Abstract
We report the synthesis of block copolymers of mono-methoxylated polyethylene glycol and poly(glycerol carbonate) (mPEG-b-PGC) via the ring-opening polymerization of benzyl glycidyl ether, mono-methoxylated polyethylene glycol, and carbon dioxide using a cobalt salen catalyst. The resulting block copolymers display high polymer/cyclic carbonate selectivity (>99%) and, if two oxirane monomers are used, random incorporation into the polymer feed. The resulting di-block mPEG-b-PGC polymer shows promise as a nanocarrier for surfactant free, sustained chemotherapeutic delivery. mPEG-b-PGC, with paclitaxel conjugated to the pendant primary alcohol of the glycerol polymer backbone, readily forms 175 nm diameter particles in solution and contains 4.6 wt% PTX which is released over 42 days. The mPEG-b-PGC polymer itself is non-cytotoxic, whereas the PTX-loaded nanoparticles are cytotoxic to lung, breast, and ovarian cancer cell lines.
Graphical Abstract
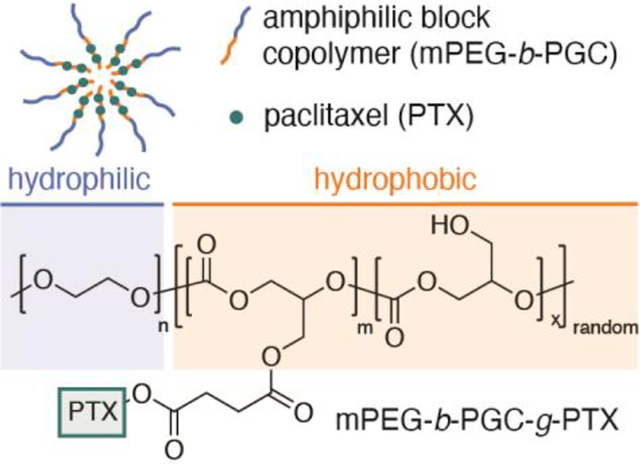
Block copolymers are versatile materials across industry sectors.1, 2 In the pharmaceutical arena, amphiphilic block copolymers readily form polymeric micelles in aqueous solution and enable encapsulation of hydrophobic agents to increase their aqueous solubility.3, 4 A classic example of a block copolymer is monomethoxy-poly(ethylene glycol)-co-poly(lactide) (mPEG-PLL), first reported in 1988.5 mPEG-PLL encapsulates a large number of agents6–12 including paclitaxel (PTX),13–15 a microtubule stabilizing agent used clinically for the treatment of breast, lung, and ovarian cancers.16 The poor aqueous solubility of PTX necessitates the use of such carriers or solubilizing agents like Cremophor EL™, which can lead to complications in patients such as anaphylaxis hypersensitivity.17, 18 From a polymer perspective, the hydrophilic and hydrophobic domains of the polymeric micelle are highly tunable, enabling optimization of the chemical, physical, and pharmacological properties. Genexol®PM represents an important milestone achievement in amphiphilic block copolymers, and is a clinically used PTX-loaded monomethoxy-poly(ethylene glycol)-block-poly(D,L-lactide) micelle.19 Compared to conventional PTX/Cremophor EL, Genexol®PM, with encapsulated PTX, exhibits reduced toxicity and a higher maximum tolerated dose (390 mg/m2 vs 175 mg/m2) in patients.20
Three shortcomings of drug-loaded polymeric micelles include: 1) poor encapsulation yields affording low drug weight percent from 1–3%; 2) burst release of the encapsulated drug (> 60% within 24 hours); and 3) the use of polyesters as the hydrophobic segment, which degrades into acidic byproducts.21 We envision that a polymeric micelle composed of PTX conjugated to a non-polyester, but degradable, block copolymer will address some of these issues and be an attractive carrier system for further investigation. Glycerol-based polymers are finding ever increasing applications in medicine.22–29 We selected poly(1,2-glycerol carbonate) (PGC) for the hydrophobic domain of the block copolymer, as it degrades into the natural metabolites of glycerol and carbon dioxide30 and possesses a pendant-chain free primary hydroxyl group for facile conjugation to PTX. Drawing inspiration from the success of mPEG-PLL nanocarriers, the incorporation of mPEG as the hydrophilic segment will allow for the formation of nanoparticles that are stable in aqueous solution without the use of surfactant (Figure 1).
Figure 1.
