Abstract
Rapid and accurate identification of Salmonella enterica serotypes Typhi and Paratyphi (A, B and C), the causal agents of enteric fever, is critical for timely treatment, case management and evaluation of health policies in low and middle-income countries where the disease still remains a serious public health problem. The present study describes the development of a multiplex assay (EFMAtyping) for simultaneous identification of pathogens causing typhoid and paratyphoid fever in a single reaction by the MeltArray approach, which could be finished within 2.5 h. Seven specific genes were chosen for differentiation of typhoidal and nontyphoidal Salmonella. All gene targets were able to be detected by the EFMAtyping assay, with expected Tm values and without cross-reactivity to other relevant Salmonella serovars. The limit of detection (LOD) for all gene targets was 50 copies per reaction. The LOD reached 102–103 CFU/ml for each pathogen in simulated clinical samples. The largest standard deviation value for mean Tm was below 0.5 °C. This newly developed EFMAtyping assay was further evaluated by testing 551 clinical Salmonella isolates, corroborated in parallel by the traditional Salmonella identification workflow, and serotype prediction was enabled by whole-genome sequencing. Compared to the traditional method, our results exhibited 100% of specificity and greater than 96% of sensitivity with a kappa correlation ranging from 0.96 to 1.00. Thus, the EFMAtyping assay provides a rapid, high throughput, and promising tool for public health laboratories to monitor typhoid and paratyphoid fever.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13099-024-00636-6.
Keywords: Typhoid and paratyphoid fever, Melting curve analysis, Rapid identification, Multiplex PCR
Introduction
Enteric fever (EF), caused by Salmonella enterica serovars Typhi and Paratyphi (A, B and C), remains a significant health problem in low and middle-income countries, especially in Southeast Asia and Africa [1], which was estimated having caused 14 million illnesses and 136,000 deaths globally in 2017 [2]. Typhoid/paratyphoid fever can cause bacteremia and inflammatory destruction of the intestine and other organs, which is fatal both for adults and children, in contrast to the less severe and self-limiting gastroenteritis caused by non-typhoidal Salmonella (NTS). Since antimicrobial therapy is crucial for treating typhoid/paratyphoid fever, early and accurate identification of the causal pathogens is critical for timely and effective disease management.
Although still the gold standard for diagnosing typhoid/paratyphoid fever, isolation and identification of the causal pathogens by culturing and biochemical tests is time-consuming, expensive and labor-intensive, requiring a minimum of at least 4–5 days. Thus, development of a rapid alternative method is needed. Compared to conventional methods, multiplex polymerase chain reaction (PCR) or real-time PCR [3–6] are cost-effective, highly accurate and convenient to use. However, PCR is of limited use as a standalone tool for routine surveillance of serovar typhi and paratyphi, because it requires various post-PCR manipulations, or distinguishes only one or two dominant disease-causing targets once a time, or targets only one gene. Genotyping based on whole genome sequencing (WGS), such as SISTR2 [7], SeqSero2 [8], Genotyphi [9], Paratype [10], has emerged as an alternative to replace current molecular typing methods for EF surveillance. However, routine use of WGS for typing is still uncommon due to the cost and need of professional instruments and bioinformatics resources.
Multicolor melting curve analysis (MMCA) is a homogeneous multiplex analytical system with fluorophore color and melting temperature (Tm) value as the two-dimensional label [11]. MMCA has been successfully used for simultaneous identification of 30 common Salmonella serotypes or 11 clinically commonest V. parahaemolyticus serotypes by a multiplex ligation reaction based on probe MCA [12, 13], for identification of clinically relevant Mycobacterium species [14], and for identification of 92 pneumococcal serotypes [15]. In this study, we developed a revised version of MMCA, EFMAtyping (enteric fever MeltArray typing), a single PCR reaction assay which is more specific for identifying both typhoidal and paratyphoidal Salmonella, and its performance was further evaluated by testing 551 clinical Salmonella isolates collected over a 17-year period (2001–2017) in Shenzhen city.
Materials and methods
Reference and clinical bacterial isolates
The initial development and evaluation of the EFMAtyping assay used a total of 42 reference bacterial isolates (Table S1), comprising strains of four positive reference Salmonella and other common Salmonella serovars, and other intestinal pathogens from American Type Culture Collection (ATCC) or National Center for Medical Culture Collections of China (CMCC) or clinical isolates, for the purpose of detecting any possible cross-reactions. The 551 clinical isolates from the stool or blood samples of patients were collected during a 17-year period (2001–2017) for a multicenter (11 sentinel hospitals in Shenzhen) study.
Bacterial culture and conventional serotyping
In the multicenter study, every blood sample (8 mL each) was injected into a single BacTec culture bottle (Biomerieux, Durham, NC, USA), and every stool specimen was enriched in 10 mL selenite cystine broth (Guangdong Huankai Microbial Science & Technology, Guangzhou, China), incubated at 37 °C for 12–18 h, then streaked onto BBL CHROMagar Salmonella agar (Bectin Dickinson, Shanghai, China) for single colonies, and incubated another 12 h. Suspected colonies were picked and streaked onto Triple Sugar Iron slants (Guangdong Huankai Microbial Science & Technology, Guangzhou, China), incubated at 37 °C for 12–18 h, and checked with serotyping by the serum agglutination using the White Kauffman Le-Minor scheme according to the Chinese National Food Safety Standards (Food Microbiological Examination Salmonella Testing, GB 4789.4-2016).
