Abstract
The biology of trophoblast cell lineage development and placentation is characterized by the involvement of several known transcription factors. Central to the action of a subset of these transcriptional regulators is CBP-p300 interacting transactivator with Glu/Asp-rich carboxy-terminal domain 2 (CITED2). CITED2 acts as a co-regulator modulating transcription factor activities and affecting placental development and adaptations to physiological stressors. These actions of CITED2 on the trophoblast cell lineage and placentation are conserved across the mouse, rat, and human. Thus, aspects of CITED2 biology in hemochorial placentation can be effectively modeled in the mouse and rat. In this review, we present information on the conserved role of CITED2 in the biology of placentation and discuss the use of CITED2 as a tool to discover new insights into regulatory mechanisms controlling placental development.
Keywords: CITED2, trophoblast, placentation, TFAP2C, HIF1, modulator
Graphical Abstract
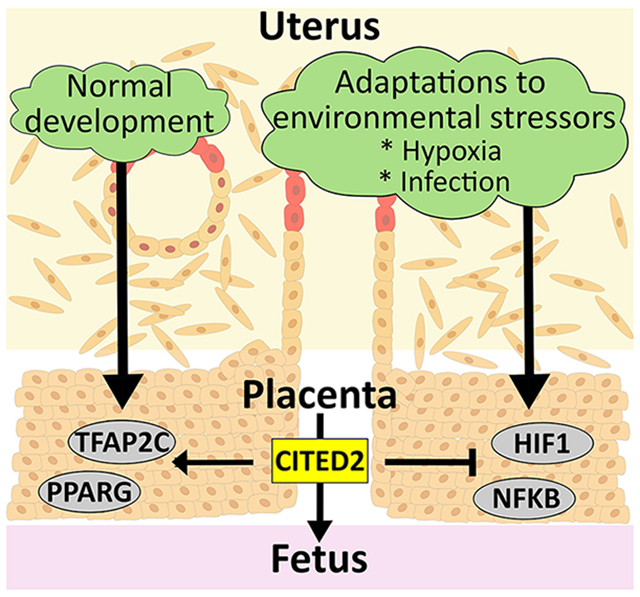
Hemochorial placentation in the human and rat is characterized by deep intrauterine trophoblast cell invasion and extensive trophoblast cell-guided spiral artery remodeling, which facilitates nutrient delivery to the fetus. CITED2 is a co-regulator modulating transcription factors central to hemochorial placentation, placental adaptations to environmental stressors, fetal development, and pregnancy outcome.
INTRODUCTION
CBP-p300 interacting transactivator with Glu/Asp-rich carboxy-terminal domain 2 (CITED2) is a transcriptional co-regulator[1-8]. CITED2 was first identified in melanocytes and given the name melanocyte-specific gene related 1 (MRG1)[1]. It was later shown, by Bhattacharya and coworkers that CITED2 acts a co-activator for the transcription factor AP-2 (TFAP2) family[4]. CITED2 belongs to the CITED protein family. Other members of the CITED family in mammals include CITED1[9-14] and CITED4[15-18]. Members of the CITED protein family each share conserved regions, including a domain at their carboxy terminus, which interacts with CBP (also referred to as CREB-binding protein, CREBBP) and p300 (also referred to as EIA-binding protein p300; EP300)[3,19]. The region of CREBBP and EP300 proteins that interacts with CITED2 also interacts with transcription factors.
CITED2 has been most extensively studied for its potential involvement in development, cancer, inflammation, metabolic, and congenital heart disease[19-33]. Here, we present an assessment of CITED2 involvement in placental development.
OVERVIEW OF PLACENTATION
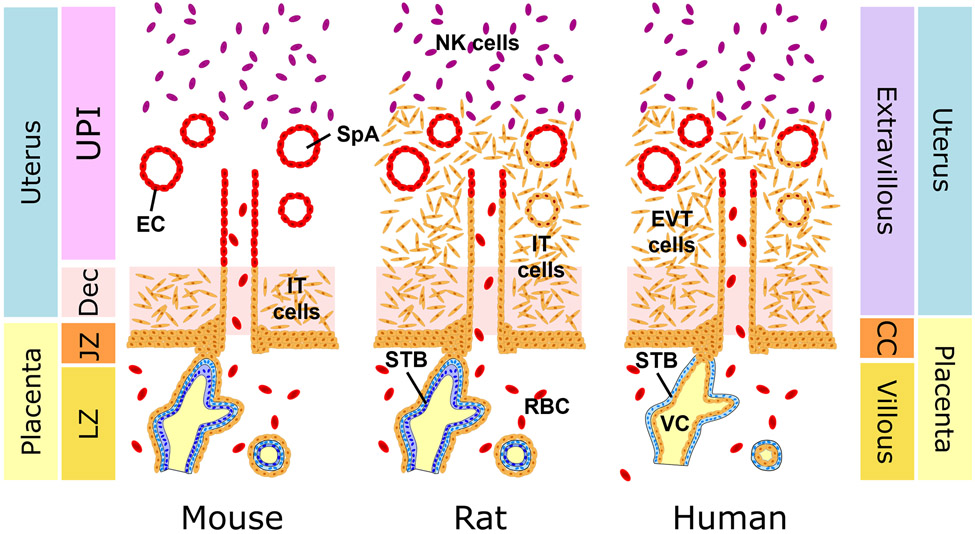
Our analysis focuses on the role of CITED2 in the process of hemochorial placentation, which is observed in the mouse, rat, and human[34]. The hemochorial placenta exhibits extensive erosion of the uterine endometrium, placing maternal blood in direct contact with the extraembryonic epithelium of the placenta, which is referred to as trophoblast[35,36]. The trophoblast cell lineage arises from the outer trophectoderm cell compartment of the blastocyst stage embryo[37]. Trophoblast cells contribute to placental structures interfacing with the uterine vasculature and the fetal vasculature. These two main trophoblast cell populations are specialized to remodel the uterine vasculature, facilitating nutrient entry into the placenta, or alternatively, to act as a barrier, enabling or restricting the delivery of nutrients and harmful compounds, respectively, to the fetus[35,36]. In the mouse and the rat, trophoblast cells engaged with the uterine vascular are referred to as invasive trophoblast cells and in the human, they are called extravillous trophoblast (EVT) cells[35,36,38]. Invasive trophoblast cells arise from a structure termed the junctional zone in the mouse and rat, while EVT cells arise from cell columns in the human placenta[35,36,38]. Trophoblast cell invasion in the mouse is shallow, whereas the rat and human exhibit deep intrauterine trophoblast cell invasion (Fig. 1)[35,39]. The uterine-placental interface defines the site where invasive trophoblast cells are distributed within the uterus. The junctional zone also contains endocrine cells referred to as trophoblast giant cells and spongiotrophoblast cells and a population of cells that accumulate glycogen termed glycogen cells[38]. Invasive/extravillous trophoblast cells come in two types based on their spatial location relative to the uterine vasculature: i) endovascular; ii) interstitial[35,36]. Endovascular invasive trophoblast cells supplant the endothelium, whereas interstitial invasive trophoblast cells are positioned between blood vessels. The barrier component of the rodent placenta is referred to as the labyrinth zone, whereas in the human placenta it is termed the villous compartment[35,36]. Syncytiotrophoblast (ST) contribute to the barrier of both rodent and human placentas and arise via fusion of progenitor trophoblast cell populations often referred to as cytotrophoblast, especially in human villous structures. Although there are prominent nomenclature differences, fundamental similarities exist in the structure and function of the mouse, rat, and human placentation sites. Thus, the mouse and rat can serve as effective models for investigating in vivo mechanisms regulating placental development, which have relevance to human placentation.
Fig. 1. Hemochorial placentation.
Schematic diagrams showing placentation sites of the mouse, rat, and human. The mouse exhibits shallow intrauterine trophoblast cell invasion, whereas the rat and human possess deep intrauterine trophoblast cell invasion. Invasive trophoblast (IT) and extravillous trophoblast (EVT) cells are functional equivalents, and the junctional zone (JZ) of the rodent placentation site is homologous to the EVT cell column (CC) of human placentation site. Uterine-placental interface (UPI), labyrinth zone (LZ), decidua (Dec), natural killer (NK) cells, villous core (VC), syncytiotrophoblast (STB), cytotrophoblast (CTB), basal CTB (bCTB), villous CTB (vCTB), spiral arteries (SpA), endothelial cells (EC), and red blood cells (RBC). Modified from Soares et al., 2017 with permission.
CITED2 AND PLACENTATION: ANIMAL MODELS
Roles for CITED2 in the biology of trophoblast cells and in placentation have been modeled in the mouse and rat (Table 1). In both species, CITED2 has been shown to possess a fundamental role in regulating placenta development and function.
Table 1.
CITED family and normal placentation.
| Family member |
Placental expression | Mutant rodent placenta phenotype | References |
|---|---|---|---|
| CITED1 | X-linked gene, expression overlap with CITED2, low level expression in human trophoblast cells | Expanded junctional zone, enlarged maternal blood sinusoids, fetal growth restriction and embryonic death on the day of birth or shortly thereafter | [10,44] |
| CITED2 | Highly expressed in both rodent and human placentas; expression increases with rat and human trophoblast stem cell differentiation | Placental and fetal growth restriction, abnormal trophoblast cell invasion, and prenatal (mouse) or immediate postnatal (rat) lethality, exaggerated responses to physiological stressors | [41,42,44,45,155] |
| CITED4 | No reported placental expression | No reported involvement in placental biology | No reports on placental biology |
Mouse
Global CITED2 deficiency mouse models have been generated[4,21,40]. Disrupting CITED2 results in prenatal lethality, cardiac malformations, lung developmental anomalies, embryonic left-right patterning and neural crest defects, adrenal agenesis, exencephaly, and placental abnormalities[4,20-22,40,41]. CITED2 deficiency leads to placental and fetal growth restriction[41]. Both the junctional and labyrinth zones are affected by the absence of CITED2. Junctional zones lacking CITED2 possess fewer spongiotrophoblast, glycogen, and trophoblast giant cells, while labyrinth zones without CITED2 exhibit abnormal fetal vasculature characterized by stunted and irregular capillaries[41]. Dunwoodie and coworkers specifically inactivated Cited2 in different cell types of the mouse placenta[42]. They utilized a Tek-Cre recombinase mouse model to disrupt Cited2 in endothelial cells of the fetal vasculature and Tpbpa-Cre and Cyp19a1-Cre recombinase mouse models to disrupt Cited2 in subsets of trophoblast cells. Disrupting CITED2 in endothelial cells or subsets of trophoblast cells adversely affected placental and fetal growth, but in contrast to global CITED2 deficiency these conditionally mutant models were compatible with postnatal survival. Interestingly, CITED2 also participates in preparing the uterus for pregnancy[43]. CITED2 is a progesterone responsive gene, and its uterine-specific ablation abrogates uterine stromal cell decidualization, which is essential for the establishment of pregnancy and the uterine-placental interface. Thus, CITED2 may directly contribute to both uterine and placental biology required for a successful pregnancy outcome.