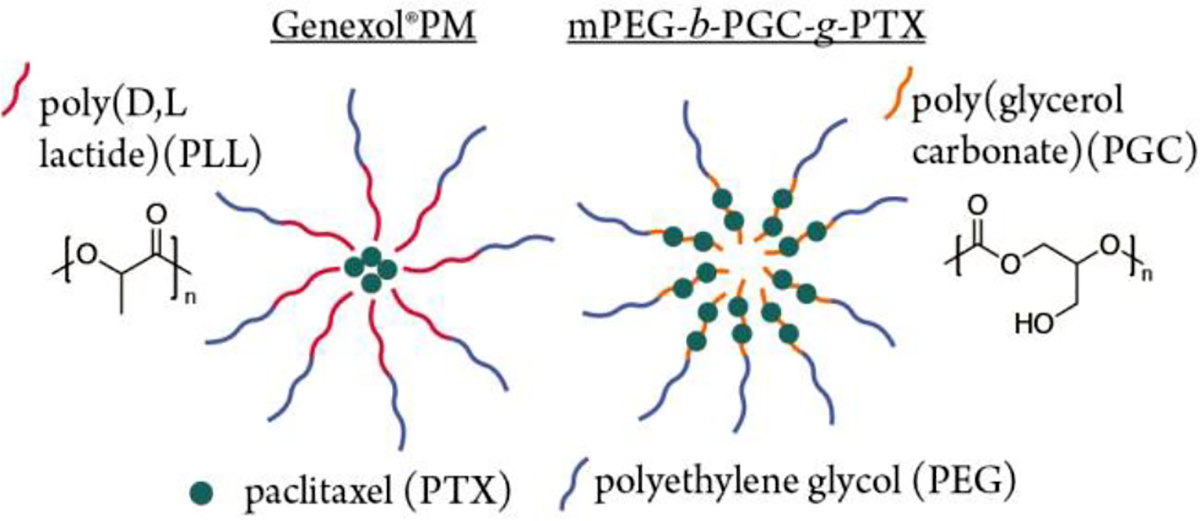
Cartoon of Genexol®PM and mPEG-b-PGC-g-PTX nanoparticle assemblies.
Herein we report the synthesis of PGC block copolymers using mPEG, monohydroxyl terminated polystyrene, or benzyl alcohol as the chain transfer agent via a cobalt salen catalyzed copolymerization with benzyl glycidyl ether (BGE) and CO2 with high turnover numbers (> 500) and polymer selectivity (99%). The mPEG-PGC-OH polymers, grafted with PTX via succinate linker (mPEG-b-PGC-g-PTX) form 175 nm diameter micelles in aqueous solution. Lastly, mPEG-b-PGC-g-PTX micelles release paclitaxel over 42 days and are cytotoxic to lung, breast, and ovarian cancer cell lines.
Specifically, we synthesized amphiphilic mPEG-b-PGC copolymers via the cobalt catalyzed ring opening polymerization of benzyl glycidyl ether (BGE) and mono-methoxylated PEG (mPEG), the chain transfer agent, at 50°C and 220 psi CO2 (Scheme 1). The thermally stable cobalt-based catalyst, SalcyCoIIITBDDNP, was selected for its high selectivity of polymerization (>99%).31 The turnover frequency (TOF) was calculated as [product]/[product+monomer]*catalyst loading*h−1. Polymer selectivity was also determined by using 1H NMR to define the ratio of the polymeric methine hydrogen to the cyclic carbonate. The number average molecular weight and dispersity were determined via GPC analysis in tetrahydrofuran against polystyrene standards.
Scheme 1.
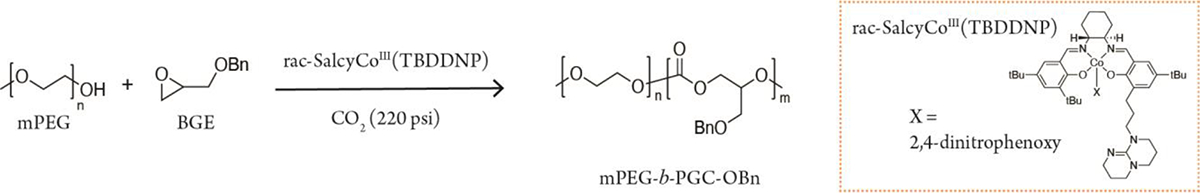
Polymerization of mPEG-b-PGC-OBn.
We prepared the above mPEG-b-PGC polymers with mPEG of three molecular weights (Mn 1.9 kDa, 5.0 kDa, 10.0 kDa). The polymerizations proceed with high TOF to afford terpolymers with narrow dispersities (Ð <1.25) (Table 1). As anticipated, increasing the amount of chain transfer agent (mPEG) in the monomer feed lowers the molecular weight of the resulting polymer.32 To demonstrate the generality of the approach using a compositionally different chain transfer agent, we investigated the polymerization with both benzyl alcohol and monohydroxy-terminated polystyrene. The polymerization using benzyl alcohol affords copolymers consistent in percent conversion with reported literature.33 With monohydroxy-terminated polystyrene, the narrow dispersity and high TOF obtained are similar to that of the copolymer using mPEG.
Table 1.
