Background:
When taken together, opioids and benzodiazepines can result in synergistic respiratory depression, which increases overdose risk (1). The percentage of all opioid overdose deaths involving benzodiazepines increased from 8.7% in 1999 to 21% in 2017 (1). Benzodiazepines were involved in 1-in-3 prescription opioid overdose deaths in 2017 (1). The fraction of patients receiving concurrent opioids and benzodiazepines increased 41% from 2002 to 2014 in the United States (2).
Objective:
This study aims at examining trends in patients receiving concurrent opioid and benzodiazepine prescriptions from 2016 to 2019 at national and state levels.
Methods and Findings:
Opioid and benzodiazepine prescriptions dispensed to adults aged 18 years and older were obtained from the all-payer IQVIA Longitudinal Prescription (LRx) database. The LRx contains prescription records from a sample of approximately 49 900 retail pharmacies that dispense nearly 92% of retail pharmacy prescriptions in the United States. Dispensing date and days’ supply of a patient’s prescriptions were used to estimate whether the patient had concurrent prescriptions. Trends of concurrent prescriptions were analyzed by patient demographics, geographic location, prescriber source (concurrent prescriptions from same or different clinician), and new to concurrent prescriptions (patients without concurrent prescriptions in the prior year). To compare trends across states, the percentage of patients with concurrent prescriptions for each state was estimated and age adjusted as annual total patients with concurrent prescriptions divided by annual total patients receiving an opioid in the state. The relative change of the percentage between 2019 and 2016 was also estimated.
The numbers of patients with opioid, benzodiazepine, or concurrent prescriptions all declined from 2016 to 2019, with relative changes of 22.5%, 13.5%, and 41.8%, respectively (Table); patients with concurrent prescriptions declined across all subgroups examined and especially among younger adults (Table).
Table.
Numbers and Characteristics of Patients With Opioid, Benzodiazepine, or Concurrent Prescriptions, From 2016 to 2019*
| Patient Characteristics | 2016, n (%) | 2017, n (%) | 2018, n (%) | 2019, n (%) | Relative Change: 2019 Versus 2016, % |
|---|---|---|---|---|---|
|
| |||||
| Patients with ≥1 opioid † | 47 688 914 | 44 680 444 | 40 129 333 | 36 969 632 | −22.5 |
| Among these, patients new to opioid | 25 348 761 (53.2) | 23 779 810 (53.2) | 19 764 007 (49.3) | 17 335 105 (46.9) | −31.6 |
