Abstract
Methionine, an indispensable amino acid crucial for dietary balance, intricately governs metabolic pathways. Disruption in its equilibrium has the potential to heighten homocysteine levels in both plasma and tissues, posing a conceivable risk of inducing inflammation and detriment to the integrity of vascular endothelial cells. The intricate interplay between methionine metabolism, with a specific focus on S-adenosyl-L-methionine (SAM), and the onset of thoracic aortic dissection (TAD) remains enigmatic despite acknowledging the pivotal role of inflammation in this vascular condition. In an established murine model induced by β-aminopropionitrile monofumarate (BAPN), we delved into the repercussions of supplementing with S-adenosyl-L-methionine (SAM) on the progression of TAD. Our observations uncovered a noteworthy improvement in aortic dissection and rupture rates, accompanied by a marked reduction in mortality upon SAM supplementation. Notably, SAM supplementation exhibited a considerable protective effect against BAPN-induced degradation of elastin and the extracellular matrix. Furthermore, SAM supplementation demonstrated a robust inhibitory influence on the infiltration of immune cells, particularly neutrophils and macrophages. It also manifested a notable reduction in the inflammatory polarization of macrophages, evident through diminished accumulation of MHC-IIhigh macrophages and reduced expression of inflammatory cytokines such as IL1β and TNFα in macrophages. Simultaneously, SAM supplementation exerted a suppressive effect on the activation of CD4 + and CD8 + T cells within the aorta. This was evidenced by an elevated proportion of CD44- CD62L + naïve T cells and a concurrent decrease in CD44 + CD62L- effector T cells. In summary, our findings strongly suggest that the supplementation of SAM exhibits remarkable efficacy in alleviating BAPN-induced aortic inflammation, consequently impeding the progression of thoracic aortic dissection.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12986-024-00837-5.
Keywords: Aortic dissection, S-adenosyl-L-methionine, Inflammation, Macrophage polarization, T cell activation
Introduction
The weakening of the vascular wall under high intraluminal pressure can lead to the development of aortic aneurysm/dissection (AAD). This condition involves the dilation of the aortic wall (aneurysm), disruption of the medial layer (dissection), or, in severe instances, rupture of the aneurysm, potentially resulting in sudden death [1]. In males aged 65 years or older, the occurrence of AAD can reach as high as 9%2. Immediate surgical intervention is critical, given that AAD rupture leads to mortality rates exceeding 80% in the absence of such intervention [2]. Several factors, including hypertension, dyslipidemia, atherosclerosis, smoking, and male gender, contribute to AAD development. [1, 3–5]. Current clinical management heavily relies on surgical procedures, underscoring the imperative to explore new therapeutic avenues due to the limited efficacy of existing pharmacological treatments.
Research indicates that inflammatory processes play a role in the restructuring of arterial walls [6–8], including those in the ascending portion of the thoracic aorta [9, 10]. Various inflammatory cells, such as neutrophils, T cells, B cells, macrophages, mast cells, and NK cells, infiltrate both the luminal thrombus and all layers of the arterial wall [11, 12]. These cells discharge soluble inflammatory substances like cytokines, chemokines, leukotrienes, reactive oxygen species, and immunoglobulins. The vasa vasorum vessels act as conduits facilitating the infiltration of inflammatory cells into the aortic intima and media [13]. In individuals with type-A Stanford dissection, there is an increased expression of pro-inflammatory cytokine genes [10], suggesting that inflammation might play a role in weakening the aortic wall at various dissection sites.
Methionine (Met), as an essential amino acid in animals, holds significant relevance to physiological processes such as immune function, protein synthesis, growth promotion, and detoxification [14]. Through conversion to S-adenosylmethionine (SAM), Met serves as a crucial methyl donor, playing a pivotal role in immune disorders due to its involvement in epigenetic regulation, particularly DNA methylation [15, 16]. DNA methylation, a key mammalian epigenetic mechanism, is implicated in inflammatory responses, a vital biological defense mechanism against harmful intruders. Methylation profiles on cytosine-phosphate–guanine (CpG) regions are linked to genes associated with inflammatory responses [17, 18]. Macrophages, integral components of the innate immune system, play crucial roles in responding to bacterial and viral pathogens [19]. Activation of macrophages by external stimuli, including lipopolysaccharides (LPS), a Gram-negative bacterial outer membrane component, triggers the Toll-like receptor-4 signaling pathway, activating mitogen-activated protein kinases (MAPKs) and leading to the secretion of inflammatory cytokines [20]. Previous studies have highlighted the regulatory role of DNA methylation in LPS-induced macrophage inflammatory responses, maintaining intestinal homeostasis and regulating mucosal inflammation in the gut [21]. Furthermore, Methionine has demonstrated the ability to inhibit LPS-induced expression of inflammation-related genes in macrophage cells [22]. SAM exerts its anti-inflammatory effects by downregulating the expression of the proinflammatory cytokine tumor necrosis factor-alpha (TNF) [23] and upregulating the anti-inflammatory cytokine interleukin 10 (IL-10) [24]. Acting as a primary methyl donor in cellular processes, SAM influences gene expression through epigenetic mechanisms, including DNA, RNA, and histone methylation. However, the formation of S-adenosylhomocysteine (SAH) [25], an inhibitor of SAM-dependent methylation processes, poses a regulatory challenge. SAM is also involved in polyamine biosynthesis and the transsulfuration pathway, contributing to the production of the antioxidant glutathione. SAM’s anti-inflammatory effects may be mediated through modifications in histone methylation and binding to the promoter regions of cytokine genes [23]. Specifically, SAM influences histone H3 methylation and binding to the promoter region of the phosphodiesterase 4B gene (PDE4B), affecting cAMP-mediated TNF expression [26].
Although methionine metabolites have been extensively examined in the context of inflammation, the role of SAM in aortic dissection (AD) remains notably underexplored. Our study is dedicated to investigating the potential involvement of SAM in the progression of aortic dissection.