Catalyst, monomer, and chain transfer agent influence on polymerization characteristics.
| Chain Transfer Agent (-OH) | BGE : Catalyst : -OH | TOF (h−1) | Polymer Selectivity | Mn (NMR) | Mn (theoretical) | Mn (GPC) | Ð |
|---|---|---|---|---|---|---|---|
| mPEG, 1.9k | 2000:1:40 | 570 | >99% | 5.9k | 5.9k | 4.3k | 1.03 |
| 2000:1:20 | 600 | >99% | 11.1k | 12.4k | 8.8k | 1.02 | |
| 2000:1:10 | 630 | >99% | 24.9k | 26.0k | 13.1k | 1.04 | |
| mPEG, 5.0k | 2000:1:20 | 580 | >99% | 14.5k | 16.3k | 9.9k | 1.03 |
| mPEG, 10.0k | 2000:1:20 | 370 | >99% | 10.5k | 14.0k | 13.6k | 1.25 |
| benzyl alcohol | 2000:1:40 | 630 | >99% | 6.0k | 6.4k | 6.8k | 1.03 |
| 2000:1:20 | 670 | >99% | 13.6k | 13.6k | 9.3k | 1.03 | |
| 4000:1:40 | 630 | >99% | 12.6k | 12.4k | 9.4k | 1.04 | |
| polystyrene, 6.0k | 2000:1:20 | 580 | >99% | 14.7k | 12.1k | 10.3k | 1.04 |
| 2000:1:13 | 558 | >99% | 19.6k | 17.3k | 11.3k | 1.04 |
Next, we investigated the terpolymerization of mPEG, BGE, and propylene oxide (PO) with CO2 using SalcyCoIIIDNP, PPNDNP as the catalyst in order to assess the kinetic preference of monomers in the reaction mixture (Scheme 2). Again, the polymerization proceeds with high TOF and results in polymers with low dispersity. We then determined the mole fractions of monomers incorporated into the resulting tetrapolymer via NMR. We fitted the data for the mole fraction of PO in the feed and the glycerol carbonate unit to the Fineman-Ross model (R2 = 0.96) (Figure 2). The monomer reactivity ratio of BGE (rBGE = k11/k12) and PO (rPO = k22/k21) are 0.98 and 0.97, respectively. The relatively small difference in monomer reactivity ratios indicates negligible preference of monomer incorporation into the growing polymer chain. This is consistent with literature, in which a random distribution of monomeric units is defined as having r1 and r2 approximately equal to 1.34
Scheme 2.
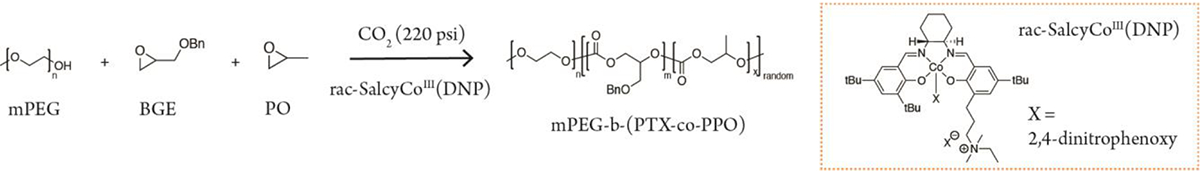
Terpolymerization of mPEG, CO2, BGE, and PO.
Figure 2.
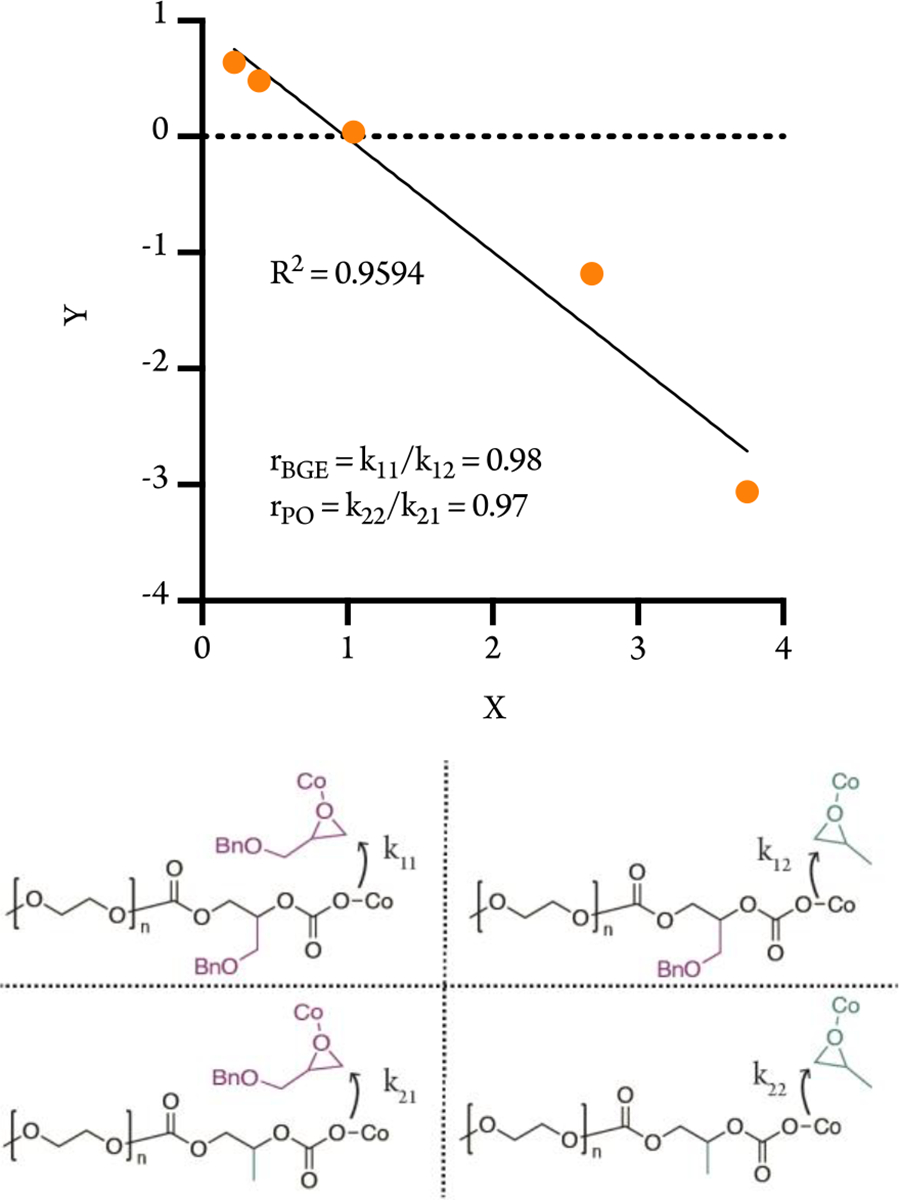
Fineman-Ross analysis for BGE, PO, and mPEG terpolymerization (Y = [fBGE/(1-fBGE)][1–2FBGE)/FBGE]; X = fBGE2/(1-fBGE)2][(1–2FBGE)/FBGE]).