Rat
A global CITED2 deficient rat model was generated utilizing CRISPR/Cas9 technology[44]. Phenotypes for the Cited2 null rat and Cited2 null mouse were compared. Some similarities and differences in the rat and mouse Cited2 null phenotypes were observed. CITED2 deficient mice and rats shared heart, lung, placental abnormalities, and fetal growth restriction. However, deficits in adrenal gland morphogenesis, aberrations in neural tube development, and prenatal death were unique to the mouse. The explanation for the species differences is unknown, as is which species may best model CITED2 deficiencies in the human. In contrast, the placenta is a common site of action for CITED2 in the mouse, rat, and human[41,42,44]. Furthermore, it is evident that placental and fetal growth restricted phenotypes are intrinsic to CITED2 actions within trophoblast cells[44]. CITED2 is expressed prominently in cells of the rat junctional zone and in invasive trophoblast cells of the uterine-placental interface (Fig. 2A and B)[44,45]. These tissue compartments also represent sites of deficits within the Cited2 null placentation site[44]. CITED2 deficiency resulted in death within hours of delivery, most likely due to cardiac malformations, including ventral septal defects and double outlet right ventricle, and/or because of a delay in lung development. Within the placenta, CITED2 deficiency affected the junctional zone transcriptome, resulting in downregulation of transcripts such as matrix metallopeptidase 9 (Mmp9) and insulin like growth factor 2 (Igf2), and the upregulation of several interferon-responsive transcripts, including ISG15 ubiquitin like modifier (Isg15). MMP9 and IGF2 are established regulators of placental development[46-49]. CITED2 deficiency also results in a delay in the onset of intrauterine trophoblast cell invasion. Furthermore, CITED2 deficient invasive trophoblast cells exhibit a distinct transcriptome, consistent with CITED2’s role as a co-regulator[44]. Finally, CITED2 deficiency affects the ability of the placentation site to adapt to physiological stressors (see below). Thus, it is evident that the rat is a useful model for investigating conserved roles for CITED2 in placentation.
Fig. 2. CITED2 is expressed in both rat and human placentation sites.
A) Schematic showing the late gestation rat placentation site. Invasive trophoblast cells are depicted in green. B) In situ hybridization showing Cited2 transcript localization in a rat gestation day (gd) 18.5 placentation site (Scale bar, 500 μm). C) Schematic showing the late gestation human extravillous trophoblast (EVT) cell column (CC). Invasive EVT cells are depicted in green. D) In situ hybridization showing CITED2 transcript localization in first trimester (12 week) human placenta: CITED2, CDH1 (marker of basal cytotrophoblast, bCTB), NOTUM (marker of EVT cells) (Scale bar, 50 μm). Uterine-placental interface (UPI), junctional zone (JZ), labyrinth zone (LZ), decidua (Dec), natural killer (NK) cells, villous core (VC), syncytiotrophoblast (STB), cytotrophoblast (CTB), and villous CTB (vCTB). Modified from Kuna et al., 2023 with permission.
CITED2 AND PLACENTATION: HUMAN
CITED2 is prominently expressed in central and distal regions within the EVT cell column of first trimester human placenta (Fig. 2C and D)[44]. Consistent with these observations, CITED2 exhibits an EVT cell differentiation-dependent increase in expression[44]. In contrast, CITED2 shows limited expression in ST[44]. Selective shRNA-mediated inhibition of CITED2 in human trophoblast stem (TS) cells interferes with normal EVT cell development, including their acquisition of invasive capabilities[44]. Thus, CITED2 is poised to contribute to the regulation of trophoblast cell invasion and the actions of invasive trophoblast cells on the uterine vasculature.
CITED2 has also been implicated in disease states, such as diabetes[50,51], congenital heart malformations[21,24,28-30,52-57], and cardiovascular dysfunction[58-60]. Some of these disease states may be secondary to or exacerbated by CITED2 insufficiency leading to disruptions in placentation (see below). In fact, dysregulation of CITED2 expression has been associated with preeclampsia[61]. CITED2 protein expression is decreased in the endoplasmic reticulum (ER) of stress-induced BeWo choriocarcinoma cells[62]. ER stress has been implicated in preeclampsia[63]. Whether non-transformed trophoblast cells exhibit a similar relationship of CITED2 protein expression and ER stress is unknown. Table 2 summarizes literature implicating CITED2 dysregulation in various disease states.
Table 2.
CITED2 involvement in disease states.
| Disease states | CITED2 relevance | References |
|---|---|---|
| Glucose homeostasis and diabetes | Required for the regulation of hepatic gluconeogenesis | [50,156] |
| Downregulation mediates high glucose-induced endoplasmic reticulum stress-dependent apoptosis | [51] | |
| Upregulated in vascular tissue of patients with obesity and type 2 diabetes | [157] | |
| Impairs insulin signaling in endothelial cells | [158] | |
| Decreased in heart tissue of maternal diabetic-exposed embryos | [58] | |
| Increased expression in adipose-derived stem cells from diabetic patients, which exhibit decreased proliferation and wound healing abilities | [159] | |
| Inflammation | Potent repressor of macrophage proinflammatory activation | [23] |
| Myeloid deficiency significantly increases macrophage and neutrophil recruitment | [32] | |
| Restrains signal transducer and activator of transcription 1 (STAT1) and interferon regulatory factor 1 (IRF1) signaling in macrophages and limits development of atherosclerotic plaques | [73] | |
| Deficiency results in enhanced placenta interferon-responsive gene expression | [44] | |
| Pregnancy related disorders | Lower levels in cumulus cells are associated with higher rates of oocyte fertilization and embryo implantation | [161] |
| Dysregulated in the placenta from mothers with hypertension | [160] | |
| Upregulated in placentas from women with severe preeclampsia | [61] | |
| Upregulated in placentas from growth restricted fetuses | [87] | |
| Decreased expression in endoplasmic-reticulum stress-induced BeWo choriocarcinoma cells (note: CITED2 may be restricted to the transformed character of the cells and not their trophoblast origin) | [62] | |
| Cardiovascular disease | Essential for heart development | [20,21,26,27,44,162] |
| Mutations contribute to congenital heart disease | [24,28-30,52-57] | |
| Lung development | Dysregulation results in aberrations in lung development | [44,163,164] |
| Neurodevelopmental disorders | Deficiency results in an abnormal neural development (exencephaly) in the mouse, a phenotype reduced with folic acid treatment | [4,21,40] |
EP300, an established partner in CITED2 action, has been implicated in the pathology of pregnancy disorders. The Rubinstein-Taybi syndrome is caused by disruptions in CREBBP or EP300. The subset of cases of the Rubinstein-Taybi syndrome affected by EP300 dysregulation are directly connected to pregnancy complications such as preeclampsia, HELLP syndrome, and fetal growth restriction[64,65]. Meta-analysis of placental transcriptome data has also identified CREBBP/EP300 as a hub in preeclampsia-related pathways[66]. Other anomalies in the EP300 gene have been linked to pregnancy miscarriages[67]. These observations highlight the importance of a functional EP300 protein for the execution of key pregnancy-related events. Most interestingly, EP300 regulates human TS cell differentiation[68]. Depletion of EP300 through short hairpin RNAs or CRISPR/Cas9 gene targeting inhibits TS cell differentiation and leads to retention of TS cells in a proliferative state. At least part of the mechanism of action of EP300 appears to be through inhibition of epidermal growth factor receptor (EGFR) signaling. Activation of EGFR is required for TS cell proliferation[69]. Furthermore, EP300 is required for EVT and ST development[68]. CITED2 shares involvement in regulating TS cell proliferation and in promoting EVT cell differentiation with EP300, but unlike EP300, is not involved in driving ST differentiation[44]. Finally, actions on trophoblast cell development appear unique to EP300 and are not shared by the related protein, CREBBP[68].
CITED2 AS A MODULATOR OF PLACENTAL PLASTICITY
A healthy placenta can adapt to environmental challenges affecting pregnancy[35,70]. Stimuli driving adaptations, include nutrition status, oxygen tension, exposure to pathogens, inflammation, and many others[38,71,72]. CITED2 has been implicated in cellular adaptations to hypoxia and to inflammation[23,31,32,73-79]. CITED2 modulates placental adaptations to hypoxia and to a viral mimic, and thus is in an excellent position to affect placental health[44]. In both cases, hypoxia and exposure to a viral mimic, the presence of CITED2 restrains trophoblast cell responses to the physiological stressor. Failure to attenuate these adaptive responses can lead to fetal growth restriction and fetal death[44]. CITED2 may modulate trophoblast cell adaptations to other physiological stressors, and hence, potentially broadening the scope of its importance as a regulator of placentation.
INDIRECT ACTIONS OF PLACENTAL CITED2 ON FETAL DEVELOPMENT
There are many genes affecting placental development that also affect fetal heart and brain development[80]. Some of these genes act within the placenta, heart, and brain, while it is apparent that the impact of other genes on the fetal heart and possibly brain is secondary to deficits in placental development and function[80-82]. Cited2 represents a gene impacting the fetal heart and potentially the fetal brain both directly within each fetal tissue and indirectly through its actions on the placenta.
OTHER CITED FAMILY MEMBERS AND PLACENTATION
There are two other CITED family members: CITED1 and CITED4. CITED1 shows some overlap in its expression with CITED2, including expression in the embryo and the placenta, as well as the heart, mammary glands, and melanocytes[10,83,84]. Dunwoodie and co-workers showed that disruption of the X-linked Cited1 gene in the mouse results in aberrant placental development[11]. Cited1 mutants have an irregular and expanded junctional zone and enlarged maternal blood sinusoids within the labyrinth zone. The fetal vasculature component of the placenta is also disrupted due to junctional zone projections into the labyrinth zone. CITED1 deficiency also resulted in fetal growth restriction and fetal death on the day of birth or shortly thereafter[11]. Little is known about a role for CITED1 in human placenta development. CITED1 does not appear relevant in human TS cells or their differentiation to ST or EVT cells[44]. Some limited insight has emerged from CITED1 and placenta disease. CITED1 expression is increased in placenta of pregnant women with HELLP syndrome[85]. CITED4 was identified as a co-activator for TFAP2 actions in heart development and function[86]. CITED4 has not been directly implicated as a regulator of placentation but is downregulated in placentas from growth restricted fetuses[87].