| Patients with ≥1 benzodiazepine ‡ | 21 473 507 | 20 778 972 | 19 573 156 | 18 583 244 | −13.5 |
| Among these, patients new to benzodiazepine | 8 792 232 (40.9) | 8 292 278 (39.9) | 7 098 082 (36.3) | 6 395 014 (34.4) | −27.3 |
| Patients with concurrent prescriptions § | 5 286 603 | 4 681 001 | 3 804 308 | 3 077 293 | −41.8 |
| Characteristics of patients with concurrent prescriptions | |||||
| Sex | |||||
| Female | 3 521 133 (66.6) | 3 128 262 (66.8) | 2 557 896 (67.2) | 2 074 132 (67.4) | −41.1 |
| Male | 1 765 470 (33.4) | 1 552 739 (33.2) | 1 246 412 (32.8) | 1 003 161 (32.6) | −43.2 |
| Age group, y | |||||
| 18–34 | 406 969 (7.7) | 314 793 (6.7) | 207 495 (5.5) | 145 571 (4.7) | −64.2 |
| 35–49 | 1 166 840 (22.1) | 980 671 (21.0) | 745 062 (19.6) | 563 042 (18.3) | −51.7 |
| 50–64 | 2 040 927 (38.6) | 1 807 384 (38.6) | 1 468 043 (38.6) | 1 171 857 (38.1) | −42.6 |
| ≥65 | 1 671 867 (31.6) | 1 578 153 (33.7) | 1 383 708 (36.4) | 1 196 823 (38.9) | −28.4 |
| Urban/rural status∥ | |||||
| Metropolitan | 4 487 081 (84.9) | 3 970 677 (84.8) | 3 236 424 (85.1) | 2 625 216 (85.3) | −41.5 |
| Micropolitan | 496 038 (9.4) | 440 191 (9.4) | 353 575 (9.3) | 282 791 (9.2) | −43.0 |
| Noncore | 474 541 (5.7) | 270 133 (5.8) | 214 309 (5.6) | 169 286 (5.5) | −44.2 |
| Patient’s concurrent prescriptions from same prescriber | 3 533 770 (66.8) | 3 055 977 (65.3) | 2 430 120 (63.9) | 1 918 857 (62.4) | −45.7 |
| New to concurrent prescriptions | 2 138 439 (40.5) | 1 787 677 (38.2) | 1 171 458 (30.8) | 876 122 (28.5) | −59.0 |
Source: Our analysis of the all-payer IQVIA Longitudinal Prescription (LRx) database 2015 to 2019. The 2015 data were only used for identifying patients new to concurrent prescriptions in 2016. Prescription information from the LRx data used for this analysis included dispensing date, days’ supply, National Drug Code, patient identifier (ID), patient demographics, prescriber ID (unique ID created by IQVIA based on information on the prescription), and prescriber practice location (state and county). Only prescriber practice location had 0.6% missing values in the 2015 to 2019 data, and these records were excluded from the analysis.
Opioid products included in the analysis: codeine, fentanyl, hydrocodone, hydromorphone, methadone, morphine, oxycodone, oxymorphone, propoxyphene, tapentadol, tramadol, and buprenorphine (only buprenorphine products indicated for pain). Opioid products excluded from the analysis were buprenorphine formulations indicated for treating opioid use disorder and opioid formulations indicated for cold and cough.