To functionalize the mPEG-b-PGC polymer into a drug delivery vehicle, we synthesized nanoparticles using the mPEG(1.9k)-b-PGC polymer and PTX. Building upon previous work with PGC-PTX nanoparticles23, we hypothesized that PTX could be similarly conjugated to the pendant chain of the mPEG-b-PGC copolymer. We deprotected the benzyl terminated mPEG-b-PGC-OBn using high pressure hydrogenolysis in which mPEG-b-PGC-OBn was dissolved in 3:1 v/v ethyl acetate:methanol, pressurized to 600 psi H2, and stirred at room temperature for 16 hours. To chemically conjugate PTX to mPEG-b-PGC-OH, we performed an acylation reaction with 4-dimethylaminopyridine (DMAP) and installed a succinic anhydride onto the free hydroxyl of the PGC’s pendant chain. We then employed N,N’-dicyclohexylcarbodiimide (DCC) and DMAP to couple PTX to the succinate linker following a published procedure.23 The chemically conjugated mPEG-b-PGC-g-PTX was synthesized at two different mol percents: 11 and 48 (Scheme 3).
Scheme 3.
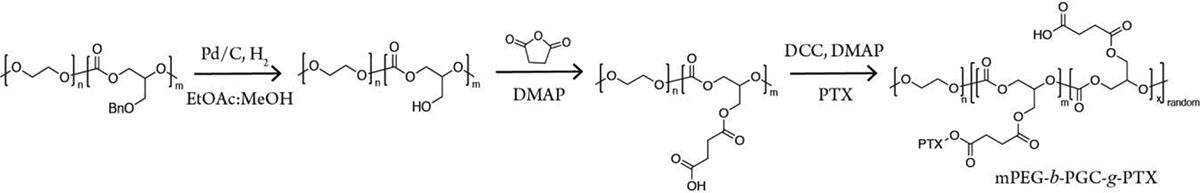
Conjugation of PTX to mPEG-b-PGC.
We prepared nanoparticles from mPEG-b-PGC-g-PTX (11 mol % PTX) using an oil-in-water mini-emulsion sonication method. It is important to note that the hydrophobicity of the paclitaxel payload is necessary for nanoparticle formation. Without PTX, the mPEG-b-PGC-OBn or mPEG-b-PGC-OH polymers do not form micelles. Scanning electron microscopy (SEM) confirmed the spherical morphology of the nanoparticles (Figure 3a). The nanoparticles are, on average, 175 nm in diameter (polydispersity 0.12) by dynamic light scattering (DLS), a size which decreases over the course of 42 days of release in 10 mM phosphate buffer with 0.3% SDS (pH 7.4, 37°C) (Figure 3b). The delivery efficiency of nanoparticle payloads to tumors depends on particle size, with 10–100 nm particles delivering 0.7% of the injected IV dose and 100–200 nm particles delivering a comparable 0.6%.35 Therefore, the mPEG-b-PGC-g-PTX nanoparticles, which are 175 nm in size are well-suited for tumor accumulation and additionally will be able to traverse the 100–500 nm endothelial gaps in tumor vasculature.
Figure 3.