CITED2 MECHANISM OF ACTION - A MODULATOR OF TRANSCRIPTION FACTOR FUNCTION
The most well studied aspect of CITED2 action is its modulation of CREBBP and EP300 activities. CREBBP and EP300 are acetyltransferases that catalyze histone 3 lysine 27 acetylation at regulatory elements positively associated with transcriptional activation[88]. All CITED family members possess three conserved regions (CR1, CR2, CR3) (Fig. 3A). CR2 is located at the carboxy terminus of each CITED family member and contains a potent transactivation domain (TAD) (Fig. 3B), which physically interacts with the Zn2+ binding cysteine/histidine-rich 1(CH1) domains of CREBBP and EP300 and is also called the transcription adaptor putative zinc finger 1 (TAZ1) domain[3,9]. The CH1/TAZ1 domain is the region within CREBBP and EP300 that interacts with transcription factors such as hypoxia inducible factor 1 subunit alpha (HIF1A)[19,75,89-91], tumor protein p53 (TP53)[92,93], RELA proto-oncogene, NF-kB subunit (RELA)[94,95], and signal transducer and activator of transcription 2 (STAT2)[96,97]. CITED2 can modulate the activity of transcription factors that interact with CH1/TAZ1. This interaction has been best demonstrated through the ability of CITED2 to effectively compete for HIF1A binding to CREBPP and EP300 resulting in the inhibition of HIF1A transcriptional activity[75,76,78,79,98-101]. CITED2 blocks the HIF1A-EP300 interaction, most likely due to direct occupancy of the CH1/TAZ1 domain of EP300[75]. These efforts have been extended to show that CITED2 controls hypoxia signaling by capturing EP300 from two distinct activation domains of HIF1A[76]. CITED2 also has a higher affinity than HIF1A for EP300, and unidirectionally displaces HIF1A from its association with EP300[102].
Fig. 3. The CITED protein family conserved domains.
A) Location of conserved regions (CR) within the CITED family. B) Amino acid sequence alignment of CR2 domains for each member of the CITED family.
CITED2 has also been shown to interact with other proteins, including LIM homeobox 2[2], TFAP2 family transcription factors[5], SMAD family members 2 and 3[103], peroxisome proliferator-activated receptor gamma (PPARG)[7], estrogen receptor[104], MYC proto-oncogene, bHLH transcription factor[105], and nucleolin[106]. Several parameters of CITED family protein interactions with the TFAP2 family have been discerned[86]. CITED2 exhibits stronger interactions with the TFAP2 family than does CITED1 or CITED4. Additionally, CITED protein interactions with TFAP2 proteins are within the region of TFAP2 proteins that binds to the CH1/TAZ1 domain of CREBBP and EP300. CITED2 may also interact with the dimerization domain of TFAP2C[5]. In general, the mechanistic significance of CITED2 interactions with these other proteins is less than that currently understood about CITED2 interactions with CREBBP and EP300. Nonetheless, collectively, our knowledge of CITED2-protein interactions are all consistent with CITED2 functioning as a modulator of transcription.
In the following paragraphs, we discuss some examples of the potential involvement of CITED2 in modulating activities of specific transcription factors previously implicated in the regulation of placentation. CITED2 can act as either a co-activator or a co-repressor depending on the transcription factor.
CITED2 as a co-activator
TFAP2C.
TFAP2C is a member of the TFAP2 family of transcription factors. TFAP2C plays an important role in trophectoderm specification and TS cell self-renewal in the mouse[107-111] and human[108,111-114]. TFAP2C deficient mice fail to establish a proper maternal-fetal interface with malformations arising during the earliest stages of placentation[115,116]. TFAP2C is also essential for junctional zone development[117]. Potential TFAP2C gene targets have been identified in various trophoblast cell lineages[108,111,113,114,118]. TFAP2C and CITED2 are co-expressed in the junctional zone and in invasive trophoblast cells situated within the rat uterine-placental interface[44,119]. CITED2 may act as a rheostat to modulate TFAP2C transcriptional activities within the developing placenta. The negative aspects of CITED2 deficiency on placentation may be a consequence, at least in part, of less-than-optimal TFAP2C driven gene expression. TFAP2C dosage appears to be critical regarding its actions in trophoblast cells[120]. As indicated above, CITED2 has been shown to physically and functionally interact with members of the TFAP2 family[4,5]. More specifically, in the developing heart, CITED2 and TFAP2C cooperate in the transactivation of the paired-like homeodomain 2 gene[121]. Target genes shared by TFAP2C and CITED2 in invasive trophoblast cells can be inferred from single cell RNA sequencing (scRNA-seq) and single nucleus assay for transposase-accessible chromatin using sequencing (snATAC-seq) datasets[119,122] but have not been empirically verified.
PPARG.
PPARG is a ligand activated nuclear receptor with a role in a variety of biological processes such as lipid and glucose homeostasis, metabolic disease, and inflammation[123,124]. PPARG is expressed in both rodent and human placentas[81,125-128]. Disruption of PPARG in the mouse results in prominent labyrinth zone dysfunction[81]. The labyrinth zone does not represent a significant site of CITED2 expression and thus, CITED2 may not be a relevant co-regulator of PPARG action within the labyrinth zone. However, PPARG and CITED2 are co-expressed in invasive trophoblast cells, where their cooperativity may be more relevant[119]. CITED2 is an established co-activator for PPARG[7]. There is evidence that CITED2 partners with PPARG in other cell types, including PPARG’s involvement in regulating cortical neuron death associated with DNA damage[129]. CITED2 is also required for optimal PPARG regulation of anti-inflammatory gene expression in macrophages[23]. Target genes shared by PPARG and CITED2 in invasive trophoblast cells can be predicted from scRNA-seq and snATAC-seq datasets[119,122].
CITED2 as a co-repressor
HIF1.
HIF1 is primarily a transcriptional activator and the main factor mediating adaptations to low oxygen tension in cells and tissues[130]. HIF1 is composed of two subunits, an alpha or A subunit, which is regulated by oxygen tension, and a beta or B subunit, which is constitutively expressed[131,132]. HIF1B is also called the aryl hydrocarbon receptor nuclear translocator (ARNT). In response to hypoxia, HIF1 is stabilized and promotes transcriptional changes in genes that are involved in angiogenesis, iron and glucose metabolism, cell proliferation, and cell survival. Early in gestation prior to uterine spiral artery remodeling intrauterine oxygen tensions are low and HIF1 is proposed to facilitate adaptations required to promote normal placenta development[70,133-139].
Disruption of HIF signaling impairs TS cell responses to hypoxia and differentiation into the invasive trophoblast cell lineage[140]. HIF signaling has also been identified as an important regulator of trophoblast cell plasticity and placental adaptations during low oxygen conditions[141]. CITED2 is a negative regulator of HIF1. As described above, CITED2 prevents HIF1 from binding to CREBBP and EP300, and thus attenuates gene activation following exposure to hypoxic environments[3,76,79,98-100]. CITED2 serves a critical role in modulating placental adaptations to hypoxia.
Nuclear factor kappa B (NFKB).
NFKB is a transcription factor involved in regulating innate immunity and inflammatory responses[142,143]. The NFKB signaling pathway is involved in organizing cellular resistance to pathogens[144-146]. NFKB possesses roles in trophoblast cell responses to pathogens[147-151]. CITED2 has been identified as a repressor of NFKB[152]. It prevents RELA from binding to its promoters and attenuates RELA acetylation by preventing its access to EP300. CITED2 has also been identified as a critical molecular switch that limits a broad proinflammatory gene network in macrophages by restraining inflammatory cytokine-induced NFKB activation[32].
FINAL THOUGHTS
CITED2 has a pivotal and conserved role in regulating placenta development, especially at the uterine-placental interface, and in ensuring plasticity of the placentation site. These actions are accomplished through cooperation with other parts of the transcriptional regulatory apparatus. In general, CITED2 promotes placental growth and development through synergism with transcription factors such as TFAP2C and PPARG, while restraining responses to physiological stressors via antagonizing the actions of other transcription factors such as HIF1 and NFKB.
CITED2 can also be used as a tool to discover new transcription factor involvement in placental development. CITED2 has been shown to modulate the activity of several different transcription factors. As described above, a subset of these transcription factors has been implicated in the regulation of placentation (TFAP2C, PPARG) and placental plasticity (HIF1, NFKB). Other transcription factors are certainly involved in modulating aspects of placentation; however, the involvement of CITED2 as a modulator of any of these transcription factors is unknown. The existence of CITED2 deficient TS cells and animal models provides tools to discover new transcription factors targeting trophoblast cells and placental biology. Furthermore, examination of scRNA-seq and snATAC-seq in control versus CITED2 deficiency provides a strategy for identifying regulatory regions and transcription factor binding motifs specifically connected to CITED2. Such experimentation can be followed by chromatin immunoprecipitation, evaluation of the involvement of CREBBP and EP300, and assessment of direct physical interactions with CITED2. Any transcription factor utilizing CREBBP or EP300 is a potential target of CITED2 modulation. Such a strategy will expand our knowledge of the transcriptional control of trophoblast cell development, placentation, and adaptations of the placenta to physiological stressors.
The placenta is not the sole target of CITED2 actions. Other organs are dependent on CITED2 for orchestrating temporally and spatially appropriate morphogenesis. Several disease processes involve the dysregulation of CITED2. This represents a challenge that has experimentally been addressed using conditional mutagenesis[42] and other methodologies to specifically manipulate the trophoblast cell lineage[44]. Such approaches are dependent upon the availability of effective high-fidelity trophoblast driven Cre recombinase strains, which has represented a concern for in vivo manipulation of trophoblast cell lineages[35,42,153], and/or the expertise in genetically manipulating and handling embryos for the creation of new trophoblast cell lineage- and placenta-specific mutant models. The precision of these approaches will improve and roles for CITED2 in specific trophoblast cell lineages will be addressed. The recent establishment of a highly specific invasive trophoblast cell-specific Cre recombinase rat model should facilitate understanding in vivo roles for CITED2 in the biology of invasive trophoblast cell-guided uterine transformation[154].
It is apparent that there are species differences associated with the biology of CITED2. Genetic disruption of the Cited2 gene in the mouse and rat yield similarities but also pronounced phenotypic differences[4,21,40,44]. The comparative biology of CITED2 reflects species-specific aspects of its expression pattern, the profile of other CITED family members, and undoubtedly the expression and activities of its many transcription factor targets. Making the leap from the mouse or rat to human CITED2 biology represents a challenge for understanding the biology of some organ systems; however, not for the placenta where CITED2 is a conserved core actor in the regulatory hierarchy directing trophoblast cell lineage development. This conservation likely emanates from the known actions of CITED2 modulated transcription factors in trophoblast cell development. We do not yet know the molecular targets of CITED2 action in mouse, rat, or human trophoblast cells. We expect to observe aspects of conservation and, also species specificity as we learn more about the involvement of CITED2 in trophoblast cell development and placentation.