Materials and methods
Animals
Four-week-old male C57BL/6 N mice were sourced from Shanghai Biomodel Organism Co, Shanghai, China, and housed in ventilated cages under sterile conditions with free access to food and water. Continuous monitoring of their weight, behavior, and food and water intake ensured their well-being throughout the study. Ethical approval for all animal procedures was obtained from the Committee on the Ethics of Animal Experiments of The First Affiliated Hospital of Nanchang University. In experiments involving SAM supplementation, mice were orally administered commercial ademetionine 1,4-butanedisulfonate enteric coated tablets (XiMeiXin, SFDA approval number: H20133197) at a dosage of 500 mg/kg (effective concentration is 50 mg/kg) for one week prior to modeling [27]. SAM supplementation continued daily during the induction period with β-aminopropionitrile monofumarate (BAPN) until the designated observation and sampling time. To induce aortic dissection (TAD), four-week-old male C57BL/6 mice were provided with drinking water containing BAPN (0.5%; A3134, Sigma-Aldrich, St. Louis, MO, USA) for 28 days. [28]. The mice were categorized into four groups: Control (n = 5), SAM (n = 5), BAPN (n = 20), and BAPN + SAM (n = 20). Following the 28-day period, the mice were euthanized, and their aortas were harvested. All animals used in the experiments were male to minimize variations in sex hormones and to enhance the incidence of TAD.
Tissue fixation and paraffin embedding
After isolating the aorta, submerge it in 4% neutral buffered formalin (NBF) at a volume at least ten times that of the tissue for 24–48 h at room temperature to ensure proper fixation. Rinse the fixed tissue in running water for 1–2 h to remove excess fixative, then dehydrate it through a graded ethanol series (70%, 80%, 95%, 100%). Clear the tissue in xylene to remove the ethanol and achieve transparency, followed by immersion in molten paraffin wax for thorough impregnation over several hours. Embed the tissue in a paraffin wax block, ensuring correct orientation in the mold, and allow it to solidify at room temperature. Trim the excess paraffin to create a flat surface, secure the block on a microtome chuck, and cut thin Sects. (4–5 micrometers) with a sharp blade. Float the sections on a warm water bath to flatten and stretch them, transfer onto glass slides, and let them air dry overnight at room temperature.
Hematoxylin and eosin (HE) staining, elastic van gieson (EVG) staining and immunofluorescence staining (IF)
Sections of mouse aortic tissues, cut into 5 μm thickness, underwent paraffin section staining post-deparaffinization. Hematoxylin and eosin staining (G1120, Solarbio) and elastic Van Gieson staining (G1593, Solarbio) were executed adhering to the manufacturer’s guidelines. Initially, deparaffinization and rehydration of aortic tissue sections mounted on slides were performed. Subsequently, the sections were stained with hematoxylin solution to highlight nuclei (blue color), followed by a water wash. Further differentiation was carried out with a 1% acid alcohol solution, and slides were rinsed with water. Eosin solution was then applied for counterstaining to visualize cytoplasm and extracellular matrix (pink color). Slides were dehydrated in alcohol, cleared in xylene, and mounted with a mounting medium. For EVG staining, tissue sections were deparaffinized and hydrated before staining with Weigert’s iron hematoxylin for nuclear visualization (blue-black color). Following a water wash, differentiation with 1% acid alcohol solution was performed, followed by rinsing with water. Van Gieson’s solution (comprising acid fuchsin and picric acid) was then applied to stain elastic fibers (dark brown color). Slides were dehydrated in alcohol, cleared in xylene, and mounted with a mounting medium. Imaging of the stained sections was conducted using a bright field camera attached to a microscope (Leica, Wetzlar, Germany).
For the immunofluorescence staining of collagen, antigen retrieval was performed by heating in citrate buffer at approximately 90 degrees Celsius (pH 6.0). After this step, sections were blocked with 1% bovine serum albumin in PBS for 30 min at room temperature. Subsequently, the sections were subjected to overnight incubation at 4 °C with rabbit anti-COL1A1 primary antibodies (Novus, NBP1-30054, diluted 1:50). Following PBS rinsing of the slides, the sections were treated with Alexa Fluor 488-conjugated secondary antibodies (donkey anti-rabbit, Invitrogen, A32790, diluted 1:500) at room temperature for 2 h. After another round of PBS rinsing, the slides were mounted with a DAPI medium. Imaging was performed using an Olympus BX43 fluorescent microscope, and analysis was conducted using the ImageJ Analysis software.
Blood pressure measurement
Following a 4-week BAPN modeling period, mice underwent noninvasive tail-cuff blood pressure measurements utilizing the Softron bp-2010 mouse blood pressure monitor, following procedures outlined in a previous study [29]. Before conducting measurements, it’s important to acclimate the mice to the restraint device and the operator’s handling, which helps minimize stress during the procedure. Ensure the room is adequately warmed to maintain the mice’s body temperature. Place the mouse in a suitable restrainer, ensuring it is comfortable and its tail is immobilized. Select an appropriately sized cuff for the mouse’s tail, ensuring a snug but not overly tight fit. Position the cuff correctly around the base of the mouse’s tail. Initiate the blood pressure monitoring system and allow it to stabilize. Inflate the cuff to a predetermined pressure level, usually above systolic pressure, to temporarily halt blood flow in the tail artery. Gradually deflate the cuff while monitoring blood flow using a sensor placed distally to the cuff. Record the pressure when blood flow is detected, indicating the systolic blood pressure (SBP). Continue deflating until blood flow is fully restored, noting the pressure at which blood flow first returns, indicating the diastolic blood pressure (DBP). Conduct at least three measurements to ensure accuracy and calculate the average. Exclude any measurements affected by movement artifacts or irregularities.
Flow cytomertry
Cells were isolated from the aorta, and flow cytometry was performed following established protocols. Briefly, the aorta was minced into small pieces and subjected to digestion in 5 ml of collagenase digestion buffer (HBSS with Ca++/Mg + + from Life Technologies Corporation, supplemented with 1 mg/dL collagenase D from Roche Diagnostics, Germany) at 37 °C for 30 min using a cell dissociator (Miltynyi Biotec). The resulting cells were filtered through a 100 μm cell strainer. Red blood cells (RBC) were lysed using 2 ml of RBC lysis buffer from Life Technologies Corporation. The remaining cells were enumerated using an automated cell counter from Bio-Rad Technologies, Inc. The cell suspensions underwent pre-incubation with anti-mouse CD16/32 antibody to prevent nonspecific binding of antibodies to FcRγ and were then stained with a combination of antibodies (Table 1). To assess cytokine production, single-cell suspensions were stimulated with a cell stimulation cocktail (eBioscience, MA) in RPMI medium containing 10% FBS for two hours at 37 °C with 5% CO2. After stimulation, cells were collected, stained with FVS440UV, blocked with anti-mouse CD16/32, and labeled with fluorescence-conjugated antibodies (Table 1). Samples were then analyzed using the BD FACSymphony™ A3 Cell Analyzer (BD Biosciences, CA), and data were processed using FlowJo_V10 software (FlowJo, OR) by using the gating strategy (Supplementary Figure S1 and S2).