DNA extraction
For initial development and evaluation of the analytical performance of EFMAtyping, bacterial genomic DNA was isolated using a QIAamp DNA Mini Kit (QIAGEN; Hilden, Germany) following the manufacturer’s protocol. The genomic DNA of the clinical isolates (from the multicenter study) was obtained by heating lysis as follows. A single colony was suspended in 50 μL of distilled water, boiled at 100 °C for 8 min, centrifuged at 12,000 × g for 5 min, and the supernatant was quantified using a Nanodrop spectrophotometer (Nanodrop Products, Wilmington, Delaware, USA), and stored at − 20 °C for future use as DNA templates.
Primers and probe design
In this study, 7 genes (SPA2308, SPC0869, srfJ, sseJ, staG, ttr and tviB; in alphabetic order) were chosen as target genes to specifically differentiate typhoidal or non-typhoidal Salmonella: ttr (encoding tetrathionate reductase) for identification of Salmonella species [16]; tviB (encoding a Vi polysaccharide capsule present in S. Typhi and S. Paratyphi C but absent in S. Paratyphi A or B) to determine whether a Salmonella strain is typhoidal or not [17]; staG (encoding a putative fimbrial protein) for detection of S. Typhi [18]; and SPA2308 (encoding a hypothetical protein) for detection of S. Paratyphi A; sseJ and srfJ, two type III secretion system (TTSS) effector genes, to discriminate D-tartrate (dT) fermenting from non-fermenting strains of S. Paratyphi B and S. Paratyphi B var. Java, because S. Paratyphi B (dT-) possesses only srfJ, but S. Java (dT +) possesses both [19]; and SPC0869 (encoding a hypothetical protein) for detection of S. Paratyphi C [20]. All primers and probes were synthesized by Sangon Biotech Co. Ltd after designing using the respective software (Primer Premier v5.0; and DNA folding software, online web server: http://unafold.rna.albany.edu/?q=mfold/DNA-Folding-Form), and evaluated using BlastN algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Fluorogenic probes were labeled at 5′ end either with carboxy fluorescein (FAM) or carboxy-X-rhodamine (ROX), and at the 3′ end with the Black Hole Quencher sequestering molecule.
Development of the EFMAtyping assay
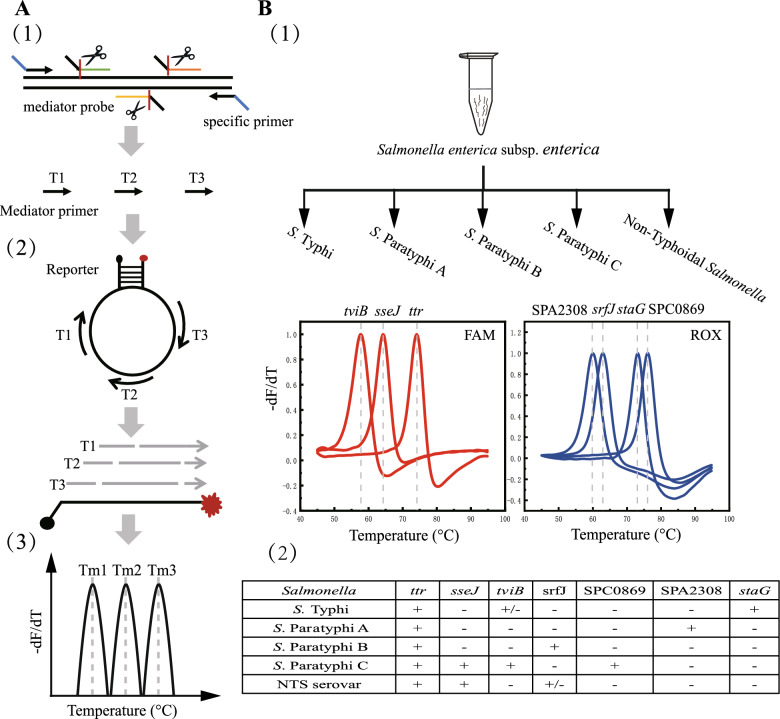
The flowchart of EFMAtyping assay is schematically shown in Fig. 1. Briefly, a common tag sequence lacking homology to any known bacterial genome by BLAST analysis is added to all target-specific primers. The universal primers will attach to the amplicons possessing a universal primer binding sequence and resulting from the first few rounds of amplification, and efficiently amplify the target gene product. During the annealing stage, the 5′-flap endonuclease activity of Taq DNA polymerase will cleave the mediator probe into a mediator primer upon its hybridization with the DNA target sequence (Fig. 1A(1)), and the resulting primer will bind to the loop of the molecular beacon reporter (Fig. 1A(2)). Amplification products with different lengths will result in unique Tm values in the respective fluorophore channels which can be used for melting curve analysis (Figs. 1A(3) and B(1)). Finally, identification results for each target gene are determined on the basis of the Tm value (Fig. 1B(2)).
Fig. 1.