Finally, it is evident that CITED2 dysregulation is linked to the etiology of an assortment of disease states not involving pregnancy or placentation. The involvement of CITED2 in both embryogenesis and disease should provide significant motivation for elucidating mechanisms underlying CITED2 action. Understanding the involvement of CITED2 in disease processes will provide insights into the role of CITED2 in placenta development. Similarly, knowledge of CITED2 in placentation should provide new directions for understanding the involvement of CITED2 in the etiology of diseases that impact human health. Not surprisingly, several pregnancy-related diseases involve the dysfunction of biological processes directly regulated by CITED2.
Fig. 4. Schematic of CITED2 positive and negative actions on gene regulation.
A) CITED2 facilitation of transcription factor (TF) interaction with CREBBP /EP300 promoting gene activation. B) CITED2 interference of TF interaction with CREBBP /EP300 preventing TF-mediated gene activation.
ACKNOWLEDGEMENTS
The research was supported by NIH grants (HD020676, HD099638, HD105734), and the Sosland Foundation. We also thank Stacy Oxley, Brandi Miller, and Leslie Tracy for administrative assistance.
Footnotes
CONFLICT OF INTEREST
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Sun HB, Zhu YX, Yin T, Sledge G, & Yang YC (1998). MRG1, the product of a melanocyte-specific gene related gene, is a cytokine-inducible transcription factor with transformation activity. Proceedings of the National Academy of Sciences of the United States of America, 95(23), 13555–13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glenn DJ, & Maurer RA (1999). MRG1 binds to the LIM domain of Lhx2 and may function as a coactivator to stimulate glycoprotein hormone alpha-subunit gene expression. The Journal of Biological Chemistry, 274(51), 36159–36167. 10.1074/jbc.274.51.36159 [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharya S, Michels CL, Leung MK, Arany ZP, Kung AL, & Livingston DM (1999). Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes & Development, 13(1), 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bamforth SD, Bragança J, Eloranta JJ, Murdoch JN, Marques FI, Kranc KR, Farza H, Henderson DJ, Hurst HC, & Bhattacharya S (2001). Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nature Genetics, 29(4), 469–474. 10.1038/ng768 [DOI] [PubMed] [Google Scholar]
- 5.Bragança J, Eloranta JJ, Bamforth SD, Ibbitt JC, Hurst HC, & Bhattacharya S (2003). Physical and functional interactions among AP-2 transcription factors, p300/CREB-binding protein, and CITED2. The Journal of Biological Chemistry, 278(18), 16021–16029. 10.1074/jbc.M208144200 [DOI] [PubMed] [Google Scholar]
- 6.Freedman SJ, Sun Z-YJ, Kung AL, France DS, Wagner G, & Eck MJ (2003). Structural basis for negative regulation of hypoxia-inducible factor-1alpha by CITED2. Nature Structural Biology, 10(7), 504–512. 10.1038/nsb936 [DOI] [PubMed] [Google Scholar]
- 7.Tien ES, Davis JW, & Vanden Heuvel JP (2004). Identification of the CREB-binding protein/p300-interacting protein CITED2 as a peroxisome proliferator-activated receptor alpha coregulator. The Journal of Biological Chemistry, 279(23), 24053–24063. 10.1074/jbc.M401489200 [DOI] [PubMed] [Google Scholar]
- 8.Chou Y-T, & Yang Y-C (2006). Post-transcriptional control of Cited2 by transforming growth factor beta. Regulation via Smads and Cited2 coding region. The Journal of Biological Chemistry, 281(27), 18451–18462. 10.1074/jbc.M601720200 [DOI] [PubMed] [Google Scholar]
- 9.Shioda T, Fenner MH, & Isselbacher KJ (1997). MSG1 and its related protein MRG1 share a transcription activating domain. Gene, 204(1–2), 235–241. 10.1016/s0378-1119(97)00551-9 [DOI] [PubMed] [Google Scholar]
- 10.Dunwoodie SL, Rodriguez TA, & Beddington RS (1998). Msg1 and Mrg1, founding members of a gene family, show distinct patterns of gene expression during mouse embryogenesis. Mechanisms of Development, 72(1–2), 27–40. 10.1016/s0925-4773(98)00011-2 [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez TA, Sparrow DB, Scott AN, Withington SL, Preis JI, Michalicek J, Clements M, Tsang TE, Shioda T, Beddington RSP, & Dunwoodie SL (2004). Cited1 is required in trophoblasts for placental development and for embryo growth and survival. Molecular and Cellular Biology, 24(1), 228–244. 10.1128/MCB.24.1.228-244.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyle S, Shioda T, Perantoni AO, & de Caestecker M (2007). Cited1 and Cited2 are differentially expressed in the developing kidney but are not required for nephrogenesis. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 236(8), 2321–2330. 10.1002/dvdy.21242 [DOI] [PubMed] [Google Scholar]
- 13.González-García I, García-Clavé E, Cebrian-Serrano A, Le Thuc O, Contreras RE, Xu Y, Gruber T, Schriever SC, Legutko B, Lintelmann J, Adamski J, Wurst W, Müller TD, Woods SC, Pfluger PT, Tschöp MH, Fisette A, & García-Cáceres C (2023). Estradiol regulates leptin sensitivity to control feeding via hypothalamic Cited1. Cell Metabolism, 35(3), 438–455.e7. 10.1016/j.cmet.2023.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramani A, Hite MEL, Garcia S, Maxwell J, Kondee H, Millican GE, McClelland EE, Seipelt-Thiemann RL, & Nelson DE (2023). Regulation of macrophage IFNγ-stimulated gene expression by the transcriptional coregulator CITED1. Journal of Cell Science, 136(1), jcs260529. 10.1242/jcs.260529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yahata T, Takedatsu H, Dunwoodie SL, Bragança J, Swingler T, Withington SL, Hur J, Coser KR, Isselbacher KJ, Bhattacharya S, & Shioda T (2002). Cloning of mouse Cited4, a member of the CITED family p300/CBP-binding transcriptional coactivators: Induced expression in mammary epithelial cells. Genomics, 80(6), 601–613. 10.1006/geno.2002.7005 [DOI] [PubMed] [Google Scholar]
- 16.Lerchenmüller C, Rabolli CP, Yeri A, Kitchen R, Salvador AM, Liu LX, Ziegler O, Danielson K, Platt C, Shah R, Damilano F, Kundu P, Riechert E, Katus HA, Saffitz JE, Keshishian H, Carr SA, Bezzerides VJ, Das S, & Rosenzweig A (2020). CITED4 Protects Against Adverse Remodeling in Response to Physiological and Pathological Stress. Circulation Research, 127(5), 631–646. 10.1161/CIRCRESAHA.119.315881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bezzerides VJ, Platt C, Lerchenmüller C, Paruchuri K, Oh NL, Xiao C, Cao Y, Mann N, Spiegelman BM, & Rosenzweig A (2016). CITED4 induces physiologic hypertrophy and promotes functional recovery after ischemic injury. JCI Insight, 1(9), e85904. 10.1172/jci.insight.85904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boström P, Mann N, Wu J, Quintero PA, Plovie ER, Panáková D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, & Spiegelman BM (2010). C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell, 143(7), 1072–1083. 10.1016/j.cell.2010.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandes MT, Calado SM, Mendes-Silva L, & Bragança J (2020). CITED2 and the modulation of the hypoxic response in cancer. World Journal of Clinical Oncology, 11(5), 260–274. 10.5306/wjco.v11.i5.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weninger WJ, Lopes Floro K, Bennett MB, Withington SL, Preis JI, Barbera JPM, Mohun TJ, & Dunwoodie SL (2005). Cited2 is required both for heart morphogenesis and establishment of the left-right axis in mouse development. Development (Cambridge, England), 132(6), 1337–1348. 10.1242/dev.01696 [DOI] [PubMed] [Google Scholar]
- 21.Yin Z, Haynie J, Yang X, Han B, Kiatchoosakun S, Restivo J, Yuan S, Prabhakar NR, Herrup K, Conlon RA, Hoit BD, Watanabe M, & Yang Y-C (2002). The essential role of Cited2, a negative regulator for HIF-1alpha, in heart development and neurulation. Proceedings of the National Academy of Sciences, 99(16), 10488–10493. 10.1073/pnas.162371799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bamforth SD, Bragança J, Farthing CR, Schneider JE, Broadbent C, Michell AC, Clarke K, Neubauer S, Norris D, Brown NA, Anderson RH, & Bhattacharya S (2004). Cited2 controls left-right patterning and heart development through a Nodal-Pitx2c pathway. Nature Genetics, 36(11), 1189–1196. 10.1038/ng1446 [DOI] [PubMed] [Google Scholar]
- 23.Kim G-D, Das R, Rao X, Zhong J, Deiuliis JA, Ramirez-Bergeron DL, Rajagopalan S, & Mahabeleshwar GH (2018). CITED2 restrains pro-inflammatory macrophage activation and response. Molecular and Cellular Biology, 38(5), e00452–17. 10.1128/MCB.00452-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sperling S, Grimm CH, Dunkel I, Mebus S, Sperling H-P, Ebner A, Galli R, Lehrach H, Fusch C, Berger F, & Hammer S (2005). Identification and functional analysis of CITED2 mutations in patients with congenital heart defects. Human Mutation, 26(6), 575–582. 10.1002/humu.20262 [DOI] [PubMed] [Google Scholar]
- 25.Val P, Martinez-Barbera J-P, & Swain A (2007). Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development (Cambridge, England), 134(12), 2349–2358. 10.1242/dev.004390 [DOI] [PubMed] [Google Scholar]
- 26.MacDonald ST, Bamforth SD, Chen C-M, Farthing CR, Franklyn A, Broadbent C, Schneider JE, Saga Y, Lewandoski M, & Bhattacharya S (2008). Epiblastic Cited2 deficiency results in cardiac phenotypic heterogeneity and provides a mechanism for haploinsufficiency. Cardiovascular Research, 79(3), 448–457. 10.1093/cvr/cvn101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopes Floro K, Artap ST, Preis JI, Fatkin D, Chapman G, Furtado MB, Harvey RP, Hamada H, Sparrow DB, & Dunwoodie SL (2011). Loss of Cited2 causes congenital heart disease by perturbing left–right patterning of the body axis. Human Molecular Genetics, 20(6), 1097–1110. 10.1093/hmg/ddq554 [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Wang F, Wu Y, Tan S, Wen Q, Wang J, Zhu X, Wang X, Li C, Ma X, & Pan H (2014). Variations of CITED2 are associated with congenital heart disease (CHD) in Chinese population. PloS One, 9(5), e98157. 10.1371/journal.pone.0098157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Su Z, Tan S, Ni B, Pan H, Liu B, Wang J, Xiao J, & Chen Q (2017). Functional Analyses of a Novel CITED2 Nonsynonymous Mutation in Chinese Tibetan Patients with Congenital Heart Disease. Pediatric Cardiology, 38(6), 1226–1231. 10.1007/s00246-017-1649-y [DOI] [PubMed] [Google Scholar]