Table 1.
Primary antibodies used for flow cytometry analysis
| Primary antibody | Conjugate | Clone | Vender | Catalog # |
|---|---|---|---|---|
| CD3e | BUV737 | 145-2C11 | BD Biosciences | 612,771 |
| CD4 | BUV496 | GK1.5 | BD Biosciences | 612,952 |
| CD8α | BB790 | 53 − 6.7 | BD Biosciences | 624,296* |
| CD11b | BV650 | M1/70 | Biolegend | 101,259 |
| CD16/32 | - | 93 | Biolegend | 101,302 |
| CD44 | FITC | IM7 | BD Biosciences | 553,133 |
| CD45 | BUV805 | 30-F11 | BD Biosciences | 748,370 |
| CD62L | PE-Cy7 | MEL-14 | Biolegend | 104,418 |
| F4/80 | BUV563 | T45-2342 | BD Biosciences | 749,284 |
| I-A/I-E (MHC-II) | APC-Cy7 | M5/114.15.2 | Biolegend | 107,628 |
| IL-1β | PE-Cy7 | NJTEN3 | eBioscience | 25-7114-82 |
| Ly6G | BV605 | 1A8 | BD Biosciences | 563,005 |
| TNF-α | BV650 | MP6-XT22 | BD Biosciences | 563,943 |
| NK1.1 | APC | PK136 | eBioscience | 17-5941-82 |
| CD19 | BUV395 | 1D3 | BD Biosciences | 563,557 |
| CD11c | BV711 | N418 | Biolegend | 117,349 |
Statistical analysis
The data presented represents the average of five or more biological replicates or independent experiments. Statistical analysis was performed using GraphPad Prism 9.0 software from GraphPad in San Diego, CA, USA. Group differences were assessed using a one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test for experiments with three or more groups. Survival rates were evaluated using the Kaplan-Meier method, and comparisons were made using the log-rank test. Significance levels were indicated as * for p < 0.05, ** for p < 0.01, and *** for p < 0.001.
Results
S-adenosyl-L-methionine (SAM) exhibits pronounced protection against thoracic aortic dissection (TAD) initiated by β-aminopropionitrile monofumarate (BAPN) in mice
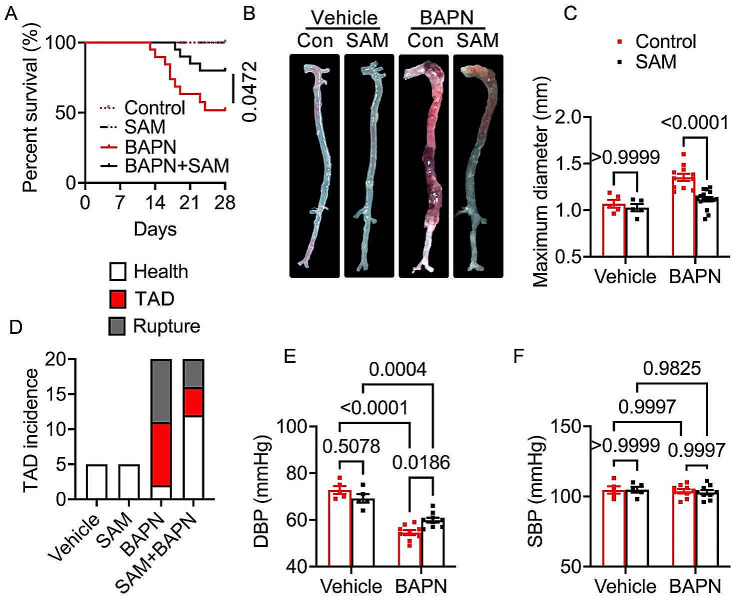
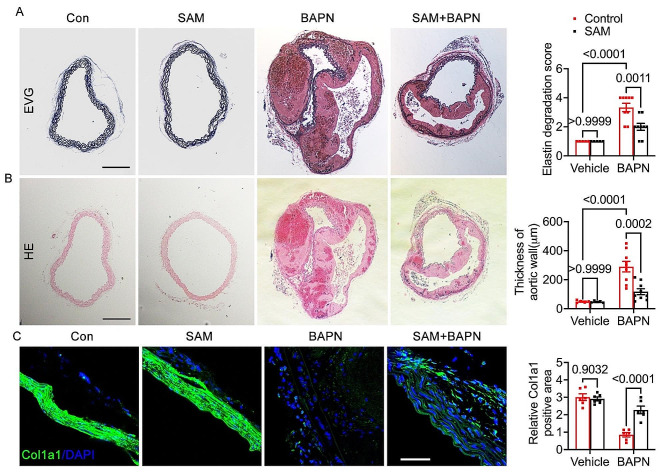
In vivo experiments were conducted to investigate the effects of SAM on TAD using a BAPN-induced TAD mouse model. The mice were divided into four groups: Control group (n = 5), SAM treated group (n = 5), BAPN group (n = 20), and SAM + BAPN group (n = 20). The survival analysis revealed a noteworthy enhancement in survival rate with SAM treatment compared to the BAPN group (Fig. 1A). SAM treatment also significantly attenuated TAD development, resulting in a reduction in maximal aortic diameters (Fig. 1B, C). Throughout the 28-day BAPN administration period, 45% (n = 9) of the BAPN group mice and 20% (n = 4) of the BAPN + SAM group mice experienced rupture (Fig. 1D). Additionally, 45% (n = 9) of the BAPN group mice and 20% (n = 4) of the BAPN + SAM group mice exhibited TAD without rupture (Fig. 1D). Furthermore, BAPN treatment led to a decrease in diastolic blood pressure (DBP) (Fig. 1E) due to aortic sclerosis post-TAD, while systolic blood pressure (SBP) remained relatively stable (Fig. 1F). SAM treatment effectively restored DBP in mice (Fig. 1E). Elastic fibers and collagen fibers are crucial components of the extracellular matrix (ECM) in arteries. TAD occurrence is associated with a decrease in total elastin content and elastic fiber cross-links. Collagen expression diminishes, and the resulting disordered deposition may indicate a gradual reparative process triggered by elastic fiber fragmentation and depletion [28]. Consequently, a pathological staining analysis was conducted. EVG staining revealed that SAM treatment mitigated BAPN-induced elastic fiber fragmentation and disarray (Fig. 2A). HE staining demonstrated alleviation of BAPN-induced dissecting aneurysm formation in the BAPN + SAM group compared to the BAPN group (Fig. 2B). BAPN-induced mice exhibited characteristic features of ECM degradation, and immunofluorescence staining of COL1A1 indicated that SAM treatment alleviated these features (Fig. 2C). These findings underscore the beneficial impact of SAM treatment in preventing TAD formation.