Benzodiazepine products included in the analysis: alprazolam, chlordiazepoxide, clobazam, clonazepam, clorazepate, diazepam, estazolam, flurazepam, lorazepam, oxazepam, quazepam, temazepam, and triazolam.
Dispensing date and days’ supply on prescriptions were used for calculating days of concurrent use for a patient.
Only prescriber practice location (state and county) is available in the data, so prescriber’s state and county were used to obtain geographic location of prescriptions.
The percentage of patients receiving concurrent prescriptions at the state level ranged from 6.8% in Washington, DC to 15.2% in Arkansas in 2016. A decline in this percentage was observed across all states. The relative change in 2019 versus 2016 ranged from −52.0% in Mississippi to −6.9% in New Hampshire (Figure).
Figure. Age-adjusted percentage of patients with concurrent opioid and benzodiazepine prescriptions in 2016 and relative reduction in the percentage in 2019 versus 2016 by state.
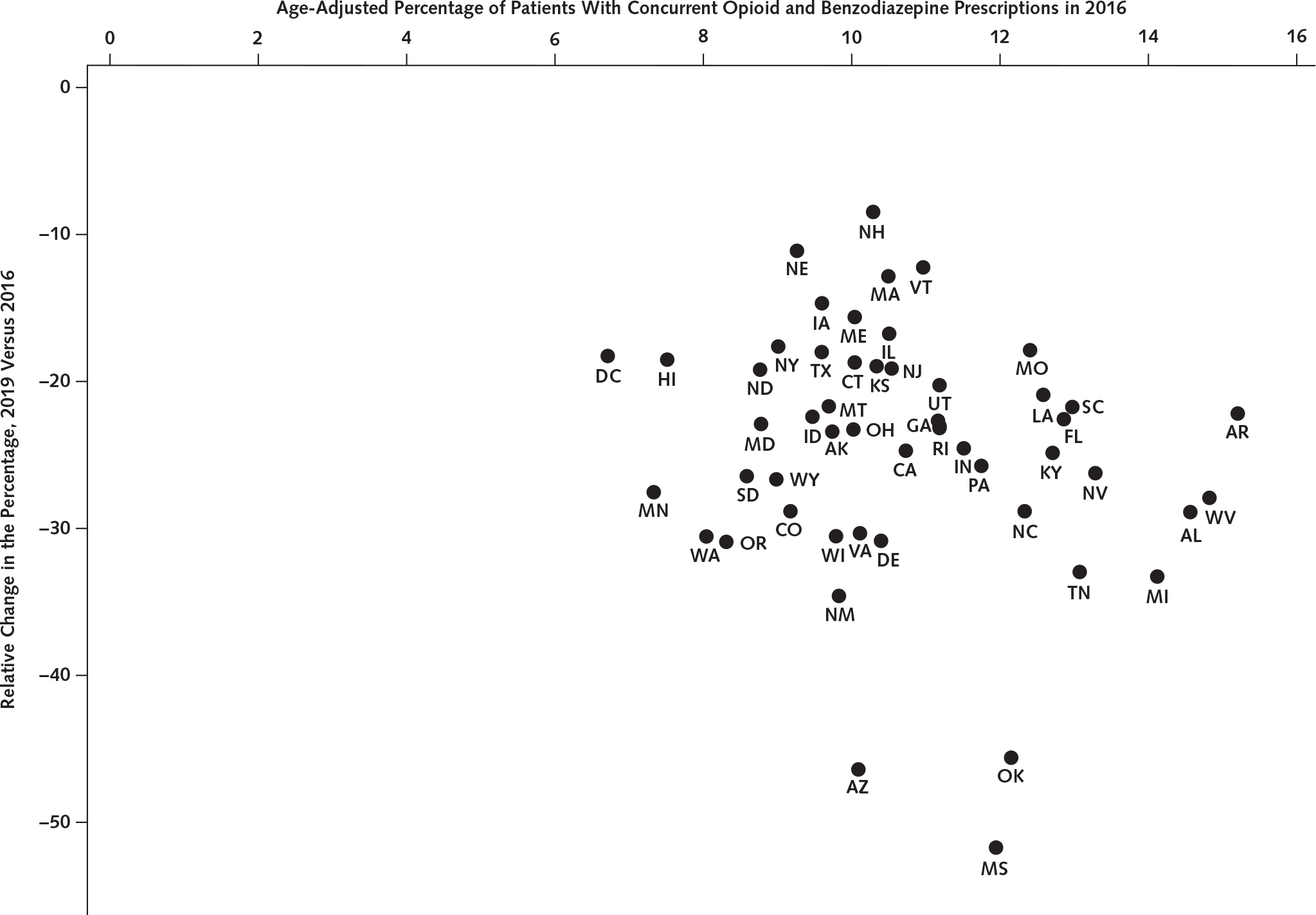
Source: Our analysis of the IQVIA Longitudinal Prescription (LRx) database 2016 to 2019. Geographic location of prescriptions was based on prescriber practice location (state). The percentage of patients with concurrent prescriptions for each state was estimated as annual total patients with concurrent prescriptions divided by annual total patients receiving an opioid in the state. The age adjustment was done based on the age group distribution of the denominator (number of patients receiving an opioid) for each state. AK = Alaska; AL = Alabama; AR = Arkansas; AZ = Arizona; CA = California; CO = Colorado; CT = Connecticut; DC = District of Columbia; DE = Delaware; FL = Florida; GA = Georgia; HI = Hawai’i; IA = Iowa; ID = Idaho; IL = Illinois; IN = Indiana; KS = Kansas; KY = Kentucky; LA = Louisiana; MA = Massachusetts; MD = Maryland; ME = Maine; MI = Michigan; MN = Minnesota; MO = Missouri; MS = Mississippi; MT = Montana; NC = North Carolina; ND = North Dakota; NE = Nebraska; NH = New Hampshire; NJ = New Jersey; NM = New Mexico; NV = Nevada; NY = New York; OH = Ohio; OK = Oklahoma; OR = Oregon; PA = Pennsylvania; RI = Rhode Island; SC = South Carolina; SD = South Dakota; TN = Tennessee; TX = Texas; UT = Utah; VA = Virginia; VT = Vermont; WA = Washington; WI = Wisconsin; WV = West Virginia; WY = Wyoming.