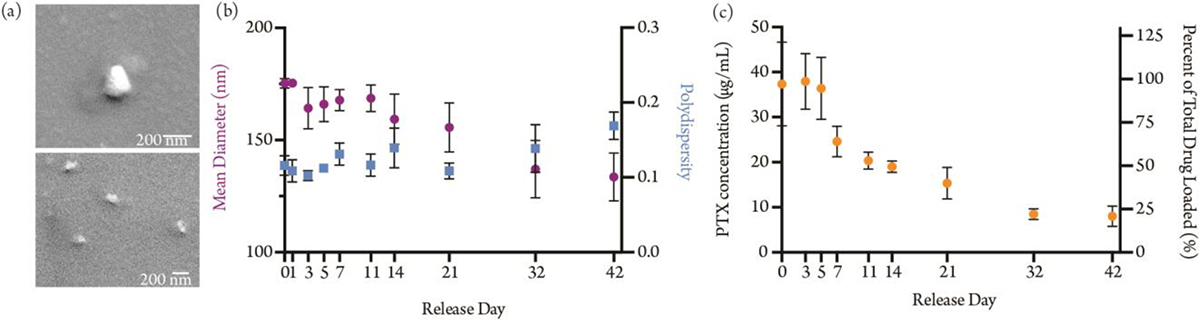
(a) SEM images of mPEG-b-PGC-g-PTX nanoparticles, (b) mPEG-b-PGC-g-PTX nanoparticle diameter, polydispersity, and (c) PTX release, over 42 days.
We assessed the release profile of the mPEG-b-PGC-g-PTX nanoparticles in 10 mM phosphate buffer, 0.3% SDS at 37°C over the course of 42 days. PTX concentration was analyzed using absorbance at 230 nm. mPEG-b-PGC-g-PTX nanoparticles release PTX in a sustained manner, with minimal drug release from days 1 through 5 and about 50% of loaded drug lost between days 5 and 14 (Figure 3c). The nanoparticles continue to release drug slowly over 42 days, at which point we terminated the experiment. This is a marked improvement over the release profile of similar systems like the mPEG-PLL nanoparticle Genexol®PM, which show a PTX burst release of 65% in the first 24 hours and 95% in the first 48 hours.36 Previously reported PGC-g-PTX nanoparticles, without the PEG block, displayed sustained PTX release for longer than 70 days.23 The faster release rate of the mPEG-b-PGC-g-PTX nanoparticles is likely a consequence of the increased hydrophilicity of the nanoparticle due to the mPEG block. The hydrophilic mPEG outer shell of the particle promotes diffusion of water into the material, accelerating hydrolytic degradation of the succinic acid-paclitaxel ester linkage and subsequent release of the payload.
We determined in vitro cytotoxicity of both the unloaded mPEG-b-PGC-OBn polymer and drug-loaded mPEG-b-PGC-g-PTX nanoparticles against NIH 3T3 murine fibroblast, SKOV3 human ovarian adenocarcinoma, A549 human lung carcinoma, and MDA-MB-231 human breast adenocarcinoma cell lines. The unloaded mPEG-b-PGC-OBn polymer is not cytotoxic (cell viability > 80%) below 0.6 mg/mL of polymer for any of the cell lines. For the drug-loaded mPEG-b-PGC-g-PTX nanoparticles, the formulation is cytotoxic to all groups with half maximal inhibitory concentration (IC50) values of 13.5 (NIH 3T3), 2.3 (A549), 1.5 (SKOV3), and 1.5 ng/mL (MDA-MB-231) (Figure 4). The result is expected and offers further confirmation to PTX’s release, since PTX inhibits cell division and affords cell death. These IC50 values are slightly less than those reported for free paclitaxel against these cancer cell lines (3.5 ng/mL, A549;2.7 ng/mL, SKOV3; 2.1 ng/mL, MDA-MB-231).37–39 Importantly, the concentration of polymer delivered to the cells is, at most, 0.4 mg/mL, which is below the observed cytotoxicity limit for mPEG-b-PGC-OBn without drug.
Figure 4.