- 30.Li B, Pu T, Liu Y, Xu Y, & Xu R (2017). CITED2 Mutations in Conserved Regions Contribute to Conotruncal Heart Defects in Chinese Children. DNA and Cell Biology, 36(7), 589–595. 10.1089/dna.2017.3701 [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Chen W, Liu W, Li D, & Shen W (2022). CITED2 alleviates lipopolysaccharide-induced inflammation and pyroptosis in—Human lung fibroblast by inhibition of NF-κB pathway. Allergologia Et Immunopathologia, 50(4), 64–70. 10.15586/aei.v50i4.628 [DOI] [PubMed] [Google Scholar]
- 32.Pong Ng H, Kim G-D, Ricky Chan E, Dunwoodie SL, & Mahabeleshwar GH (2020). CITED2 limits pathogenic inflammatory gene programs in myeloid cells. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 34(9), 12100–12113. 10.1096/fj.202000864R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.An B, Ji X, & Gong Y (2020). Role of CITED2 in stem cells and cancer. Oncology Letters, 20(4), 1–1. 10.3892/ol.2020.11968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts RM, Green JA, & Schulz LC (2016). The evolution of the placenta. Reproduction (Cambridge, England), 152(5), R179–R189. 10.1530/REP-16-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soares MJ, Varberg KM, & Iqbal K (2018). Hemochorial placentation: Development, function, and adaptations. Biology of Reproduction, 99(1), 196–211. 10.1093/biolre/ioy049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knöfler M, Haider S, Saleh L, Pollheimer J, Gamage TKJB, & James J (2019). Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cellular and Molecular Life Sciences, 76(18), 3479–3496. 10.1007/s00018-019-03104-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemberger M, Hanna CW, & Dean W (2020). Mechanisms of early placental development in mouse and humans. Nature Reviews. Genetics, 21(1), 27–43. 10.1038/s41576-019-0169-4 [DOI] [PubMed] [Google Scholar]
- 38.Shukla V, & Soares MJ (2022). Modeling Trophoblast Cell-Guided Uterine Spiral Artery Transformation in the Rat. International Journal of Molecular Sciences, 23(6), 2947. 10.3390/ijms23062947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soares MJ, Chakraborty D, Rumi MAK, Konno T, & Renaud SJ (2012). Rat placentation: An experimental model for investigating the hemochorial maternal-fetal interface. Placenta, 33(4), 233–243. 10.1016/j.placenta.2011.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbera JPM, Rodriguez TA, Greene NDE, Weninger WJ, Simeone A, Copp AJ, Beddington RSP, & Dunwoodie S (2002). Folic acid prevents exencephaly in Cited2 deficient mice. Human Molecular Genetics, 11(3), 283–293. 10.1093/hmg/11.3.283 [DOI] [PubMed] [Google Scholar]
- 41.Withington SL, Scott AN, Saunders DN, Lopes Floro K, Preis JI, Michalicek J, Maclean K, Sparrow DB, Barbera JPM, & Dunwoodie SL (2006). Loss of Cited2 affects trophoblast formation and vascularization of the mouse placenta. Developmental Biology, 294(1), 67–82. 10.1016/j.ydbio.2006.02.025 [DOI] [PubMed] [Google Scholar]
- 42.Moreau JLM, Artap ST, Shi H, Chapman G, Leone G, Sparrow DB, & Dunwoodie SL (2014). Cited2 is required in trophoblasts for correct placental capillary patterning. Developmental Biology, 392(1), 62–79. 10.1016/j.ydbio.2014.04.023 [DOI] [PubMed] [Google Scholar]
- 43.Yoo J-Y, Kim TH, Lee JH, Dunwoodie SL, Ku BJ, & Jeong J-W (2015). Mig-6 regulates endometrial genes involved in cell cycle and progesterone signaling. Biochemical and Biophysical Research Communications, 462(4), 409–414. 10.1016/j.bbrc.2015.04.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuna M, Dhakal P, Iqbal K, Dominguez EM, Kent LN, Muto M, Moreno-Irusta A, Kozai K, Varberg KM, Okae H, Arima T, Sucov HM, & Soares MJ (2023). CITED2 is a conserved regulator of the uterine-placental interface. Proceedings of the National Academy of Sciences of the United States of America, 120(3), e2213622120. 10.1073/pnas.2213622120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kent LN, Konno T, & Soares MJ (2010). Phosphatidylinositol 3 kinase modulation of trophoblast cell differentiation. BMC Developmental Biology, 10, 97. 10.1186/1471-213X-10-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker J, Liu JP, Robertson EJ, & Efstratiadis A (1993). Role of insulin-like growth factors in embryonic and postnatal growth. Cell, 75(1), 73–82. [PubMed] [Google Scholar]
- 47.Constância M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, & Reik W (2002). Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature, 417(6892), 945–948. 10.1038/nature00819 [DOI] [PubMed] [Google Scholar]
- 48.Kent LN, Ohboshi S, & Soares MJ (2012). Akt1 and insulin-like growth factor 2 (Igf2) regulate placentation and fetal/postnatal development. The International Journal of Developmental Biology, 56(4), 255–261. 10.1387/ijdb.113407lk [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plaks V, Rinkenberger J, Dai J, Flannery M, Sund M, Kanasaki K, Ni W, Kalluri R, & Werb Z (2013). Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proceedings of the National Academy of Sciences of the United States of America, 110(27), 11109–11114. 10.1073/pnas.1309561110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakai M, Matsumoto M, Tujimura T, Yongheng C, Noguchi T, Inagaki K, Inoue H, Hosooka T, Takazawa K, Kido Y, Yasuda K, Hiramatsu R, Matsuki Y, & Kasuga M (2012). CITED2 links hormonal signaling to PGC-1α acetylation in the regulation of gluconeogenesis. Nature Medicine, 18(4), 612–617. 10.1038/nm.2691 [DOI] [PubMed] [Google Scholar]
- 51.Gu H, Yu J, Dong D, Zhou Q, Wang J-Y, Fang S, & Yang P (2016). High Glucose-Repressed CITED2 Expression Through miR-200b Triggers the Unfolded Protein Response and Endoplasmic Reticulum Stress. Diabetes, 65(1), 149–163. 10.2337/db15-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu M, Wu X, Li Y, Yang X, Hu J, Zheng M, & Tian J (2014). CITED2 mutation and methylation in children with congenital heart disease. Journal of Biomedical Science, 21(1), 7. 10.1186/1423-0127-21-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen C, Bentham J, Cosgrove C, Braganca J, Cuenda A, Bamforth SD, Schneider JE, Watkins H, Keavney B, Davies B, & Bhattacharya S (2012). Functional Significance of SRJ Domain Mutations in CITED2. PLoS ONE, 7(10), e46256. 10.1371/journal.pone.0046256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dianatpour S, Khatami M, Heidari MM, & Hadadzadeh M (2020). Novel Point Mutations of CITED2 Gene Are Associated with Non-familial Congenital Heart Disease (CHD) in Sporadic Pediatric Patients. Applied Biochemistry and Biotechnology, 190(3), 896–906. 10.1007/s12010-019-03125-8 [DOI] [PubMed] [Google Scholar]
- 55.Yadav ML, Jain D, Neelabh, Agrawal D, Kumar A, & Mohapatra B (2021). A gain-of-function mutation in CITED2 is associated with congenital heart disease. Mutation Research, 822, 111741. 10.1016/j.mrfmmm.2021.111741 [DOI] [PubMed] [Google Scholar]
- 56.Chen Z, Chen H-X, Hou H-T, Yin X-Y, Yang Q, Han J, & He G-W (2022). Genetic Variants of CITED2 Gene Promoter in Human Atrial Septal Defects: Case-Control Study and Cellular Functional Verification. Journal of Cardiovascular Development and Disease, 9(10), 321. 10.3390/jcdd9100321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Z, Chen H-X, Hou H-T, Yin X-Y, Yang Q, & He G-W (2022). Pathophysiological Role of Variants of the Promoter Region of CITED2 Gene in Sporadic Tetralogy of Fallot Patients with Cellular Function Verification. Biomolecules, 12(11), 1644. 10.3390/biom12111644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su D, Song J-X, Gao Q, Guan L, Li Q, Shi C, & Ma X (2016). Cited2 participates in cardiomyocyte apoptosis and maternal diabetes-induced congenital heart abnormality. Biochemical and Biophysical Research Communications, 479(4), 887–892. 10.1016/j.bbrc.2016.09.101 [DOI] [PubMed] [Google Scholar]
- 59.Wu Q, Liu Q, Zhan J, Wang Q, Zhang D, He S, Pu S, & Zhou Z (2019). Cited2 regulates proliferation and survival in young and old mouse cardiac stem cells. BMC Molecular and Cell Biology, 20(1), 25. 10.1186/s12860-019-0207-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu M, Liu Y, Xian Z, Yu X, Chen J, Tan S, Zhang P, & Guo Y (2022). VEGF to CITED2 ratio predicts the collateral circulation of acute ischemic stroke. Frontiers in Neurology, 13, 1000992. 10.3389/fneur.2022.1000992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sitras V, Paulssen RH, Grønaas H, Leirvik J, Hanssen TA, Vårtun Å, & Acharya G (2009). Differential Placental Gene Expression in Severe Preeclampsia. Placenta, 30(5), 424–433. 10.1016/j.placenta.2009.01.012 [DOI] [PubMed] [Google Scholar]
- 62.Collett GP, Redman CW, Sargent IL, & Vatish M (2018). Endoplasmic reticulum stress stimulates the release of extracellular vesicles carrying danger-associated molecular pattern (DAMP) molecules. Oncotarget, 9(6), 6707–6717. 10.18632/oncotarget.24158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burton GJ, Yung H-W, Cindrova-Davies T, & Charnock-Jones DS (2009). Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta, 30 Suppl A(Suppl), S43–48. 10.1016/j.placenta.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roelfsema JH, & Peters DJM (2007). Rubinstein-Taybi syndrome: Clinical and molecular overview. Expert Reviews in Molecular Medicine, 9(23), 1–16. 10.1017/S1462399407000415 [DOI] [PubMed] [Google Scholar]