Identification of typhoidal and nontyphoidal Salmonella using the EFMAtyping assay. A The working principle of detecting Salmonella serotype by the EFMAtyping. (1) During the PCR amplification process, the specific primer pair will amplify the target gene, and the three mediator probes will be cleaved into specific mediator primers; (2) In the mediator primer hybridization and extension stage, three amplification products of different length will be generated; (3) During the melting curve analysis, the amplification products are identified by the predefined melting temperature (Tm) in a detection channel. B The design of Tm value and fluorescence detection channel for the EFMAtyping assay in the reaction. (1) Illustration of the sequential appearance of genes represented by their unique Tm and channeled by FAM and ROX; (2) Tabulated summary of the presence or absence of the seven genes in the five different types of Salmonella strains
The EFMAtyping assay was performed on a Slan-96S real-time PCR system (Zeesan Biotech). For sample detection, a 25-μL reaction solution contained 5 μL of genomic DNA template and 20 μL of PCR master buffer [7 mM MgCl2, 0.2 mM dNTPs, 2.5 U Taq DNA polymerase (TaKaRa), 0.8 μM universal primer, mediator probes, and molecular beacon reporters]. The sequences and concentrations of the 7 mediator probes and 4 molecular beacon reporters are listed in Table 1. The running program included 95 °C for 5 min; 40 cycles of 95 °C for 20 s, 60 °C for 1 min; and then 35 °C for 30 min, 95 °C for 2 min, 45 °C for 2 min, followed by a temperature increase from 45 °C to 95 °C (0.04 °C/step). The fluorescence intensity was measured in two detection channels [FAM at 510 nm, ROX at 620 nm] at each step of the continuous temperature increase during the melting curve analysis procedure.
Table 1.
Primers and probes used in EFMAtyping assay
| Gene | Oligo name | Sequence (5′ → 3′) | Concentration (nM) | Amplicon length (nt) | Tm value (℃) |
|---|---|---|---|---|---|
| tviB | tviB-F | Tag-GCCGATAAATACCTACAAGC | 40 | ||
| tviB-R | Tag-ATAACCGACATAGAAATCCTG | 40 | 196 | 57.6 | |
| tviB-P | ACTCTGATCTGTCACCCCACTAATCAAGGCGAGTG -C7NH2 | 400 | |||
| sseJ | sseJ-F | Tag-AATAAATCACATCCCAAGCC | 40 | ||
| sseJ -R | Tag-AACTCAGTCCAGGTAAATCC | 40 | 179 | 63.8 | |
| sseJ -P | CCTCACTCTGATATCTTACCCTCCTATGGTCAATACTTTGGCGG -C7NH2 | 400 | |||
| ttr | ttr-F | Tag-CTATACGGAGCAACGGTTA | 40 | ||
| ttr-R | Tag-GGGAAACTGGTCGTCAAT | 40 | 166 | 74.1 | |
| ttr-P | CTCACCTGTGGTCACCGGGGAGCGCTTCAGCGG -C7NH2 | 400 | |||
| SPA2308 | SPA2308-F | Tag-TCAAAGGCTCCACATCTG | 40 | ||
| SPA2308-R | Tag-GGTTTATCCTTGCCGTATT | 40 | 249 | 60.0 | |
| SPA2308-P | CCTCTCACACTCCACCATCATTCATTCCTGATGAAATCATATATA-C7NH2 | 400 | |||
| srfJ | srfJ-F | Tag-ACGATAAAGATGGCCTGGTAG | 40 | ||
| srfJ -R | Tag-GTCGATAAACCCGCTACA | 40 | 272 | 63.1 | |
| srfJ -P | CACCTCTCACACCGGCGATCATTTTTCGCAAATACAGTATCTGGC -C7NH2 | 400 | |||
| staG | staG-F | Tag-GGGTTATCAATGTAGAAATCGG | 40 | ||
| staG-R | Tag-CACCATTGAGATGACCTTCC | 40 | 267 | 73.2 | |
| staG-P | GCGCTCTCCGTCAGAGTCGACATAGGCATAGATTTTCAGGCCAT-C7NH2 | 400 | |||
| SPC0869 | SPC0869-F | Tag-CTTCTGTCTGGCTGTTTG | 40 | ||
| SPC0869-R | Tag-AGGTAGTGAATGGCTGTC | 40 | 153 | 76.4 | |
| SPC0869-P | CCAGCGCTCTCAAATACCTCAGAAATTCCGGATATATTTTGGCA-C7NH2 | 400 | |||
| Universal probe | UP1 | FAM-CGAGCAAACAGATCAGAGTGAGGAGACAGCAGCTCG-BHQ1 | 40 | ||
| UP2 | FAM-CGCGCCAGGCACGACCACAGGTGAGCACGGCAGGCAGGAGGGGGCGCG-BHQ1 | 40 | |||
| UP3 | ROX-CGAGCAAAAAGAAGTGTGAGAGGTGTGATGAGCTCG-BHQ2 | 40 | |||
| Universal probe | UP4 | ROX-CGGCGGAGTGGGCACGGAGAGCGCTGGACAGTGTGGACCCACGTCTCGCAGCAGGCCGCCG-BHQ2 | 40 | ||
| Tag | GCAAGCCCTCACGTAGCGAA | 800 |
Analytical performance of the EFMAtyping assay
To determine the limit of detection (LOD) of the assay, a series of tenfold dilutions of plasmid DNA of known concentration was detected 20 times for each concentration. The LOD for each target gene was defined as the lowest concentration that gave no more than one negative result in the 20 replicates. Each of the target genes was cloned into a pUC57 vector (Sangon Biotech Co. Ltd., Shanghai, China), and the resultant plasmid DNA (for each target gene) was extracted using the SanPrep Plasmid MiniPrep Kit (Sangon Biotech Co. Ltd., Shanghai, China) according to the instruction manual, which included six concentrations ranging from 1.0 × 106 copies/μL to 1.0 × 101 copies/μL. The reproducibility of the assay was evaluated using 104 copies/μl of DNA templates and repeated eight times. The standard deviations (SD) and average of variation (AV) values were calculated.