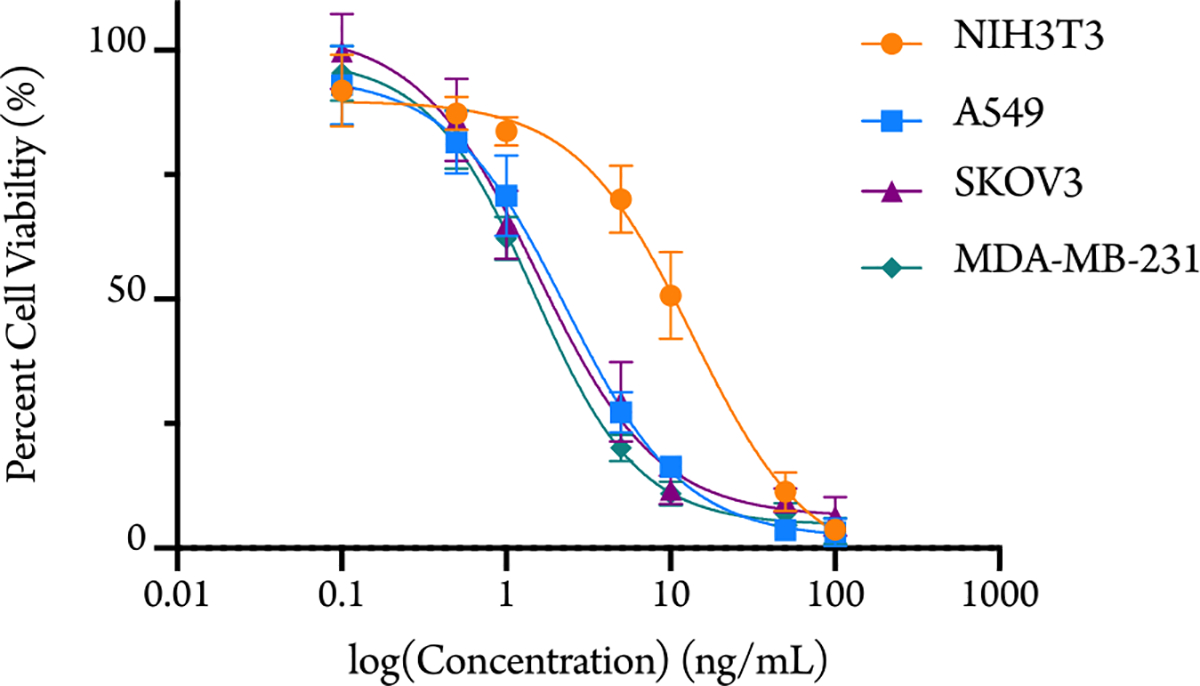
In vitro cytotoxicity of mPEG-b-PGC-g-PTX against NIH 3T3, A549, SKOV3, and MDA-MB-231 cell lines.
In conclusion, we describe an efficient synthesis to block copolymers of mono-methoxylated polyethylene glycol and poly(glycerol carbonate) (mPEG-b-PGC) via the ring-opening polymerization of benzyl glycidyl ether, mono-methoxylated polyethylene glycol, and carbon dioxide using a cobalt salen catalyst. Subsequent covalent conjugation of PTX to the copolymer enables formation PTX-loaded nanoparticles without the need of a surfactant through the use of an amphiphilic block copolymer of PEG and PGC. The nanoparticles release PTX over the course of 42 days and are toxic to human lung, breast, and ovarian cancer cell lines. The formulation is a promising start to developing surfactant-free nanoparticle formulations for the extended delivery (i.e., weeks) of hydrophobic payloads. Future work will assess the in vivo biodistribution and efficacy of the mPEG-b-PGC-g-PTX nanoparticles, as well as the loading capability of the micelles to maximize the amount of deliverable payload. With improved sustained release of PTX and the use of polymers with more biocompatible degradation products, mPEG-b-PGC-g-PTX nanocarriers address key shortcomings of conventional polymeric mPEG-PLL micelles.
Supplementary Material
ACKNOWLEDGMENT
NMR facilities at Boston University as supported by the NSF (CHE-0619339). This research is supported in part by the Biomedical Engineering Core Facilities at Boston University.
Funding Sources
This work was supported in part by the NIH (F31HL163917 (DMF), R01 CA 227433 (YLC, MWG), and R01CA232056 (YLC, MWG)) as well as Boston University.
ABBREVIATIONS
- PTX
paclitaxel
- mPEG
mono-methoxylayted polyethylene glycol
- PGC
polyglycerol carbonate
- SDS
sodium dodecyl sulfate
- IC50
half maximal inhibitory concentration
- SEM
scanning electron microscopy
- GPC
gel permeation chromatography
- DLS
dynamic light scattering
- NMR
nuclear magnetic resonance
Footnotes
Supporting Information
Please find detailed synthetic routes and procedures within the attached supplementary document
The Supporting Information is available free of charge on the ACS Publications website.
Experimental details; 1H NMR spectra; PTX absorption standard curve; in vitro results; Fineman-Ross tabulated results (PDF)
DMF, YLC, and MWG are co-inventors on patent applications describing polycarbonate-based polymers, which are available for licensing.