- 65.Fergelot P, Van Belzen M, Van Gils J, Afenjar A, Armour CM, Arveiler B, Beets L, Burglen L, Busa T, Collet M, Deforges J, de Vries BBA, Dominguez Garrido E, Dorison N, Dupont J, Francannet C, Garciá-Minaúr S, Gabau Vila E, Gebre-Medhin S, … Hennekam RC (2016). Phenotype and genotype in 52 patients with Rubinstein–Taybi syndrome caused by EP300 mutations. American Journal of Medical Genetics Part A, 170(12), 3069–3082. 10.1002/ajmg.a.37940 [DOI] [PubMed] [Google Scholar]
- 66.van Uitert M, Moerland PD, Enquobahrie DA, Laivuori H, van der Post JAM, Ris-Stalpers C, & Afink GB (2015). Meta-analysis of placental transcriptome data identifies a novel molecular pathway related to preeclampsia. PLOS ONE, 10(7), e0132468. 10.1371/journal.pone.0132468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shao Y, Yang S, Cheng L, Duan J, Li J, Kang J, Wang F, Liu J, Zheng F, Ma J, & Zhang Y (2024). Identification of chromosomal abnormalities in miscarriages by CNV-Seq. Molecular Cytogenetics, 17(1), 4. 10.1186/s13039-024-00671-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Voorden AJ, Keijser R, Veenboer GJM, Lopes Cardozo SA, Diek D, Vlaardingerbroek JA, van Dijk M, Ris-Stalpers C, van Pelt AMM, & Afink GB (2023). EP300 facilitates human trophoblast stem cell differentiation. Proceedings of the National Academy of Sciences, 120(28), e2217405120. 10.1073/pnas.2217405120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okae H, Toh H, Sato T, Hiura H, Takahashi S, Shirane K, Kabayama Y, Suyama M, Sasaki H, & Arima T (2018). Derivation of human trophoblast stem cells. Cell Stem Cell, 22(1), 50–63.e6. 10.1016/j.stem.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 70.Soares MJ, Iqbal K, & Kozai K (2017). Hypoxia and placental development. Birth Defects Research, 109(17), 1309–1329. 10.1002/bdr2.1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosario GX, Konno T, & Soares MJ (2008). Maternal hypoxia activates endovascular trophoblast cell invasion. Developmental Biology, 314(2), 362–375. 10.1016/j.ydbio.2007.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baines KJ, Rampersaud AM, Hillier DM, Jeyarajah MJ, Grafham GK, Eastabrook G, Lacefield JC, & Renaud SJ (2020). Antiviral inflammation during early pregnancy reduces placental and fetal growth trajectories. The Journal of Immunology, 204(3), 694–706. 10.4049/jimmunol.1900888 [DOI] [PubMed] [Google Scholar]
- 73.Zafar A, Pong Ng H, Diamond-Zaluski R, Kim G-D, Ricky Chan E, Dunwoodie SL, Smith JD, & Mahabeleshwar GH (2021). CITED2 inhibits STAT1-IRF1 signaling and atherogenesis. The FASEB Journal, 35(9), e21833. 10.1096/fj.202100792R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoshida T, Sekine T, Aisaki K, Mikami T, Kanno J, & Okayasu I (2011). CITED2 is activated in ulcerative colitis and induces p53-dependent apoptosis in response to butyric acid. Journal of Gastroenterology, 46(3), 339–349. 10.1007/s00535-010-0355-9 [DOI] [PubMed] [Google Scholar]
- 75.Bhattacharya S, Michels CL, Leung M-K, Arany ZP, Kung AL, & Livingston DM (1999). Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes & Development, 13(1), 64–75. 10.1101/gad.13.1.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoon H, Lim J-H, Cho C-H, Huang LE, & Park J-W (2011). CITED2 controls the hypoxic signaling by snatching p300 from the two distinct activation domains of HIF-1α. Biochimica Et Biophysica Acta, 1813(12), 2008–2016. 10.1016/j.bbamcr.2011.08.018 [DOI] [PubMed] [Google Scholar]
- 77.Machado-Oliveira G, Guerreiro E, Matias AC, Facucho-Oliveira J, Pacheco-Leyva I, & Bragança J (2015). FBXL5 modulates HIF-1α transcriptional activity by degradation of CITED2. Archives of Biochemistry and Biophysics, 576, 61–72. 10.1016/j.abb.2015.04.012 [DOI] [PubMed] [Google Scholar]
- 78.Berlow RB, Dyson HJ, & Wright PE (2017). Hypersensitive termination of the hypoxic response by a disordered protein switch. Nature, 543(7645), Article 7645. 10.1038/nature21705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruiz-Ortiz I, & Sancho DD (2020). Competitive binding of HIF-1α and CITED2 to the TAZ1 domain of CBP from molecular simulations. Physical Chemistry Chemical Physics. 10.1039/D0CP00328J [DOI] [PubMed] [Google Scholar]
- 80.Perez-Garcia V, Fineberg E, Wilson R, Murray A, Mazzeo CI, Tudor C, Sienerth A, White JK, Tuck E, Ryder EJ, Gleeson D, Siragher E, Wardle-Jones H, Staudt N, Wali N, Collins J, Geyer S, Busch-Nentwich EM, Galli A, … Hemberger M (2018). Placentation defects are highly prevalent in embryonic lethal mouse mutants. Nature, 555(7697), 463–468. 10.1038/nature26002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, & Evans RM (1999). PPAR gamma is required for placental, cardiac, and adipose tissue development. Molecular Cell, 4(4), 585–595. [DOI] [PubMed] [Google Scholar]
- 82.Radford BN, Zhao X, Glazer T, Eaton M, Blackwell D, Mohammad S, Lo Vercio LD, Devine J, Shalom-Barak T, Hallgrimsson B, Cross JC, Sucov HM, Barak Y, Dean W, & Hemberger M (2023). Defects in placental syncytiotrophoblast cells are a common cause of developmental heart disease. Nature Communications, 14(1), 1174. 10.1038/s41467-023-36740-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shioda T, Fenner MH, & Isselbacher KJ (1996). Msg1, a novel melanocyte-specific gene, encodes a nuclear protein and is associated with pigmentation. Proceedings of the National Academy of Sciences of the United States of America, 93(22), 12298–12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yahata T, de Caestecker MP, Lechleider RJ, Andriole S, Roberts AB, Isselbacher KJ, & Shioda T (2000). The MSG1 non-DNA-binding transactivator binds to the p300/CBP coactivators, enhancing their functional link to the Smad transcription factors. The Journal of Biological Chemistry, 275(12), 8825–8834. 10.1074/jbc.275.12.8825 [DOI] [PubMed] [Google Scholar]
- 85.Öcal E, Alkan Akalın S, & Aşır F (2023). Role of cited-1 and caspase-6 expression in HELLP syndrome. European Review for Medical and Pharmacological Sciences, 27(7), 3082–3087. 10.26355/eurrev_202304_31942 [DOI] [PubMed] [Google Scholar]
- 86.Bragança J, Swingler T, Marques FIR, Jones T, Eloranta JJ, Hurst HC, Shioda T, & Bhattacharya S (2002). Human CREB-binding protein/p300-interacting transactivator with ED-rich tail (CITED) 4, a new member of the CITED family, functions as a co-activator for transcription factor AP-2. The Journal of Biological Chemistry, 277(10), 8559–8565. 10.1074/jbc.M110850200 [DOI] [PubMed] [Google Scholar]
- 87.Paauw ND, Lely AT, Joles JA, Franx A, Nikkels PG, Mokry M, & van Rijn BB (2018). H3K27 acetylation and gene expression analysis reveals differences in placental chromatin activity in fetal growth restriction. Clinical Epigenetics, 10, 85. 10.1186/s13148-018-0508-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martire S, Nguyen J, Sundaresan A, & Banaszynski LA (2020). Differential contribution of p300 and CBP to regulatory element acetylation in mESCs. BMC Molecular and Cell Biology, 21(1), 55. 10.1186/s12860-020-00296-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Freedman SJ, Sun Z-YJ, Kung AL, France DS, Wagner G, & Eck MJ (2003). Structural basis for negative regulation of hypoxia-inducible factor-1alpha by CITED2. Nature Structural & Molecular Biology, 10(7), 504–512. 10.1038/nsb936 [DOI] [PubMed] [Google Scholar]
- 90.Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, Bunn HF, & Livingston DM (1996). An essential role for p300/CBP in the cellular response to hypoxia. Proceedings of the National Academy of Sciences, 93(23), 12969–12973. 10.1073/pnas.93.23.12969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dames SA, Martinez-Yamout M, De Guzman RN, Dyson HJ, & Wright PE (2002). Structural basis for Hif-1 alpha /CBP recognition in the cellular hypoxic response. Proceedings of the National Academy of Sciences of the United States of America, 99(8), 5271–5276. 10.1073/pnas.082121399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grossman SR, Perez M, Kung AL, Joseph M, Mansur C, Xiao ZX, Kumar S, Howley PM, & Livingston DM (1998). P300/MDM2 complexes participate in MDM2-mediated p53 degradation. Molecular Cell, 2(4), 405–415. 10.1016/s1097-2765(00)80140-9 [DOI] [PubMed] [Google Scholar]
- 93.Krois AS, Ferreon JC, Martinez-Yamout MA, Dyson HJ, & Wright PE (2016). Recognition of the disordered p53 transactivation domain by the transcriptional adapter zinc finger domains of CREB-binding protein. Proceedings of the National Academy of Sciences, 113(13), E1853–E1862. 10.1073/pnas.1602487113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhong H, Voll RE, & Ghosh S (1998). Phosphorylation of NF-κB p65 by PKA Stimulates Transcriptional Activity by Promoting a Novel Bivalent Interaction with the Coactivator CBP/p300. Molecular Cell, 1(5), 661–671. 10.1016/S1097-2765(00)80066-0 [DOI] [PubMed] [Google Scholar]
- 95.Mukherjee SP, Behar M, Birnbaum HA, Hoffmann A, Wright PE, & Ghosh G (2013). Analysis of the RelA:CBP/p300 interaction reveals its involvement in NF-κB-driven transcription. PLoS Biology, 11(9), e1001647. 10.1371/journal.pbio.1001647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wojciak JM, Martinez-Yamout MA, Dyson HJ, & Wright PE (2009). Structural basis for recruitment of CBP/p300 coactivators by STAT1 and STAT2 transactivation domains. The EMBO Journal, 28(7), 948–958. 10.1038/emboj.2009.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhattacharya S, Eckner R, Grossman S, Oldread E, Arany Z, D’Andrea A, & Livingston DM (1996). Cooperation of Stat2 and p300/CBP in signalling induced by interferon-alpha. Nature, 383(6598), 344–347. 10.1038/383344a0 [DOI] [PubMed] [Google Scholar]