Detection of simulated clinical samples was done as follows. Reference strains were each cultured overnight in LB broth and adjusted by diluting with enrichment broth to 106 or 107 colony forming units (CFU)/mL using an in prior empirical turbidity and verified by counting. 100 μL (104 or 101 CFU/ml) of the cultures was mixed respectively with 900 μL of blood or stool suspension sample. One mL of the pre-mixed bacterial sample for each pathogen was used for genomic DNA extraction (following the aforementioned protocol used for the DNA templates for the EFMAtyping assay) using a QIAamp Stool or Blood DNA Mini Kit (QIAGEN, Hilden, Germany).
Identification of the clinical isolates in the multicenter study
During 2001–2017, 551 clinical samples were collected from 11 sentinel hospitals in Shenzhen and used to evaluate the specificity and sensitivity of the EFMAtyping assay. All suspected isolates from stool or blood samples were in parallel analyzed by the traditional Salmonella identification methods (biochemical testing, and conventional serotyping) and the EFMAtyping assay (genomic DNA extraction from clinical isolates was carried out by heating lysis as described above). The detection procedures were performed following the aforementioned protocols. For further verification, WGS was performed on the positive strains isolated from all clinical samples. Serotypes were validated by SalmonellaIn Silico Typing Resource (SISTR). The relevant data presented in the study were deposited in the National Center for Biotechnology Information (NCBI) sequence read archive (SRA) under BioProject: PRJNA1012012. The agreement between the EFMAtyping assay and the conventional Salmonella identification method was determined by calculating the kappa value.
Results
Establishment of the EFMAtyping assay
The 7-plex EFMAtyping assay included four different molecular beacon reporters and seven different mediator probes labeled with FAM or ROX. When the corresponding target sequences of the reporters were amplified, each of them could generate a predefined Tm, and be used to identify typhoidal and nontyphoidal Salmonella by the seven different Tm combination. Three (ttr, tviB, staG) of the 7 target genes were detected in the FAM channel, and the other four (SPA2308, SPC0869, sseJ, srfJ) in the ROX channel (Figs. 1A(3) and B(1)). Detailed criteria for determining results of typhoidal and nontyphoidal Salmonella were shown in Fig. 1B(2). The experimental setup (gene loci, fluorophore channels, designated melting temperatures and probe sequences) was detailed in Table 1. The results of the established EFMAtyping assay are shown in Fig. S1.
Performance characteristics of the EFMAtyping assay
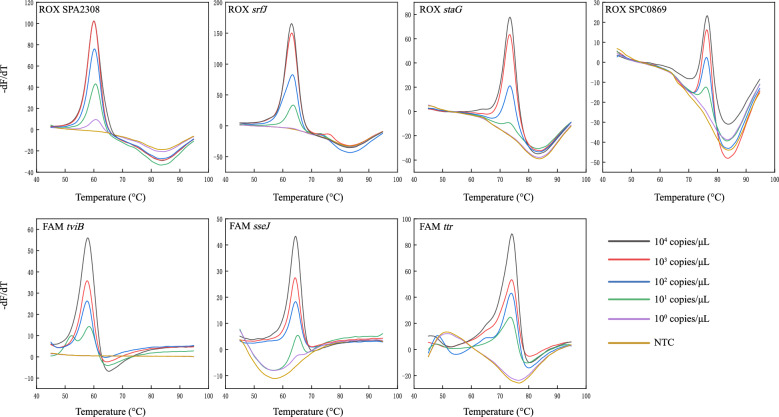
The EFMAtyping assay was able to detect all gene targets, with the expected Tm values for each gene. No cross reaction was detected when reference strains were included (Table S1). The limit of detection (LOD) was determined to be 50 copies per reaction for each target gene (Fig. 2). Tm values showed very little fluctuation in repeated experiments. The largest SD value for mean Tm was below 0.5 °C (Fig. 3, Table S3). The performance of the EFMAtyping assay was further evaluated by testing simulated stool and blood samples spiked with a fixed number of bacteria. The results (Tables S2-1 and S2-2) showed that the LOD was between 102 and 103 CFU/mL, thus demonstrating that the EFMAtyping assay has the potential to detect real clinical samples.
Fig. 2.
Analytical sensitivity analysis of the EFMAtyping assay. Tenfold serial dilutions of a DNA template ranging from 104 to 100 copies (colored lines) in 25-μL reactions were analyzed using three detection channels. A water sample was analyzed as controls
Fig. 3.

The reproducibility of the Tm value for each target gene detected in EFMAtyping assay. Black circles represent the mean Tm value for each target, and the horizontal bars show the range of eight-fold standard deviations in the mean Tm value
Evaluation of the EFMAtyping assay
A total of 551 clinical isolates were subjected to traditional way of characterization in parallel to EFMAtyping. EFMAtyping identified 271 (49.2%) isolates as S. Paratyphi A, one isolate each of S. Paratyphi B (0.2%) and C (0.2%), 135 (24.5%) isolates as S. Typhi, and 143 (25.9%) isolates as other serotypes. The EFMAtyping assay had a sensitivity of 96–100%, and a specificity of 100%, about 98–100% consistency with results from the conventional serological method. The kappa value between EFMAtyping assay and the serological method was between 0.96 and 1 (Table 2), indicating good consistency between the two methods. As detailed in Fig. S2, the 551 Salmonella strains were identified comprising 10 serotypes through WGS, which was 100% consistent with the results from EFMAtyping (Table S4). Of note, among the 21 isolates misidentified by the conventional methods, six were identified as nontyphoidal Salmonella, ten as S. Paratyphi A and five as S. Typhi by EFMAtyping (Table S5). A comparison of the performance between the conventional methods and EFMAtyping is shown in Table S6.