REFERENCES
- 1.Feng H; Lu X; Wang W; Kang N-G; Mays J, Block Copolymers: Synthesis, Self-Assembly, and Applications. Polymers.2017, 9, 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dau H; Jones GR; Tsogtgerel E; Nguyen D; Keyes A; Liu Y-S; Rauf H; Ordonez E; Puchelle V; Basbug Alhan H; Zhao C; Harth E, Linear Block Copolymer Synthesis. Chem Rev.2022, 122, 14471–14553. [DOI] [PubMed] [Google Scholar]
- 3.Ghezzi M P S; Padula C; Santi P; Del Favero E; Cantú L; Nicoli S, Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J Control Release.2021, 332, 312–336. [DOI] [PubMed] [Google Scholar]
- 4.Englert C; Brendel JC; Majdanski TC; Yildirim T; Schubert S; Gottschaldt M; Windhab N; Schubert US, Pharmapolymers in the 21st century: Synthetic polymers in drug delivery applications. Prog Polym Sci.2018, 87, 107–164. [Google Scholar]
- 5.Zhu K; Lin Z; Yang S; Li Y; Ye H, Synthesis of PEG-PLA block copolymers and determination of average block lengths of carbon-13 NMR. Gaofenzi Xuebao.1988, 4, 258–263. [Google Scholar]
- 6.Ghasemi R; Abdollahi M; Emamgholi Zadeh E; Khodabakhshi K; Badeli A; Bagheri H; Hosseinkhani S, mPEG-PLA and PLA-PEG-PLA nanoparticles as new carriers for delivery of recombinant human Growth Hormone (rhGH). Sci Rep.2018, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andima M; Costabile G; Isert L; Ndakala A; Derese S; Merkel O, Evaluation of β-Sitosterol Loaded PLGA and PEG-PLA Nanoparticles for Effective Treatment of Breast Cancer: Preparation, Physicochemical Characterization, and Antitumor Activity. Pharmaceutics.2018, 10, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Afsharzadeh M; Hashemi M; Babaei M; Abnous K; Ramezani M, PEG‐PLA nanoparticles decorated with small‐molecule PSMA ligand for targeted delivery of galbanic acid and docetaxel to prostate cancer cells. J Cell Physiol.2020, 235, 4618–4630. [DOI] [PubMed] [Google Scholar]
- 9.Wang J; Fu J; Sun W; Yin X; Lv K; Zhang J, Functionalized PEG-PLA nanoparticles for brain targeted delivery of ketoconazole contribute to pregnane X receptor overexpressing in drug-resistant epilepsy. Epilepsy Res 2022, 186, 107000. [DOI] [PubMed] [Google Scholar]
- 10.Massadeh S; Almohammed I; Barhoush E; Omer M; Aldhawi N; Almalik A; Alaamery M, Development of Epirubicin-Loaded Biocompatible Polymer PLA–PEG–PLA Nanoparticles: Synthesis, Characterization, Stability, and In Vitro Anticancerous Assessment. Polymers.2021, 13, 1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumari P; Muddineti OS; Rompicharla SVK; Ghanta P; B B N AK; Ghosh B; Biswas S, Cholesterol-conjugated poly(D, L-lactide)-based micelles as a nanocarrier system for effective delivery of curcumin in cancer therapy. Drug Deliv 2017, 24, 209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Q; Tan L; He C; Liu B; Xu Y; Zhu Z; Shao Z; Gong B; Shen Y-M, Redox-responsive micelles self-assembled from dyanmic covalent block copolymers for intracellular drug delivery Acta Biomater.2015, 17, 193–200. [DOI] [PubMed] [Google Scholar]
- 13.Xiong J; Meng F; Wang C; Cheng R; Liu Z; Zhong Z, Folate-conjugated crosslinked biodegradable micelles for receptor-mediated delivery of paclitaxel. J Mater Chem.2011, 21, 5786. [Google Scholar]
- 14.Liang H; Ren X; Qian J; Zhang X; Meng L; Wang X; Li L; Fang X; Sha X, Size-Shifting Micelle Nanoclusters Based on a Cross-Linked and pH-Sensitive Framework for Enhanced Tumor Targeting and Deep Penetration Features. ACS Appl Mater Interfaces.2016, 8, 10136–10146. [DOI] [PubMed] [Google Scholar]
- 15.Xu X; Zhang X; Wang X; Li Y; Jing X, Comparative study of paclitaxel physically encapsulated in and chemically conjugated with PEG-PLA. Polym Adv Technol.2009, 20, 843–848. [Google Scholar]
- 16.Zhu L; Chen L, Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett.2019, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jadhav SR Gary B; Mata Jitendra P.; Eldridge Daniel S.; Palombo Enzo A.; Harding Ian H.; Shah Rohan M., Structural aspects of a self-emulsifying multifunctional amphiphilic excipient: Part II. The case of Cremophor EL. J Mol Liq.2021, 344, 117881. [Google Scholar]
- 18.Gelderblom H; Verweij J; Nooter K; Sparreboom A, Cremophor EL. Eur J Cancer.2001, 37, 1590–1598. [DOI] [PubMed] [Google Scholar]
- 19.Kim SC; Kim DW; Shim YH; Bang JS; Oh HS; Kim SW; Seo MH, In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy J Control Release.2001, 72, 191–202. [DOI] [PubMed] [Google Scholar]
- 20.Kim T-Y; Kim D-W; Chung J-Y; Shin SG; Kim S-C; Heo D-S; Kim NK; Bang Y-J, Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res.2004, 10, 3708–3716. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Hydrolytic degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids J Biomed Mater Res.1999, 48, 342–353. [DOI] [PubMed] [Google Scholar]