Fig. 1.
SAM supplementation on thoracic aortic dissection incidence. (A) The survival rate was estimated by the Kaplan-Meier method and compared by log-rank test (n = 5 for vehicle and SAM group, n = 20 for BAPN and SAM + BAPN group). (B) Representative macrographs of the aorta. (C) maximum diameter. (D) TAD incidence. (E), Diastolic Blood Pressure. (F) Systolic Blood Pressure
Fig. 2.
Pathological staining analysis of 28-day animal experiment induced by BAPN. (A), representative images from elastic van Gieson (EVG) staining, along with statistical analysis of elastin degradation. (B), representative images from hematoxylin and eosin (HE) staining are presented, accompanied by statistical analysis of aortic wall thickness. The scale bar for both panels (A) and (B) is 200 μm. (C), immunofluorescence staining of Col1a1, accompanied by quantitative analysis of the Col1a1 positive area in the vascular wall, scale bar 50 μm
SAM suppresses infiltration of neutrophils and macrophages
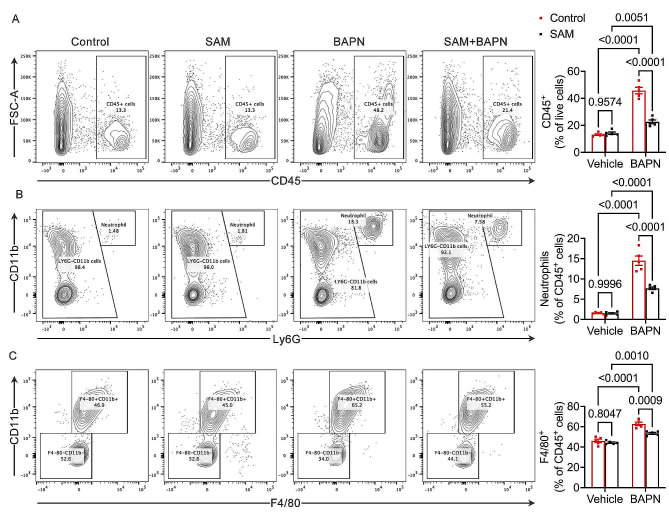
Acute aortic dissection can be triggered by Neutrophil-Derived Matrix Metalloproteinase-9 [30]. This condition is marked by the initial accumulation of macrophages in the aortic adventitia, followed by their infiltration into the media. Subsequently, this process fosters a localized inflammatory response within the aorta [31]. Our study delved into the dynamics of aortic leukocyte infiltration, including neutrophils and macrophages, in mice. In response to BAPN-induced vascular injury, a substantial accumulation of aortic CD45 + leukocytes were observed in control mice compared to the baseline group. However, intriguingly, supplementation with SAM markedly reduced the accumulation of aortic CD45 + leukocytes (Fig. 3A). Furthermore, BAPN administration elicited a significant increase in aortic neutrophil and macrophage infiltration in control mice relative to the baseline group. Remarkably, the SAM-supplemented group exhibited significant mitigation of BAPN-induced accumulation of aortic neutrophils and macrophages (Fig. 3B, C). These results collectively suggest a potential role for SAM in curbing the infiltration of inflammatory leukocytes into the aorta, thereby potentially ameliorating aortic degeneration.
Fig. 3.
SAM suppressed the leukocytes infiltration. (A), Flow cytometry plots for CD45 + leukocytes in the aorta and percentage of CD45 + cells within live cells. (B), Flow cytometry plots for CD11b + and Ly6G + positive neutrophils in the aorta and percentage of neutrophils within CD45 + leucocytes. (C), Flow cytometry plots for CD11b + and F4/80 + positive macrophages in the aorta and percentage of macrophages within CD45 + leucocytes. n = 5 for each group
SAM blunts M1 macrophage polarization in aortic wall
During periods of stress, macrophages from peripheral regions have a tendency to cluster around the aortic wall [32, 33]. M0 macrophages, in response to stimuli, undergo a shift in polarization, evolving into the pro-inflammatory M1 phenotype. These M1 macrophages play a pivotal role in breaking down the extracellular matrix by secreting matrix metalloproteinases (MMPs) and inflammatory mediators including IL-2, IL-1β, MCP-1, and IFN-γ. This process significantly contributes to the progression of aortic dissection [34].