Table 2.
Performance of the EFMAtyping assay compared with the conventional method
| Organism | EFMAtyping | Conventional method | Sensitivity | Specificity | PPV | NPV | Diagnostic accuracy | Kappa | |
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||
| S. Typhi | Positive | 135 | 0 | 96.4 (91.2–98.8) | 100 (99.1–100) | 100 (97.3–100) | 98.8 (97.2–99.4) | 99.0 (97.9–99.7) | 0.97 |
| Negative | 5 | 411 | |||||||
| S. ParatyphiA | Positive | 271 | 0 | 96.4 (93.5–98.2) | 100 (98.6–100) | 100 (98.6–100) | 96.4 (93.6–98.2) | 98.2 (96.7–99.1) | 0.96 |
| Negative | 10 | 270 | |||||||
| S. ParatyphiB | Positive | 1 | 0 | 100 (2.5–100) | 100 (99.3–100) | 100 (2.5–100) | 100 (99.3–100) | 100 (99.3–100) | 1 |
| Negative | 0 | 550 | |||||||
| S. ParatyphiC | Positive | 1 | 0 | 100 (2.5–100) | 100 (99.3–100) | 100 (2.5–100) | 100 (99.3–100) | 100 (99.3–100) | 1 |
| Negative | 0 | 550 | |||||||
| nontyphoidal Salmonella | Positive | 143 | 0 | 96 (91.4–98.5) | 100 (99–100) | 100 (97.4–100) | 98.5 (96.3–99.3) | 98.9 (97.6–99.6) | 0.97 |
| Negative | 6 | 402 | |||||||
PPV: Positive predictive values, NPV: Negative predictive values
Discussion
Typhoid/paratyphoid fever continues to be a significant public health problem for some endemic countries, and the emergence of increasing antimicrobial resistance undermines effective treatment and disease control. Rapid and accurate differentiation of pathogens of typhoid/paratyphoid fever from nontyphoidal Salmonella would guide a rational regimen of antibiotic use, and hopefully help stop the spread of drug-resistant isolates. In this study, we have developed a novel MeltArray assay, termed EFMAtyping, based on probe melting curve analysis for simultaneous identification of typhoidal and nontyphoidal Salmonella [21].
The novel EFMAtyping assay has covered S. Typhi, S. Paratyphi (either A, B or C) by detecting all 7 designed gene targets with expected Tm values and without any cross-reaction. The LOD of the assay was 50 copies per reaction for each of the target gene. Moreover, results of the reproducibility analyses showed that the assay had highly reproducible traits, with the largest SD value for the mean Tm being no more than 0.5 °C and the CVs being less than 1%. Test results of the simulated clinical samples suggested that the assay most probably could meet clinical laboratory requirement. Overall, these performance characteristics were similar to those observed in assays developed for rapid identification of SARS-CoV-2 variants [22], E. coli serotyping [21] and Streptococcus pneumoniae serotyping [23]. In the multicenter clinical study, EFMAtyping assay had shown perfect concordance with the WGS serological method. 406 isolates belonging to S. Paratyphi A and S. Typhi were correctly identified by the EFMAtyping assay and no false positives (identification of non-target serovars) were observed. The EFMAtyping assay could be completed within 2.5 h by delivering both results of Salmonella confirmation and serotyping, much faster compared to the traditional methods which often need overnight incubation of the biochemical tests for identification. Therefore, the EFMAtyping assay potentially has broad application prospects for rapid and accurate identification of pathogens that cause EF in clinical and public health laboratories.
To circumvent the limitations of conventional serotyping, many studies have focused on developing real-time PCR with multiple reactions or multiplex real-time PCR because of their ease of use [24, 25], coupling with other methods based on Luminex, CRISPR [26], or WGS. The drawbacks of these methods include either the need of bioinformatics analysis and/or gel electrophoresis after PCR amplification, or reliance on special and costly instruments, or procedures being time consuming or complex, which limit their widespread use in clinical settings. A multiplex ligation reaction based on probe melting curve analysis method was recently developed and applied [13, 27, 28], to achieve higher throughput for multiplex detection, and it could detect multiple pathogens in one reaction based on a two-dimensional labeling strategy (up to 32 targets). However, the need to open the test tube in the ligation reaction step added extra operational complexity and chances of contamination, and the relatively nonspecific amplification reduced its analytical sensitivity (LOD), which all limit its applications to clinical isolates rather than clinical samples. By combining PCR amplification and melting curve analysis into a single and closed-tube reaction, the EFMAtyping assay required only a single template addition step without any additional separate reactions before detection on the real-time PCR machine, therefore reducing the assay time to 2.5 h. Moreover, the lower LOD of the EFMAtyping assay made it feasible to detect Salmonella in clinical samples. Thus, this newly developed EFMAtyping assay is a highly practical, rapid, and robust method.