- 22.Petersen A; Chu N-Q; Fitzgerald DM; McCaslin EZ; Blessing WA; Colby AH; Colson YL; Grinstaff MW, Sustainable glycerol terpolycarbonates as temporary bioadhesives. Biomater Sci.2021, 9, 8366–8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekladious I; Liu R; Zhang H; Foil DH; Todd DA; Graf TN; Padera RF; Oberlies NH; Colson YL; Grinstaff MW, Synthesis of poly(1,2-glycerol carbonate)–paclitaxel conjugates and their utility as a single high-dose replacement for multi-dose treatment regimens in peritoneal cancer. Chem Sci.2017, 8, 8443–8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tawfeek H; Khidr S; Samy E; Ahmed S; Murphy M; Mohammed A; Shabir A; Hutcheon G; Saleem I, Poly(Glycerol Adipate-co-ω-Pentadecalactone) Spray-Dried Microparticles as Sustained Release Carriers for Pulmonary Delivery. Pharm Res.2011, 28, 2086–2097. [DOI] [PubMed] [Google Scholar]
- 25.Liu R; Wolinsky JB; Catalano PJ; Chirieac LR; Wagner AJ; Grinstaff MW; Colson YL; Raut CP, Paclitaxel-Eluting Polymer Film Reduces Locoregional Recurrence and Improves Survival in a Recurrent Sarcoma Model: A Novel Investigational Therapy. Ann Surg Oncol.2012, 19, 199–206. [DOI] [PubMed] [Google Scholar]
- 26.Zawaneh PN; Singh SP; Padera RF; Henderson PW; Spector JA; Putnam D, Design of an injectable synthetic and biodegradable surgical biomaterial. Proc Natl Acad Sci U.S.A 2010, 107, 11014–11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dernedde J; Rausch A; Weinhart M; Enders S; Tauber R; Licha K; Schirner M; Zügel U; Von Bonin A; Haag R, Dendritic polyglycerol sulfates as multivalent inhibitors of inflammation. Proc Natl Acad Sci U.S.A 2010, 107, 19679–19684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zamboulis A; Nakiou EA; Christodoulou E; Bikiaris DN; Kontonasaki E; Liverani L; Boccaccini AR, Polyglycerol Hyperbranched Polyesters: Synthesis, Properties and Pharmaceutical and Biomedical Applications. Int J Mol Sci.2019, 20, 6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolbuk D; Jeznach O; Wrzecionek M; Gadomska-Gajadhur A, Poly(Glycerol Succinate) as an Eco-Friendly Component of PLLA and PLCL Fibres towards Medical Applications. Polymers.2020, 12, 1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beharaj A; McCaslin EZ; Blessing WA; Grinstaff MW, Sustainable polycarbonate adhesives for dry and aqueous conditions with thermoresponsive properties. Nat Commun.2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H; Grinstaff MW, Synthesis of Atactic and Isotactic Poly(1,2-glycerol carbonate)s: Degradable Polymers for Biomedical and Pharmaceutical Applications. J Am Chem Soc.2013, 135, 6806–6809. [DOI] [PubMed] [Google Scholar]
- 32.Furuncuoğlu T; Uğur İ; Değirmenci İ; Aviyente V, Role of Chain Transfer Agents in Free Radical Polymerization Kinetics. Macromolecules.2010, 43, 1823–1835. [Google Scholar]
- 33.Jiang D; Mallat T; Krumeich F; Baiker A, Copper-based metal-organic framework for the facile ring-opening of epoxides. J Catal.2008, 257, 390–395. [Google Scholar]
- 34.Thirumoolan D; Anver Basha K; Kanai T; Mohammed Safiullah S; Vetrivel K; Abdul Wasi K; Ranjithkumar B, Synthesis, characterization and reactivity ratios of poly N-(p-bromophenyl)-2-methacrylamide-Co-N-vinyl-2-pyrrolidone. J Saudi Chem Soc.2016, 20, 195–200. [Google Scholar]
- 35.Wilhelm S; Tavares AJ; Dai Q; Ohta S; Audet J; Dvorak HF; Chan WCW, Analysis of nanoparticle delivery to tumours. Nat Rev Mater.2016, 1, 16014. [Google Scholar]
- 36.Werner ME; Cummings ND; Sethi M; Wang EC; Sukumar R; Moore DT; Wang AZ, Preclinical Evaluation of Genexol-PM, a Nanoparticle Formulation of Paclitaxel, as a Novel Radiosensitizer for the Treatment of Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys.2013, 86, 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liebmann J; Cook J; Lipschultz C; Teague D; Fisher J; Mitchell J, Cytotoxic studies of paclitaxel (Taxol®) in human tumour cell lines. Br J Cancer.1993, 68, 1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim G; Jang S-K; Kim YJ; Jin H-O; Bae S; Hong J; Park I-C; Lee JH, Inhibition of glutamine uptake resensitizes paclitaxel resistance in SKOV3-TR ovarian cancer cell via mTORC1/S6K signaling pathway Int J Mol Sci.2022, 23, 8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakayama S; Torikoshi Y; Takahashi T; Yoshida T; Sudo T; Matsushima T; Kawasaki Y; Katayama A; Gohda K; Hortobagyi GN; Noguchi S; Sakai T; Ishihara H; Ueno NT, Prediction of paclitaxel sensitivity by CDK1 and CDK2 activity in human breast cancer cells. Breast Cancer Research.2009, 11, R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