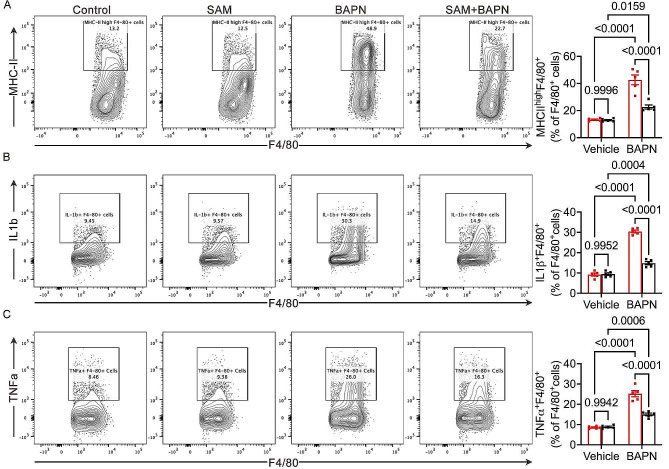
To delve deeper into the mechanism by which SAM reduces the risk of aortic dissection (AD) formation, we assessed the inflammatory status of macrophages. MHCII expression in macrophages mirrors their antigen-presenting capacity and polarization. We determined macrophage MHCII expression and the percentage of MHCIIhighF4/80 + cells. Intriguingly, we observed a significant increase in the percentages of aortic MHCIIhighF4/80 + macrophages within F4/80 + cells in control mice after BAPN treatment. However, SAM supplementation reversed the BAPN-induced increase in the percentage of MHCIIhighF4/80 + macrophages (Fig. 4A). Furthermore, we aimed to identify macrophage subsets producing IL-1β and TNF-α. Freshly isolated aortic cells were stimulated with a PMA cocktail, and IL-1β + and TNF-α + macrophages were determined using flow cytometry. We found that the percentage of aortic IL-1β + and TNF-α + F4/80 + macrophages significantly increased in BAPN-induced control mice compared to the control baseline group. However, SAM supplementation markedly reduced the BAPN-induced elevation in the percentage of IL-1β + and TNF-α + F4/80 + macrophages (Fig. 4B, C). These results indicate that SAM treatment can mitigate the inflammatory phenotype of macrophages, thereby attenuating the development of thoracic aortic dissection (TAD).
Fig. 4.
SAM blunts M1 macrophage polarization. (A), Flow cytometry plots for the identification of MHCIIhighF4/80 + macrophages and Quantified percentage of MHCIIhighF4/80 + macrophages within F4/80 + macrophages. (B), Representative images of flow cytometry plots of and relative percentage of IL1b + cells in the F4/80 + subset. (C), Representative images of flow cytometry plots of and relative percentage of TNFα + cells in the F4/80 + subset. n = 5 per group, two-way ANOVA with Bonferroni multiple comparison test
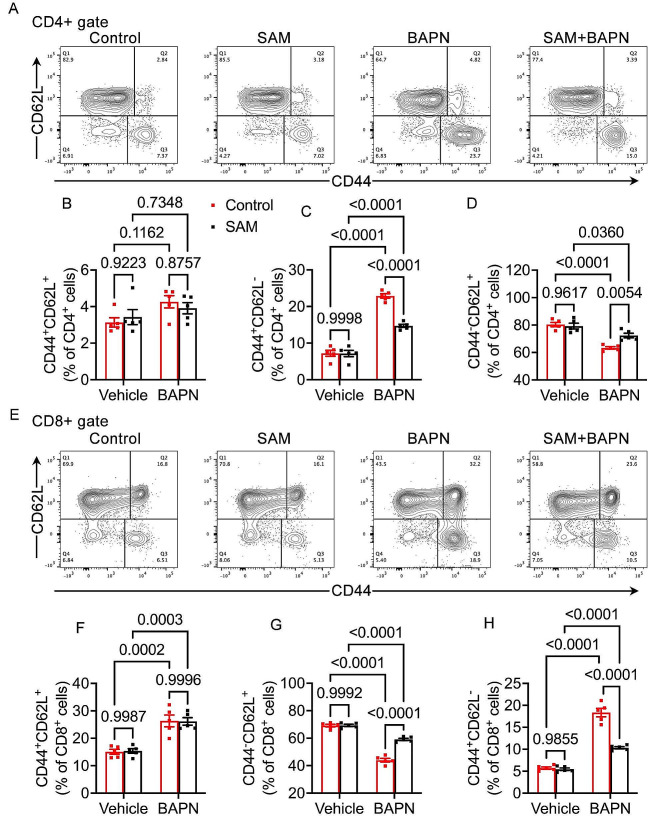
SAM alleviates CD4 + and CD8 + T cells activation in the aorta
T cells have been demonstrated to contribute to aortic dissection (AD) development [35, 36] by inducing vascular smooth muscle cell (VSMC) apoptosis and promoting matrix metalloproteinase (MMP) synthesis [37]. Clinical studies in AD patients reveal elevated levels of CD3+, CD4+, and CD8 + cells in aortic tissue, indicating the involvement of T cell activation in AD development [9]. SAM exhibits dual roles in regulating both the activation and functional capacity of T cells [38]. We delved deeper into the impact of SAM supplementation on the activation of CD4 + and CD8 + T cells. Our investigation unveiled that SAM supplementation markedly counteracted BAPN-induced activation of CD4 + T cells (Fig. 5A-D). This was evidenced by a notable increase in the proportion of effector memory CD4 + T cells (CD44 + CD62L−) (Fig. 5C) and a simultaneous decrease in the proportion of naïve CD4 + T cells (CD44-CD62L+) (Fig. 5D) within the CD4 + subset. Remarkably, central memory CD4 + T cells (CD44 + CD62L+) remained largely unaffected (Fig. 5B). Moreover, SAM supplementation exerted a significant dampening effect on BAPN-induced activation in CD8 + T cells (Fig. 5E-H), as indicated by a heightened percentage of effector memory CD8 + T cells (CD44 + CD62L−) (Fig. 5H) and a concomitant decrease in the percentage of naïve CD8 + T cells (CD44-CD62L+) (Fig. 5G) within the CD8 + subset. Interestingly, while BAPN treatment led to elevated levels of central memory CD8 + T cells, no significant disparities in central memory CD8 + T cells (CD44 + CD62L+) were observed between the control and SAM supplement groups, both under normal conditions and in pathological states (Fig. 5F). In conclusion, our study underscores the pivotal role of SAM in curbing the inflammatory response by modulating T cell activation, thereby effectively impeding the progression of aortic dissection (TAD).
Fig. 5.