Various molecular methods for identification of typhoidal/nontyphoidal Salmonella had previously developed [18, 29, 30] but most of them focused on just one or two of the EF pathogens. Many target genes are reported in literature for detecting S. Typhi but their specificity needs to be evaluated with more clinical strains. For example, five novel gene markers (STY0307, STY0322, STY0326, STY2020, and STY2021) were found to be highly specific for diagnosis of typhoid fever [31]. Only one single target gene chosen often means much lower specificity, like fimC gene for S. Typhi, which still requires improvement in its specificity [32]. The cell surface proteins, which are significantly and differentially expressed between S. Typhi and S. Paratyphi A, were considered to be relevant for the diagnosis of Salmonella [33]. However, diagnostic accuracy of the current rapid diagnostic tests was low, ranging from 31 to 97% [34]. In addition, discriminating S. Paratyphi B from S. Paratyphi B variant Java is important for the food chain industry, because the pathogenicity of these two is totally different. Unfortunately, the SPAB_01124 marker was unable to specifically differentiate all S. Paratyphi B variants from each other [35]. The rfb, fliC, fljB and viaB gene clusters encoding somatic (O) and/or flagellar (H) antigens and/or Vi antigen are often selected as target genes for identification of Salmonella serovars [4]. The caveat is that if one wants to accurately identify nontyphoidal Salmonella, S. Typhi, and S. Paratyphi (A, B, and C), more different groups of strains [at least four O groups (group A, B, C1, D), five H1 groups (phase 1 H types “a”,“b”, “c,”, “d” and “j”) and two H2 groups (phase II H types “1,5″,”1,2″)) will be needed to be differentiated, taking into account the closely related antigenic formula. Furthermore, the possibility for cross-reaction, e.g. S. Cholerasuis vs. S. Paratyphi C and S. Typhisuis, S. Paratyphi B vs. S. Paratyphi B variant Java, makes further identification necessary, which will require at least 15 or more target genes to be included. By sharp contrast, our EFMAtyping assay only needed 7 specific genes to know the presence/absence of typhoidal/nontyphoidal Salmonella and it was done in one tube compared to the mono-, du- or triplex reactions [36]. Our WGS sequencing data also confirmed these molecular markers to be serovar-specific. The EFMAtyping assay could successfully differentiate typhoidal and nontyphoidal Salmonella according to the defined gene profiles.
One limitation of this study is that only one strain each of S. Paratyphi B and C was included for the validation test of the assay sensitivity and specificity, which calls for more S. Paratyphi B and C strains to be tested in the future. Another limitation is the clinical samples (551 isolates) for evaluation were not as many as desired. A rapid and sensitive pathogen detection method would help clinical diagnosis as well as preventing the spread of outbreaks. Clinical specimens often contain a more complex microbial community, which will be a real field test of the specificity and sensitivity of this EFMAtyping method. The presence of low level of Salmonella in bacteremic typhoid patient can give a negative result, particularly if the patient has been treated with antibiotics prior to culturing. Therefore, further studies are needed to evaluate the effectiveness of EFMAtyping method in clinical practice.
Deployment of WGS is becoming increasingly feasible in public health investigations. In contrast to the current gold-standard techniques, WGS method is genetically more informative. Salmonella In Silico Typing Resource (SISTR), or SeqSero2, can be used for serovar prediction from WGS assemblies by determination of antigen gene and cgMLST gene alleles [7, 8]. GenoTyphi and Paratype were specially developed for genotyping S. Typhi and S. Paratyphi A [9, 10]. However, due to constraints of cost, time and bioinformatics resources, WGS typing method remains impractical and challenging for most public health laboratories. Thus, the EFMAtyping assay we herein developed could serve as an effective complementary tool for less developed countries.
Conclusions
In conclusion, this EFMAtyping assay is a suitable screening tool for simultaneous identification of typhoidal/nontyphoidal Salmonella due to its rapidness, ease of use, and robustness. It is anticipated that this assay could play its due role for timely and effective public health intervention of EF.
Supplementary Information
Author contributions
Yixiang Jiang and Biao Kan conceived and designed the study. Yixiang Jiang, Xiaolu Shi, Min Jiang performed the experiments and data analysis. Yixiang Jiang drafted the manuscript. Qinghua Hu revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Sanming Project of Medicine in Shenzhen (SZSM202311015).
Availability of data and materials
Sequence data that support the findings of this study have been deposited in the European Nucleotide Archive with the primary accession code PRJNA1012012.
Declarations
Ethics approval and consent to participate
Not Applicable as the work has been done using archived strains.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qinghua Hu, Email: huqinghua03@163.com.
Biao Kan, Email: kanbiao@icdc.cn.