- 98.Berlow RB, Martinez-Yamout MA, Dyson HJ, & Wright PE (2019). Role of Backbone Dynamics in Modulating the Interactions of Disordered Ligands with the TAZ1 Domain of the CREB-Binding Protein. Biochemistry, 58(10), 1354–1362. 10.1021/acs.biochem.8b01290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Guzman RN, Martinez-Yamout MA, Dyson HJ, & Wright PE (2004). Interaction of the TAZ1 domain of the CREB-binding protein with the activation domain of CITED2: Regulation by competition between intrinsically unstructured ligands for non-identical binding sites. The Journal of Biological Chemistry, 279(4), 3042–3049. 10.1074/jbc.M310348200 [DOI] [PubMed] [Google Scholar]
- 100.Matt T, Martinez-Yamout MA, Dyson HJ, & Wright PE (2004). The CBP/p300 TAZ1 domain in its native state is not a binding partner of MDM2. Biochemical Journal, 381(Pt 3), 685–691. 10.1042/BJ20040564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chu W-T, Chu X, & Wang J (2020). Investigations of the underlying mechanisms of HIF-1α and CITED2 binding to TAZ1. Proceedings of the National Academy of Sciences, 117(11), 5595–5603. 10.1073/pnas.1915333117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hóbor F, Hegedüs Z, Ibarra AA, Petrovicz VL, Bartlett GJ, Sessions RB, Wilson AJ, & Edwards TA (n.d.). Understanding p300-transcription factor interactions using sequence variation and hybridization. RSC Chemical Biology, 3(5), 592–603. 10.1039/d2cb00026a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chou Y-T, Wang H, Chen Y, Danielpour D, & Yang Y-C (2006). Cited2 modulates TGF-beta-mediated upregulation of MMP9. Oncogene, 25(40), 5547–5560. 10.1038/sj.onc.1209552 [DOI] [PubMed] [Google Scholar]
- 104.Lau WM, Doucet M, Huang D, Weber KL, & Kominsky SL (2013). CITED2 Modulates Estrogen Receptor Transcriptional Activity in Breast Cancer Cells. Biochemical and Biophysical Research Communications, 437(2), 261–266. 10.1016/j.bbrc.2013.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chou Y-T, Hsieh C-H, Chiou S-H, Hsu C-F, Kao Y-R, Lee C-C, Chung C-H, Wang Y-H, Hsu H-S, Pang S-T, Shieh Y-S, & Wu C-W (2012). CITED2 functions as a molecular switch of cytokine-induced proliferation and quiescence. Cell Death and Differentiation, 19(12), 2015–2028. 10.1038/cdd.2012.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shin S-H, Lee GY, Lee M, Kang J, Shin H-W, Chun Y-S, & Park J-W (2018). Aberrant expression of CITED2 promotes prostate cancer metastasis by activating the nucleolin-AKT pathway. Nature Communications, 9(1), 4113. 10.1038/s41467-018-06606-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kuckenberg P, Kubaczka C, & Schorle H (2012). The role of transcription factor Tcfap2c/TFAP2C in trophectoderm development. Reproductive Biomedicine Online, 25(1), 12–20. 10.1016/j.rbmo.2012.02.015 [DOI] [PubMed] [Google Scholar]
- 108.Cao Z, Carey TS, Ganguly A, Wilson CA, Paul S, & Knott JG (2015). Transcription factor AP-2γ induces early Cdx2 expression and represses HIPPO signaling to specify the trophectoderm lineage. Development (Cambridge, England), 142(9), 1606–1615. 10.1242/dev.120238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Benchetrit H, Herman S, van Wietmarschen N, Wu T, Makedonski K, Maoz N, Yom Tov N, Stave D, Lasry R, Zayat V, Xiao A, Lansdorp PM, Sebban S, & Buganim Y (2015). Extensive Nuclear Reprogramming Underlies Lineage Conversion into Functional Trophoblast Stem-like Cells. Cell Stem Cell, 17(5), 543–556. 10.1016/j.stem.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 110.Kubaczka C, Senner CE, Cierlitza M, Araúzo-Bravo MJ, Kuckenberg P, Peitz M, Hemberger M, & Schorle H (2015). Direct Induction of Trophoblast Stem Cells from Murine Fibroblasts. Cell Stem Cell, 17(5), 557–568. 10.1016/j.stem.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 111.Kim M, Adu-Gyamfi EA, Kim J, & Lee B-K (2023). Super-enhancer-associated transcription factors collaboratively regulate trophoblast-active gene expression programs in human trophoblast stem cells. Nucleic Acids Research, 51(8), 3806–3819. 10.1093/nar/gkad215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen Y, Siriwardena D, Penfold C, Pavlinek A, & Boroviak TE (2022). An integrated atlas of human placental development delineates essential regulators of trophoblast stem cells. Development (Cambridge, England), 149(13), dev200171. 10.1242/dev.200171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Krendl C, Shaposhnikov D, Rishko V, Ori C, Ziegenhain C, Sass S, Simon L, Müller NS, Straub T, Brooks KE, Chavez SL, Enard W, Theis FJ, & Drukker M (2017). GATA2/3-TFAP2A/C transcription factor network couples human pluripotent stem cell differentiation to trophectoderm with repression of pluripotency. Proceedings of the National Academy of Sciences, 114(45), E9579–E9588. 10.1073/pnas.1708341114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Varberg KM, Dominguez EM, Koseva B, Varberg JM, McNally RP, Moreno-Irusta A, Wesley ER, Iqbal K, Cheung WA, Schwendinger-Schreck C, Smail C, Okae H, Arima T, Lydic M, Holoch K, Marsh C, Soares MJ, & Grundberg E (2023). Extravillous trophoblast cell lineage development is associated with active remodeling of the chromatin landscape. Nature Communications, 14(1), 4826. 10.1038/s41467-023-40424-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Auman HJ, Nottoli T, Lakiza O, Winger Q, Donaldson S, & Williams T (2002). Transcription factor AP-2gamma is essential in the extra-embryonic lineages for early postimplantation development. Development (Cambridge, England), 129(11), 2733–2747. 10.1242/dev.129.11.2733 [DOI] [PubMed] [Google Scholar]
- 116.Werling U, & Schorle H (2002). Transcription factor gene AP-2gamma essential for early murine development. Molecular and Cellular Biology, 22(9), 3149–3156. 10.1128/MCB.22.9.3149-3156.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sharma N, Kubaczka C, Kaiser S, Nettersheim D, Mughal SS, Riesenberg S, Hölzel M, Winterhager E, & Schorle H (2016). Tpbpa-Cre-mediated deletion of TFAP2C leads to deregulation of Cdkn1a, Akt1 and the ERK pathway, causing placental growth arrest. Development (Cambridge, England), 143(5), 787–798. 10.1242/dev.128553 [DOI] [PubMed] [Google Scholar]
- 118.Kidder BL, & Palmer S (2010). Examination of transcriptional networks reveals an important role for TCFAP2C, SMARCA4, and EOMES in trophoblast stem cell maintenance. Genome Research, 20(4), 458–472. 10.1101/gr.101469.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Scott RL, Vu HTH, Jain A, Iqbal K, Tuteja G, & Soares MJ (2022). Conservation at the uterine-placental interface. Proceedings of the National Academy of Sciences of the United States of America, 119(41), e2210633119. 10.1073/pnas.2210633119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Latos PA, Sienerth AR, Murray A, Senner CE, Muto M, Ikawa M, Oxley D, Burge S, Cox BJ, & Hemberger M (2015). Elf5-centered transcription factor hub controls trophoblast stem cell self-renewal and differentiation through stoichiometry-sensitive shifts in target gene networks. Genes & Development, 29(23), 2435–2448. 10.1101/gad.268821.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Q, Pan H, Guan L, Su D, & Ma X (2012). CITED2 mutation links congenital heart defects to dysregulation of the cardiac gene VEGF and PITX2C expression. Biochemical and Biophysical Research Communications, 423(4), 895–899. 10.1016/j.bbrc.2012.06.099 [DOI] [PubMed] [Google Scholar]
- 122.Vu HTH, Scott RL, Iqbal K, Soares MJ, & Tuteja G (2023). Core conserved transcriptional regulatory networks define the invasive trophoblast cell lineage. Development (Cambridge, England), 150(15), dev201826. 10.1242/dev.201826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee C-H, & Evans RM (2002). Peroxisome proliferator-activated receptor-gamma in macrophage lipid homeostasis. Trends in Endocrinology and Metabolism: TEM, 13(8), 331–335. 10.1016/s1043-2760(02)00668-9 [DOI] [PubMed] [Google Scholar]
- 124.Semple RK, Chatterjee VKK, & O’Rahilly S (2006). PPAR gamma and human metabolic disease. The Journal of Clinical Investigation, 116(3), 581–589. 10.1172/JCI28003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barak Y, Sadovsky Y, & Shalom-Barak T (2007). PPAR signaling in placental development and function. PPAR Research, 2008, e142082. 10.1155/2008/142082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fournier T, Tsatsaris V, Handschuh K, & Evain-Brion D (2007). PPARs and the Placenta. Placenta, 28(2), 65–76. 10.1016/j.placenta.2006.04.009 [DOI] [PubMed] [Google Scholar]
- 127.Fournier T, Therond P, Handschuh K, Tsatsaris V, & Evain-Brion D (2008). PPARgamma and early human placental development. Current Medicinal Chemistry, 15(28), 3011–3024. 10.2174/092986708786848677 [DOI] [PubMed] [Google Scholar]
- 128.Nadra K, Quignodon L, Sardella C, Joye E, Mucciolo A, Chrast R, & Desvergne B (2010). PPARgamma in placental angiogenesis. Endocrinology, 151(10), 4969–4981. 10.1210/en.2010-0131 [DOI] [PubMed] [Google Scholar]
- 129.Gonzalez YR, Zhang Y, Behzadpoor D, Cregan S, Bamforth S, Slack RS, & Park DS (2008). CITED2 signals through peroxisome proliferator-activated receptor-gamma to regulate death of cortical neurons after DNA damage. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(21), 5559–5569. 10.1523/JNEUROSCI.1014-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Semenza GL (2012). Hypoxia-inducible factors in physiology and medicine. Cell, 148(3), 399–408. 10.1016/j.cell.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ke Q, & Costa M (2006). Hypoxia-inducible factor-1 (HIF-1). Molecular Pharmacology, 70(5), 1469–1480. 10.1124/mol.106.027029 [DOI] [PubMed] [Google Scholar]