Effect of SAM on CD4 + and CD8 + T cells activation in the aorta. (A-D) Representative images of flow cytometry plots of pulmonary CD4 + T cells and the relative percentage of naïve (CD44-CD62L + CD4+), effector memory (CD44 + CD62L-CD4+), and central memory (CD44 + CD62L + CD4+) subsets. (E-H) Representative images of flow cytometry plots of and the percentage of pulmonary naïve (CD44-CD62L + CD8+), effector memory (CD44 + CD62L-CD8+), and central memory cells (CD44 + CD62L + CD8+) in CD8 + T cells. n = 5 for each group
Discussion
Our study provides compelling evidence that SAM treatment effectively slows down the progression of thoracic aortic dissection (TAD) in a BAPN-induced TAD model. This research represents the investigation into SAM’s potential role in TAD progression through nutritional intervention. The evidence presented, which includes improved survival rates, reduced incidence of TAD formation and rupture, as well as diminished degradation of elastin fibers and collagen, along with attenuated leukocyte infiltration, strongly supports the effectiveness of SAM intervention in TAD progression. Moreover, our findings suggest that SAM exerts its beneficial effects by mitigating macrophage M1 polarization and T cell activation, which ultimately contributes to the reduction in elastin and collagen degradation. This study underscores the critical role of SAM in TAD progression and highlights a promising nutritional intervention strategy for TAD prevention.
Methionine adenosyltransferase 2 A (MAT2A) stands as a pivotal enzyme in the methionine cycle, chiefly orchestrating the synthesis of S-adenosylmethionine (SAM) from methionine and adenosine triphosphate (ATP) [39]. A growing body of evidence suggests that mutations in MAT2A render individuals more susceptible to thoracic aortic disease [40]. SAM, serving as a vital substrate for methyl transfers to various biomolecules, undergoes a cyclical transformation that produces S-adenosylhomocysteine (SAH). The hydrolysis of SAH then yields homocysteine and adenosine, ultimately leading to the regeneration of methionine and SAM. The balance between SAM and SAH, reflected in the SAM/SAH ratio, is crucial for maintaining appropriate cellular methylation dynamics [41].
Notably, plasma SAH has been positively linked to the risk of cardiovascular events, independent of plasma total homocysteine concentrations, especially in patients undergoing coronary angiography [42]. Previous studies have unveiled associations between elevated plasma SAH concentrations and endothelial dysfunction, atherosclerosis, involving mechanisms such as oxidative stress [43, 44] and endoplasmic reticulum stress [45]. SAM, known for its role in regulating methylation reactions and influencing gene expression in various diseases [23, 46], has been the focus of several retrospective studies in cardiovascular disease (CVD) patients. These studies indicated a decrease in plasma SAM in CVD patients, a positive association with flow-mediated vasodilation (FMD), and an inverse correlation with carotid intima-media thickness (IMT) [47–49]. Additionally, exogenous SAM supplementation has demonstrated promising outcomes by reducing injury-induced carotid intima thickness and enhancing endothelium-dependent vasodilation in mice [50, 51]. However, conflicting reports have surfaced, with some studies suggesting increased or unchanged plasma SAM in patients with CVD [52, 53]. Considering SAM’s crucial role as a methyl donor in virtually all cellular methylation reactions, its deficiency has been associated with abnormal DNA methylation in CVD, as noted in previous case-control studies [53]. Consequently, we formulated a hypothesis positing an inverse association between SAM and the risk of CVD. In this study, we embarked on a prospective evaluation of the relationship between SAM supplementation and the risk of TAD in a mouse model. In corresponding with previous study, SAM supplementation significantly decreased the incidence and mortality of TAD. Previous studies have demonstrated that SAM can partially inhibit the development and progression of AD by attenuating dysregulated cellular autophagy and phenotypic switching in VSMCs [54]. Upon exposure to inflammatory stimuli and injury, VSMCs undergo dysregulation, transitioning from a contractile to a synthetic phenotype. This phenotypic shift is evidenced by the downregulation of contractile proteins (such as α-SMA and SM22α) and a concomitant upregulation of OPN and inflammatory proteins (MMP2 and MMP9) [55, 56]. The transformation of SMC phenotype is driven by inflammatory conditions. Accordingly, our study seeks to elucidate the role of SAM in modulating inflammatory responses during the pathogenesis of TAD.
Examining the intricate connection between methionine metabolism and T cell function reveals a complex interplay that can be harnessed for potential immunomodulation strategies. Higher levels of S-adenosylmethionine (SAM) are linked to a diminished anti-tumor effector function in CD8 + T cells, influencing the histone methylation landscape and suppressing the expression of critical effector genes [57]. Strategically inhibiting the methionine cycle through methionine restriction could potentially enhance the anti-tumor response of CD8 + T cells by inducing epigenetic reprogramming. Additionally, S-adenosylhomocysteine (SAH), a byproduct of SAM, could play a pivotal role in modulating T cell responses. SAH hydrolase (SAHH) promotes the effector function of pro-inflammatory CD4 + T cells, while its product, homocysteine, supports the proliferation and effector function of T cells [58, 59]. This raises the intriguing possibility that SAHH overexpression might augment the anti-tumor effector function of T cells. Interestingly, ethanol-induced suppression of MAT2A expression in CD4 + T cells, resulting in decreased SAM levels and increased T cell activation-induced apoptosis, highlights the critical role of SAM in T cell survival during activation [16]. Conversely, in T cells deficient in autophagy, reduced SAM levels were associated with altered histone methylation patterns, increased expression of effector genes, and enhanced anti-tumor function of CD8 + T cells [57]. This intriguing duality suggests that SAM, under specific metabolic conditions, may exert suppressive functions through the regulation of histone methylation. The role of SAH, generated from SAM by methyltransferases, in regulating the effector function of CD4 + T cells adds another layer of complexity. SAHH inhibitors have shown promise in modulating inflammatory T cells and regulatory T cells in various mouse models, indicating potential therapeutic applications [60–62]. In summary, the interplay between methionine metabolism and T cell function presents a complex landscape with potential implications for immunomodulation. In our study, we detected decreased CD4 + T cells and CD8 + T cells activation which is demonstrated by decreased CD44 + CD62L- effector T cells proportion and increased CD44- CD62L + naive T cells. However, the precise mechanism by which SAM influences the activation of T cells remains to be fully elucidated, warranting further investigation.
A previous investigation explored the effects of SAM on cytokine expression in human THP1 macrophage cells, suggesting its involvement in genome-wide and gene-specific epigenetic modifications. The study proposed that SAM could rapidly influence epigenetic gene regulation within 24 h of exposure, affecting the expression of genes encoding specific inflammatory mediators. Our current study provides further support for these findings, demonstrating a significant reduction in BAPN-induced expression of proinflammatory cytokines TNFα and IL1β by SAM, indicating its broad-ranging impact on inflammation. These results are consistent with earlier research demonstrating SAM’s anti-inflammatory effects in murine macrophages within a BAPN-induced aortic dissection model [63, 64].