References
- 1.Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive salmonella infections. Clin Microbiol Rev. 2015;28(4):901–37. 10.1128/CMR.00002-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanaway JD, Reiner RC, Blacker BF, Goldberg EM, Khalil IA, Troeger CE, et al. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2019;19(4):369–81. 10.1016/S1473-3099(18)30685-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabagaran SR, Kalaiselvi V, Chandramouleeswaran N, Deepthi KNG, Brahmadathan KN, Mani M. Molecular diagnosis of Salmonella typhi and its virulence in suspected typhoid blood samples through nested multiplex PCR. J Microbiol Methods. 2017;139:150–4. 10.1016/j.mimet.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 4.Hirose K, Itoh K, Nakajima H, Kurazono T, Yamaguchi M, Moriya K, et al. Selective amplification of tyv (rfbE), prt (rfbS), viaB, and fliC genes by multiplex PCR for identification of Salmonella enterica serovars Typhi and Paratyphi A. J Clin Microbiol. 2002;40(2):633–6. 10.1128/JCM.40.02.633-636.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.HosuruSubramanya S, Khokhar F, Pickard D, Dyson Z, Iqbal J, Pragasam A, et al. Multiplex PCR assay to detect high risk lineages of salmonella Typhi and Paratyphi A. PLoS ONE. 2022;17(7):e0267805. 10.1371/journal.pone.0267805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur A, Ruhela A, Sharma P, Khariwal H, Seth S, Kumar A, et al. Simultaneous and high sensitive detection of Salmonella typhi and Salmonella paratyphi a in human clinical blood samples using an affordable and portable device. Biomed Microdevices. 2019;21(4):95. 10.1007/s10544-019-0441-6 [DOI] [PubMed] [Google Scholar]
- 7.Hensel M, Yoshida CE, Kruczkiewicz P, Laing CR, Lingohr EJ, Gannon VPJ, et al. The salmonella in silico typing resource (SISTR): an open web-accessible tool for rapidly typing and subtyping draft salmonella genome assemblies. PLoS ONE. 2016;11(1):e0147101. 10.1371/journal.pone.0147101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S, den Bakker HC, Li S, Chen J, Dinsmore BA, Lane C, et al. SeqSero2: rapid and improved salmonella serotype determination using whole-genome sequencing data. Appl Environ Microbiol. 2019;85(23):e01746-e1819. 10.1128/AEM.01746-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong VK, Baker S, Connor TR, Pickard D, Page AJ, Dave J, et al. An extended genotyping framework for Salmonella enterica serovar Typhi, the cause of human typhoid. Nat Commun. 2016;7(1):12827. 10.1038/ncomms12827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanmoy AM, Hooda Y, Sajib MSI, da Silva KE, Iqbal J, Qamar FN, et al. Paratype: a genotyping tool for Salmonella Paratyphi A reveals its global genomic diversity. Nat Commun. 2022;13(1):7912. 10.1038/s41467-022-35587-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao Y, Wang X, Sha C, Xia Z, Huang Q, Li Q. Combination of fluorescence color and melting temperature as a two-dimensional label for homogeneous multiplex PCR detection. Nucleic Acids Res. 2013;41(7):e76–e76. 10.1093/nar/gkt004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuo L, Jiang M, Jiang Y, Shi X, Li Y, Lin Y, et al. Multiplex ligation reaction based on probe melting curve analysis: a pragmatic approach for the identification of 30 common Salmonella serovars. Ann Clin Microbiol Antimicrob. 2019;18(1):1–10. 10.1186/s12941-019-0338-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Jiang Y, Shi X, Li Y, Jiang M, Lin Y, et al. Simultaneous identification of clinically common vibrio parahaemolyticus serotypes using probe melting curve analysis. Front Cell Infect Microbiol. 2019;9:385. 10.3389/fcimb.2019.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Liang B, Du C, Tian X, Cai X, Hou Y, et al. Rapid identification of clinically relevant mycobacterium species by multicolor melting curve analysis. J Clin Microbiol. 2019;57(1):e01096-e1118. 10.1128/JCM.01096-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou S, Che J, Wang X, Lin Y, Niu J, Liang W, et al. Identification of pneumococcal serotypes with individual recognition of vaccine types by a highly multiplexed real-time PCR-based MeltArray approach. J Microbiol Immunol Infect. 2023;57(1):107–17. 10.1016/j.jmii.2023.10.008 [DOI] [PubMed] [Google Scholar]
- 16.Hopkins KL, Peters TM, Lawson AJ, Owen RJ. Rapid identification of Salmonella enterica subsp. arizonae and S. enterica subsp diarizonae by real-time polymerase chain reaction. Diagn Microbiol Infect Dis. 2009;64(4):452–4. 10.1016/j.diagmicrobio.2009.03.022 [DOI] [PubMed] [Google Scholar]
- 17.Nair S, Alokam S, Kothapalli S, Porwollik S, Proctor E, Choy C, et al. Salmonella enterica serovar Typhi strains from which SPI7, a 134-kilobase island with genes for Vi exopolysaccharide and other functions, has been deleted. J Bacteriol. 2004;186(10):3214–23. 10.1128/JB.186.10.3214-3223.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nga TVT, Karkey A, Dongol S, Thuy HN, Dunstan S, Holt K, et al. The sensitivity of real-time PCR amplification targeting invasive Salmonellaserovars in biological specimens. BMC Infect Dis. 2010;10(1):1–9. 10.1186/1471-2334-10-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connor TR, Owen SV, Langridge G, Connell S, Nair S, Reuter S, et al. What’s in a Name? Species-wide whole-genome sequencing resolves invasive and noninvasive lineages of salmonella enterica serotype Paratyphi B. MBio. 2016;7(4):e00527-e616. 