- 132.Ziello JE, Jovin IS, & Huang Y (2007). Hypoxia-Inducible Factor (HIF)-1 Regulatory Pathway and its Potential for Therapeutic Intervention in Malignancy and Ischemia. The Yale Journal of Biology and Medicine, 80(2), 51. [PMC free article] [PubMed] [Google Scholar]
- 133.Cowden Dahl KD, Fryer BH, Mack FA, Compernolle V, Maltepe E, Adelman DM, Carmeliet P, & Simon MC (2005). Hypoxia-inducible factors 1alpha and 2alpha regulate trophoblast differentiation. Molecular and Cellular Biology, 25(23), 10479–10491. 10.1128/MCB.25.23.10479-10491.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fryer BH, & Simon MC (2006). Hypoxia, HIF and the placenta. Cell Cycle (Georgetown, Tex.), 5(5), 495–498. 10.4161/cc.5.5.2497 [DOI] [PubMed] [Google Scholar]
- 135.Galbiati S, Inversetti A, Causarano V, Stenirri S, Soriani N, Ambrosi A, Valsecchi L, Candiani M, Cremonesi L, Ferrari M, & Smid M (2015). HIF1A and MIF as potential predictive mRNA biomarkers of pre-eclampsia: A longitudinal prospective study in high risk population. Clinical Chemistry and Laboratory Medicine (CCLM), 53(9), 1339–1347. 10.1515/cclm-2014-0745 [DOI] [PubMed] [Google Scholar]
- 136.Robb KP, Cotechini T, Allaire C, Sperou A, & Graham CH (2017). Inflammation-induced fetal growth restriction in rats is associated with increased placental HIF-1α accumulation. PLOS ONE, 12(4), e0175805. 10.1371/journal.pone.0175805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shenoy V, Kanasaki K, & Kalluri R (2010). Pre-eclampsia: Connecting angiogenic and metabolic pathways. Trends in Endocrinology and Metabolism: TEM, 21(9), 529–536. 10.1016/j.tem.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 138.Zamudio S, Wu Y, Ietta F, Rolfo A, Cross A, Wheeler T, Post M, Illsley NP, & Caniggia I (2007). Human placental hypoxia-inducible factor-1alpha expression correlates with clinical outcomes in chronic hypoxia in vivo. The American Journal of Pathology, 170(6), 2171–2179. 10.2353/ajpath.2007.061185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rajakumar A, Brandon HM, Daftary A, Ness R, & Conrad KP (2004). Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta, 25(10), 763–769. 10.1016/j.placenta.2004.02.011 [DOI] [PubMed] [Google Scholar]
- 140.Chakraborty D, Rumi MAK, & Soares MJ (2012). NK cells, hypoxia and trophoblast cell differentiation. Cell Cycle (Georgetown, Tex.), 11(13), 2427–2430. 10.4161/cc.20542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chakraborty D, Cui W, Rosario GX, Scott RL, Dhakal P, Renaud SJ, Tachibana M, Rumi MAK, Mason CW, Krieg AJ, & Soares MJ (2016). HIF-KDM3A-MMP12 regulatory circuit ensures trophoblast plasticity and placental adaptations to hypoxia. Proceedings of the National Academy of Sciences of the United States of America, 113(46), E7212–E7221. 10.1073/pnas.1612626113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, & Suuronen T (2008). Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Research Reviews, 7(2), 83–105. 10.1016/j.arr.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 143.Baltimore D. (2009). Discovering NF-κB. Cold Spring Harbor Perspectives in Biology, 1(1), a000026. 10.1101/cshperspect.a000026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Albensi BC, & Mattson MP (2000). Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse (New York, N.Y.), 35(2), 151–159. [DOI] [PubMed] [Google Scholar]
- 145.Kaltschmidt B, & Kaltschmidt C (2009). NF-κB in the Nervous System. Cold Spring Harbor Perspectives in Biology, 1(3), a001271. 10.1101/cshperspect.a001271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Karin M. (2009). NF-κB as a Critical Link Between Inflammation and Cancer. Cold Spring Harbor Perspectives in Biology, 1(5), a000141. 10.1101/cshperspect.a000141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Botelho RM, Tenorio LPG, Silva ALM, Tanabe ELL, Pires KSN, Gonçalves CM, Santos JC, Marques ALX, Allard MJ, Bergeron JD, Sebire G, Silva ECO, Souza ST, Fonseca EJS, Borbely AU, & Borbely KSC (2019). Biomechanical and functional properties of trophoblast cells exposed to Group B Streptococcus in vitro and the beneficial effects of uvaol treatment. Biochimica et Biophysica Acta (BBA) - General Subjects, 1863(9), 1417–1428. 10.1016/j.bbagen.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 148.Liu Y, Yang J, Bao J, Li X, Ye A, Zhang G, & Liu H (2017). Activation of the cholinergic anti-inflammatory pathway by nicotine ameliorates lipopolysaccharide-induced preeclampsia-like symptoms in pregnant rats. Placenta, 49, 23–32. 10.1016/j.placenta.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 149.Tilburgs T, Meissner TB, Ferreira LMR, Mulder A, Musunuru K, Ye J, & Strominger JL (2017). NLRP2 is a suppressor of NF-ƙB signaling and HLA-C expression in human trophoblasts†,‡. Biology of Reproduction, 96(4), 831–842. 10.1093/biolre/iox009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kadam L, Kilburn B, Baczyk D, Kohan-Ghadr HR, Kingdom J, & Drewlo S (2019). Rosiglitazone blocks first trimester in-vitro placental injury caused by NF-κB-mediated inflammation. Scientific Reports, 9(1), 2018. 10.1038/s41598-018-38336-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xue P, Zheng M, Gong P, Lin C, Zhou J, Li Y, Shen L, Diao Z, Yan G, Sun H, & Hu Y (2015). Single administration of ultra-low-dose lipopolysaccharide in rat early pregnancy induces TLR4 activation in the placenta contributing to preeclampsia. PloS One, 10(4), e0124001. 10.1371/journal.pone.0124001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lou X, Sun S, Chen W, Zhou Y, Huang Y, Liu X, Shan Y, & Wang C (2011). Negative feedback regulation of NF-κB action by CITED2 in the nucleus. The Journal of Immunology, 186(1), 539–548. 10.4049/jimmunol.1001650 [DOI] [PubMed] [Google Scholar]
- 153.Anamthathmakula P, Shallie PD, Nayak N, Dhal S, Vivian JL, Mor G, Soares MJ, & Nayak NR (2023). Variable Cre Recombination Efficiency in Placentas of Cyp19-Cre ROSAmT/mG Transgenic Mice. Cells, 12(16), 2096. 10.3390/cells12162096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Iqbal K, Dominguez EM, Nixon B, Moreno-Irusta A, Crnkovich B, Scott RL, Vu HTH, Tuteja G, Vivian JL, & Soares MJ (2024). Conditionally mutant animal model for investigating the invasive trophoblast cell lineage. Development (Cambridge, England), 151(2), dev202239. 10.1242/dev.202239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Imakawa K, Dhakal P, Kubota K, Kusama K, Chakraborty D, Karim Rumi MA, & Soares MJ (2016). CITED2 modulation of trophoblast cell differentiation: Insights from global transcriptome analysis. Reproduction (Cambridge, England), 151(5), 509–516. 10.1530/REP-15-0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sakai M, Tujimura-Hayakawa T, Yagi T, Yano H, Mitsushima M, Unoki-Kubota H, Kaburagi Y, Inoue H, Kido Y, Kasuga M, & Matsumoto M (2016). The GCN5-CITED2-PKA signalling module controls hepatic glucose metabolism through a cAMP-induced substrate switch. Nature Communications, 7, 13147. 10.1038/ncomms13147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang X, Lockhart SM, Rathjen T, Albadawi H, Sørensen D, O’Neill BT, Dwivedi N, Preil SR, Beck HC, Dunwoodie SL, Watkins MT, Rasmussen LM, & Rask-Madsen C (2016). Insulin Downregulates the Transcriptional Coregulator CITED2, an Inhibitor of Proangiogenic Function in Endothelial Cells. Diabetes, 65(12), 3680–3690. 10.2337/db16-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kunkemoeller B, Chen K, Lockhart SM, Wang X, & Rask-Madsen C (2021). The transcriptional coregulator CITED2 suppresses expression of IRS-2 and impairs insulin signaling in endothelial cells. American Journal of Physiology. Endocrinology and Metabolism, 321(2), E252–E259. 10.1152/ajpendo.00435.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xiao S, Zhang D, Liu Z, Jin W, Huang G, Wei Z, Wang D, & Deng C (2020). Diabetes-induced glucolipotoxicity impairs wound healing ability of adipose-derived stem cells-through the miR-1248/CITED2/HIF-1α pathway. Aging, 12(8), 6947–6965. 10.18632/aging.103053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Valenzuela-Melgarejo FJ, Lagunas C, Carmona-Pastén F, Jara-Medina K, & Delgado G (2021). Supraphysiological Role of Melatonin Over Vascular Dysfunction of Pregnancy, a New Therapeutic Agent? Frontiers in Physiology, 12, 767684. 10.3389/fphys.2021.767684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fang Y, Shang W, Wei D-L, & Zeng S-M (2016). Cited2 protein level in cumulus cells is a biomarker for human embryo quality and pregnancy outcome in one in vitro fertilization cycle. Fertility and Sterility, 105(5), 1351–1359.e4. 10.1016/j.fertnstert.2015.12.137 [DOI] [PubMed] [Google Scholar]
- 162.MacDonald ST, Bamforth SD, Bragança J, Chen C-M, Broadbent C, Schneider JE, Schwartz RJ, & Bhattacharya S (2013). A cell-autonomous role of Cited2 in controlling myocardial and coronary vascular development. European Heart Journal, 34(32), 2557–2565. 10.1093/eurheartj/ehs056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu B, Qu X, Gu S, Doughman Y-Q, Watanabe M, Dunwoodie SL, & Yang Y-C (2008). Cited2 is required for fetal lung maturation. Developmental Biology, 317(1), 95–105. 10.1016/j.ydbio.2008.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jank M, Schwartz J, Miyake Y, Ozturk Aptekmann A, Patel D, Boettcher M, & Keijzer R (2024). Dysregulation of CITED2 in abnormal lung development in the nitrofen rat model. Pediatric Surgery International, 40(1), 43. 10.1007/s00383-023-05607-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.