Although our research sheds light on the role of SAM in TAD progression through in vivo experiments, uncertainties linger regarding its corresponding role in in vitro cell experiments. The complexities of SAM regulation in TAD, involving immune cells, inflammation-related factors, and VSMCs, pose challenges in conducting meaningful in vitro studies. Furthermore, our deliberate decision to utilize male mice aimed to reduce hormonal variability, suggesting that the results may not directly translate to female mice. Nonetheless, our findings strongly underscore SAM’s pivotal role in improving TAD outcomes.
To summarize, our study presents compelling evidence supporting the protective effects of SAM in TAD development. SAM treatment effectively mitigates leukocyte infiltration, particularly macrophages and neutrophils, reduces aortic inflammation, and suppresses T cell activation, ultimately contributing to the attenuation of TAD progression. These results position SAM as a promising and effective strategy for both the prevention and treatment of TAD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
Author Contributions: Conceptualization, Q.W., A.L. and J.Z.; methodology, Q.W., J.A., W.Z., Y.Z. and J.H.; validation, Q.W., A.L. and J.Z.; formal analysis, Q.W.; investigation, Q.W., J.A., W.Z., Y.Z., J.H. and G.L.; resources, Q.W., A.L. and J.Z.; writing—original draft preparation, Q.W.; writing—review and editing, Q.W., M.W. and L.X.; visualization, Q.W., A.L. and J.Z.; supervision, A.L. and J.Z.; project administration, Q.W., A.L.; funding acquisition, Q.W. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the high-end talent project of science and technology innovation of Jiangxi “double thousand plan” (No. jxsq2023201106), Natural Science Foundation of Jiangxi Province (Grant No. 20204BCJ23017), Science and technology innovation base plan of Jiangxi Province (20212BCD42006), National Natural Science Foundation of China (Grant No.81960061), and Science Foundation of Traditional Chinese Medicine of Jiangxi Provincial Health Commission (Grant No.2022B981).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The animal study protocol (2021-12-002) was approved by the Institutional Review Board of the Animal Care and Use Committee of First Affiliated Hospital of Nanchang University, Jiangxi, China.
Informed consent
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aiping Le, Email: ndyfy00973@ncu.edu.cn.
Jianbing Zhu, Email: ndyfy05336@ncu.edu.cn.
References
- 1.Kim HW, Stansfield BK. Genetic and epigenetic regulation of aortic aneurysms. Biomed Res Int. 2017;2017:7268521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson RW. Detection and management of small aortic aneurysms. N Engl J Med. 2002;346:1484–6. 10.1056/NEJM200205093461910 [DOI] [PubMed] [Google Scholar]
- 3.Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwoger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ, Guidelines ESCCfP. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–926. 10.1093/eurheartj/ehu281 [DOI] [PubMed] [Google Scholar]
- 4.Lee AJ, Fowkes FG, Carson MN, Leng GC, Allan PL. Smoking, atherosclerosis and risk of abdominal aortic aneurysm. Eur Heart J. 1997;18:671–6. 10.1093/oxfordjournals.eurheartj.a015314 [DOI] [PubMed] [Google Scholar]
- 5.Bossone E, LaBounty TM, Eagle KA. Acute aortic syndromes: diagnosis and management, an update. Eur Heart J. 2018;39:739–d749. 10.1093/eurheartj/ehx319 [DOI] [PubMed] [Google Scholar]
- 6.Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111:816–28. 10.1161/01.CIR.0000154569.08857.7A [DOI] [PubMed] [Google Scholar]
- 7.Luo F, Zhou XL, Li JJ, Hui RT. Inflammatory response is associated with aortic dissection. Ageing Res Rev. 2009;8:31–5. 10.1016/j.arr.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 8.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–94. 10.1161/01.ATV.0000214999.12921.4f [DOI] [PubMed] [Google Scholar]
- 9.He R, Guo DC, Estrera AL, Safi HJ, Huynh TT, Yin Z, Cao SN, Lin J, Kurian T, Buja LM, Geng YJ, Milewicz DM. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J Thorac Cardiovasc Surg. 2006;131:671–8. 10.1016/j.jtcvs.2005.09.018 [DOI] [PubMed] [Google Scholar]
- 10.Weis-Muller BT, Modlich O, Drobinskaya I, Unay D, Huber R, Bojar H, Schipke JD, Feindt P, Gams E, Muller W, Goecke T, Sandmann W. Gene expression in acute Stanford type A dissection: a comparative microarray study. J Transl Med. 2006;4:29. 10.1186/1479-5876-4-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanderlaan PA, Reardon CA. Thematic review series: the immune system and atherogenesis. The unusual suspects:an overview of the minor leukocyte populations in atherosclerosis. J Lipid Res. 2005;46:829–38. 10.1194/jlr.R500003-JLR200 [DOI] [PubMed] [Google Scholar]
- 12.Ihara M, Urata H, Kinoshita A, Suzumiya J, Sasaguri M, Kikuchi M, Ideishi M, Arakawa K. Increased chymase-dependent angiotensin II formation in human atherosclerotic aorta. Hypertension. 1999;33:1399–405. 10.1161/01.HYP.33.6.1399 [DOI] [PubMed] [Google Scholar]
- 13.Herron GS, Unemori E, Wong M, Rapp JH, Hibbs MH, Stoney RJ. Connective tissue proteinases and inhibitors in abdominal aortic aneurysms. Involvement of the vasa vasorum in the pathogenesis of aortic aneurysms. Arterioscler Thromb. 1991;11:1667–77. 10.1161/01.ATV.11.6.1667 [DOI] [PubMed] [Google Scholar]
- 14.Dong Z, Sinha R, Richie JP. Jr. Disease prevention and delayed aging by dietary sulfur amino acid restriction: translational implications. Ann N Y Acad Sci. 2018;1418:44–55. 10.1111/nyas.13584 [DOI] [PubMed] [Google Scholar]