10.1128/mBio.00527-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horsburgh MJ, Liu W-Q, Feng Y, Wang Y, Zou Q-H, Chen F, et al. Salmonella paratyphi C: genetic divergence from salmonella choleraesuis and pathogenic convergence with Salmonella typhi. PLoS ONE. 2009;4(2):e4510. 10.1371/journal.pone.0004510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Q, Chen D, Du C, Liu Q, Lin S, Liang L, et al. Highly multiplex PCR assays by coupling the 5′-flap endonuclease activity of Taq DNA polymerase and molecular beacon reporters. Proc Natl Acad Sci U S A. 2022;119(9):e2110672119. 10.1073/pnas.2110672119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan T, Xu Y, Zheng R, Zeng X, Chen Z, Lin S, et al. Accessible and adaptable multiplexed real-time PCR approaches to identify SARS-CoV-2 variants of concern. Microbiol Spectr. 2022;10(5):e0322222. 10.1128/spectrum.03222-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou S, Che J, Wang X, Lin Y, Niu J, Liang W, et al. Identification of pneumococcal serotypes with individual recognition of vaccine types by a highly multiplexed real-time PCR-based MeltArray approach. J Microbiol Immunol Infect. 2024;57(1):107–17. 10.1016/j.jmii.2023.10.008 [DOI] [PubMed] [Google Scholar]
- 24.Nurjayadi M, Pertiwi YP, Islami N, Azizah N, Efrianti UR, Saamia V, et al. Detection of the Salmonella typhi bacteria in contaminated egg using real-time PCR to develop rapid detection of food poisoning bacteria. Biocatal Agric Biotechnol. 2019;20:10124. 10.1016/j.bcab.2019.101214 [DOI] [Google Scholar]
- 25.Yin Ngan GJ, Ng LM, Lin RTP, Teo JWP. Development of a novel multiplex PCR for the detection and differentiation of Salmonella enterica serovars Typhi and Paratyphi A. Res Microbiol. 2010;161(4):243–8. 10.1016/j.resmic.2010.03.005 [DOI] [PubMed] [Google Scholar]
- 26.Ryan ET, Fabre L, Le Hello S, Roux C, Issenhuth-Jeanjean S, Weill F-X. CRISPR is an optimal target for the design of specific PCR assays for salmonella Enterica serotypes Typhi and Paratyphi A. PLoS Negl Trop Dis. 2014;8(1):e2671. 10.1371/journal.pntd.0002671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Y, He L, Wu P, Shi X, Jiang M, Li Y, et al. Simultaneous identification of ten bacterial pathogens using the multiplex ligation reaction based on the probe melting curve analysis. Sci Rep. 2017;7(1):5902. 10.1038/s41598-017-06348-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu L, Li M, Li Y, Jiang M, Jiang Y, Shi X, et al. A novel molecular method for simultaneous identification of vibrio parahaemolyticus 57 K-serogroups using probe melting curve analysis. Front Cell Infect Microbiol. 2021;11:594808. 10.3389/fcimb.2021.594808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy H, Diallo S, Tennant SM, Livio S, Sow SO, Tapia M, et al. PCR method to identify salmonella Enterica Serovars Typhi, Paratyphi A, and Paratyphi B among salmonella isolates from the blood of patients with clinical enteric fever. J Clin Microbiol. 2008;46(5):1861–6. 10.1128/JCM.00109-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teh CSJ, Lau MY, Chong CW, Ngoi ST, Chua KH, Lee WS, et al. One-step differential detection of Salmonella enterica serovar Typhi, serovar Paratyphi A and other Salmonella spp by using a quadruplex real-time PCR assay. J Microbiol Methods. 2021;183:106184. 10.1016/j.mimet.2021.106184 [DOI] [PubMed] [Google Scholar]
- 31.Goay YX, Chin KL, Tan CL, Yeoh CY, Ja’afar JN, Zaidah AR, et al. Identification of five novel salmonella typhi-specific genes as markers for diagnosis of typhoid fever using single-gene target PCR assays. Biomed Res Int. 2016;2016:8905675. 10.1155/2016/8905675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nurjayadi M, Islami N, Pertiwi YP, Saamia V, Wirana IM. Evaluation of primer detection capabilities of fimC Salmonella typhi using real time PCR for rapid detection of bacteria causes of food poisoning. IOP Conf Series Mater Sci Eng. 2018;434:012097. 10.1088/1757-899X/434/1/012097 [DOI] [Google Scholar]
- 33.Saleh S, Van Puyvelde S, Staes A, Timmerman E, Barbé B, Jacobs J, et al. Salmonella Typhi, Paratyphi A, Enteritidis and Typhimurium core proteomes reveal differentially expressed proteins linked to the cell surface and pathogenicity. PLoS Negl Trop Dis. 2019;13(5):e0007416. 10.1371/journal.pntd.0007416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thriemer K, Ley B, Menten J, Jacobs J, van den Ende J. A systematic review and meta-analysis of the performance of two point of care typhoid fever tests, Tubex TF and Typhidot, in endemic countries. PLoS ONE. 2013;8(12):e81263. 10.1371/journal.pone.0081263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gand M, Mattheus W, Saltykova A, Roosens N, Dierick K, Marchal K, et al. Development of a real-time PCR method for the genoserotyping of Salmonella Paratyphi B variant Java. Appl Microbiol Biotechnol. 2019;103(12):4987–96. 10.1007/s00253-019-09854-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nair S, Patel V, Hickey T, Maguire C, Greig DR, Lee W, et al. Real-time PCR assay for differentiation of typhoidal and nontyphoidal salmonella. J Clin Microbiol. 2019;57(8):e00167-e219. 10.1128/JCM.00167-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data that support the findings of this study have been deposited in the European Nucleotide Archive with the primary accession code PRJNA1012012.