly/3ywfYbs
- 14.Ranganathan P, Sengar M, Chinnaswamy G. et al. Impact of COVID-19 on cancer care in India: a cohort study. Lancet Oncol. 2021;22(7):970-976. doi: 10.1016/S1470-2045(21)00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris E, Goldacre R, Spata E. et al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol Hepatol. 2021;6(3):199-208. doi: 10.1016/S2468-1253(21)00005-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin Y, Yang H, Ji W. et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:372. doi: 10.3390/v12040372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espinosa OA, Zanetti ADS, Antunes EF, Longhi FG, Matos TA, Battaglini PF. Prevalence of comorbidities in patients and mortality cases affected by SARS-CoV2: a systematic review and meta-analysis. Rev Inst Med Trop Sao Paulo. 2020;62:e43. doi: 10.1590/S1678-9946202062043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow N, Fleming-Dutra K, Gierke R, Hall A, Hughes M, Pilishvili T. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 – United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382. doi: 10.15585/mmwr.mm6913e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang J, Jin G, Liu T. et al. Clinical characteristics and risk factors for mortality in cancer patients with COVID-19. Front Med. 2021;15:264-274. doi: 10.1007/s11684-021-0845-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arayici ME, Kipcak N, Kayacik U. et al. Effects of SARS-CoV-2 infections in patients with cancer on mortality, ICU admission and incidence: a systematic review with meta-analysis involving 709,908 participants and 31,732 cancer patients. J Cancer Res Clin Oncol. 2023;149:2915-2928. doi: 10.1007/s00432-022-04191-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai A, Sachdeva S, Parekh T, Desai R. COVID-19 and cancer: lessons from a pooled meta-analysis. JCO Glob Oncol. 2020;6:557-559. doi: 10.1200/GO.20.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serraino D. COVID-19 and cancer: looking for evidence. Eur J Surg Oncol. 2020;46:929-930. doi: 10.1016/j.ejso.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, Wu Y, He Y. et al. Age-related risk factors and complications of patients with COVID-19: a population-based retrospective study. Front Med. 2021;8:757459. doi: 10.3389/fmed.2021.757459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arayici ME, Basbinar Y, Ellidokuz H. The impact of cancer on the severity of disease in patients affected with COVID-19: an umbrella review and meta-meta-analysis of systematic reviews and meta-analyses involving 1,064,476 participants. Clin Exp Med. 2023;23(6):2221-2229. doi: 10.1007/s10238-022-00911-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C, Zhao Y, Okwan-Duodu D, Basho R, Cui X. COVID-19 in cancer patients: risk, clinical features, and management. Cancer Biol Med. 2020;17:519-527. doi: 10.20892/j.issn.2095-3941.2020.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spezzani V, Piunno A, Iselin HU. Benign COVID-19 in an immunocompromised cancer patient – the case of a married couple. Swiss Med Wkly. 2020;150:w20246. doi: 10.4414/smw.2020.20246 [DOI] [PubMed] [Google Scholar]
- 27.Barlesi F, Foulon S, Bayle A, Gachot B, Pommeret F, Willekens C. Outcome of cancer patients infected with COVID-19, including toxicity of cancer treatments [abstract]. In: Proceedings of the AACR Annual Meeting, 27-28 April 2020, Philadelphia (PA: ): AACR; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during this study are available in the Dryad plataform https://datadryad.org/stash/share/bA2M_lfrZo8bLXLqVzd77qM5-phck7yDTe3GSuv4ZxU