Discussion:
Using national all-payer retail pharmacy dispensing records, we found that total patients receiving concurrent opioids and benzodiazepines declined substantially between 2016 and 2019 in the United States especially among younger adults. The period examined was after federal efforts to address opioid and benzodiazepine coprescribing, including the 2016 U.S. Food and Drug Administration (FDA) boxed warning and the Centers for Disease Control and Prevention (CDC) guideline for prescribing opioids for chronic pain (3, 4). Prior studies examining the effect of these efforts found a reduction in concurrent opioid and benzodiazepine prescriptions (3, 5).
The variation in the amount of decline across states was likely due to different levels of intervention efforts and/or policies at the state or health-system level. Future research should examine the causes of the state-level variation observed by this study. The number of patients new to concurrent prescriptions declined 59% from 2016 to 2019 and only accounted for 28.5% of total patients with concurrent prescriptions in 2019, indicating that much fewer patients initiated with concurrent prescriptions. Research has shown that clinicians may have better adherence to clinical guidelines when treating patients new to 1 of 2 or both drugs than when modifying previous regimens (3).
The limitations of this study include lack of data on: prescriptions dispensed outside of retail pharmacies (for example, hospital pharmacies), diagnosis for the prescription (for example, cannot exclude patients with cancer or those receiving palliative care), and actual prescription consumption. Also, despite extensive coverage, LRx data are unweighted and are not nationally representative.
Despite the limitations, this study provides updated estimates of the number of patients receiving concurrent opioids and benzodiazepines and, to our knowledge, is the first to use national all-payer data to examine state-level variation. Receiving concurrent opioids and benzodiazepines remains common, despite the known risks. These findings highlight the need for continued public health and clinical actions, including greater adherence to evidence-based prescribing guidelines, more patient education, and alternative pain-management options; in addition, data from prescription drug monitoring programs should be checked for medication records. The findings also highlight the need for evidence-based protocols to safely deprescribe opioids and/or benzodiazepines for patients already exposed. Together, these efforts could further improve the safe use of opioid and benzodiazepine prescriptions.
Financial support:
By the CDC via employment of Drs. Zhang, Strahan, and Guy and intergovernmental personnel agreement (IPA) no. 20IPA2009424 with Dr. Larochelle.
Footnotes
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M21-4656.
Reproducible Research Statement: Study protocol: Not available. Statistical code: Available upon request from Dr. Zhang (e-mail, kzhang@cdc.gov). Data set: IQVIA LRx data used in the analysis are proprietary and cannot be shared under the data use agreement. The data can be purchased from IQVIA.
Note: Dr. Zhang had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Contributor Information
Kun Zhang, National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, Atlanta, Georgia.
Andrea E. Strahan, National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, Atlanta, Georgia.
Gery P. Guy, National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, Atlanta, Georgia.
Marc R. Larochelle, Clinical Addiction Research and Education Unit, Section of General Internal Medicine, Department of Medicine, Boston University School of Medicine, Boston Medical Center, Boston, Massachusetts.
References
- 1.Tori ME, Larochelle MR, Naimi TS. Alcohol or benzodiazepine co-involvement with opioid overdose deaths in the United States, 1999–2017. JAMA Netw Open. 2020;3:e202361. doi: 10.1001/jamanetworkopen.2020.2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang CS, Kang EM, Kornegay CJ, et al. Trends in the concomitant prescribing of opioids and benzodiazepines, 2002–2014. Am J Prev Med. 2016;51:151–160. doi: 10.1016/j.amepre.2016.02.014 [DOI] [PubMed] [Google Scholar]
- 3.Jeffery MM, Hooten WM, Jena AB, et al. Rates of physician coprescribing of opioids and benzodiazepines after the release of the Centers for Disease Control and Prevention guidelines in 2016. JAMA Netw Open. 2019;2:e198325. doi: 10.1001/jamanetworkopen.2019.8325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624–45. doi: 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohnert ASB, Guy GP Jr, Losby JL. Opioid prescribing in the United States before and after the Centers for Disease Control and Prevention’s 2016 opioid guideline. Ann Intern Med. 2018;169:367–375. doi: 10.7326/M18-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]