- 15.Yang ML, Gee AJ, Gee RJ, Zurita-Lopez CI, Khare S, Clarke SG, Mamula MJ. Lupus autoimmunity altered by cellular methylation metabolism. Autoimmunity. 2013;46:21–31. 10.3109/08916934.2012.732133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hote PT, Sahoo R, Jani TS, Ghare SS, Chen T, Joshi-Barve S, McClain CJ, Barve SS. Ethanol inhibits methionine adenosyltransferase II activity and S-adenosylmethionine biosynthesis and enhances caspase-3-dependent cell death in T lymphocytes: relevance to alcohol-induced immunosuppression. J Nutr Biochem. 2008;19:384–91. 10.1016/j.jnutbio.2007.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Novo CA, Bachert C. DNA methylation, bacteria and airway inflammation: latest insights. Curr Opin Allergy Clin Immunol. 2015;15:27–32. 10.1097/ACI.0000000000000130 [DOI] [PubMed] [Google Scholar]
- 18.Wichnieski C, Maheshwari K, Souza LC, Nieves F, Tartari T, Garlet GP, Carneiro E. Letra A and Silva RM. DNA methylation profiles of immune response-related genes in apical periodontitis. Int Endod J. 2019;52:5–12. 10.1111/iej.12966 [DOI] [PubMed] [Google Scholar]
- 19.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–70. 10.1038/nri2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996;8:402–11. 10.1016/S0952-7915(96)80131-2 [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Sugi Y, Hosono A, Kaminogawa S. Epigenetic regulation of TLR4 gene expression in intestinal epithelial cells for the maintenance of intestinal homeostasis. J Immunol. 2009;183:6522–9. 10.4049/jimmunol.0901271 [DOI] [PubMed] [Google Scholar]
- 22.Shen Y, Yang S, Shi Z, Lin T, Zhu H, Bi F, Liu A, Ying X, Liu H, Yu K, Yan S. SeMet mediates anti-inflammation in LPS-induced U937 cells targeting NF-kappaB signaling pathway. Inflammation. 2015;38:736–44. 10.1007/s10753-014-9984-0 [DOI] [PubMed] [Google Scholar]
- 23.Ara AI, Xia M, Ramani K, Mato JM, Lu SC. S-adenosylmethionine inhibits lipopolysaccharide-induced gene expression via modulation of histone methylation. Hepatology. 2008;47:1655–66. 10.1002/hep.22231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClain CJ, Hill DB, Song Z, Chawla R, Watson WH, Chen T, Barve S. S-Adenosylmethionine, cytokines, and alcoholic liver disease. Alcohol. 2002;27:185–92. 10.1016/S0741-8329(02)00224-0 [DOI] [PubMed] [Google Scholar]
- 25.Song Z, Uriarte S, Sahoo R, Chen T, Barve S, Hill D, McClain. C. S-adenosylmethionine (SAMe) modulates interleukin-10 and interleukin-6, but not TNF, production via the adenosine (A2) receptor. Biochim Biophys Acta. 2005;1743:205–13. 10.1016/j.bbamcr.2004.12.001 [DOI] [PubMed] [Google Scholar]
- 26.Gobejishvili L, Avila DV, Barker DF, Ghare S, Henderson D, Brock GN, Kirpich IA, Joshi-Barve S, Mokshagundam SP, McClain CJ, Barve S. S-adenosylmethionine decreases lipopolysaccharide-induced phosphodiesterase 4B2 and attenuates tumor necrosis factor expression via cAMP/protein kinase A pathway. J Pharmacol Exp Ther. 2011;337:433–43. 10.1124/jpet.110.174268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li ML, Cao SY, Qu J, Zhang L, Gao Q, Wang X, Yin M, Liu Y, Lei MZ, Lei QY. S-adenosyl-L-methionine supplementation alleviates damaged intestinal epithelium and inflammatory infiltration caused by Mat2a deficiency. Development. 2023;150. [DOI] [PubMed]
- 28.Pan L, Bai P, Weng X, Liu J, Chen Y, Chen S, Ma X, Hu K. Sun A and Ge J. Legumain is an endogenous modulator of integrin alphavbeta3 triggering vascular degeneration, dissection, and rupture. Circulation. 2022;145:659–74. 10.1161/CIRCULATIONAHA.121.056640 [DOI] [PubMed] [Google Scholar]
- 29.Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–5. 10.1161/01.HYP.25.5.1111 [DOI] [PubMed] [Google Scholar]
- 30.Kurihara T, Shimizu-Hirota R, Shimoda M, Adachi T, Shimizu H, Weiss SJ, Itoh H, Hori S, Aikawa N, Okada Y. Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection. Circulation. 2012;126:3070–80. 10.1161/CIRCULATIONAHA.112.097097 [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Zhang H, Cao L, He Y, Ma A, Guo W. The role of macrophages in aortic dissection. Front Physiol. 2020;11:54. 10.3389/fphys.2020.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang T, He X, Liu X, Liu Y, Zhang W, Huang Q, Liu W, Xiong L, Tan R, Wang H, Zeng H. Weighted Gene Co-expression Network Analysis identifies FKBP11 as a Key Regulator in Acute Aortic dissection through a NF-kB dependent pathway. Front Physiol. 2017;8:1010. 10.3389/fphys.2017.01010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Son BK, Sawaki D, Tomida S, Fujita D, Aizawa K, Aoki H, Akishita M, Manabe I, Komuro I, Friedman SL, Nagai R. And Suzuki T. Granulocyte macrophage colony-stimulating factor is required for aortic dissection/intramural haematoma. Nat Commun. 2015;6:6994. 10.1038/ncomms7994 [DOI] [PubMed] [Google Scholar]
- 34.Del Porto F, Cifani N, Proietta M, Taurino M. MMP-12 and macrophage activation in acute aortic dissection. Cardiology. 2014;128:314–5. discussion 316. 10.1159/000361039 [DOI] [PubMed] [Google Scholar]
- 35.Watanabe R, Hashimoto M. Vasculitogenic T cells in large Vessel Vasculitis. Front Immunol. 2022;13:923582. 10.3389/fimmu.2022.923582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song M, Deng L, Shen H, Zhang G, Shi H, Zhu E, Xia Q, Han H. Th1, Th2, and Th17 cells are dysregulated, but only Th17 cells relate to C-reactive protein, D-dimer, and mortality risk in Stanford type A aortic dissection patients. J Clin Lab Anal. 2022;36:e24469. 10.1002/jcla.24469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian Z, Zhang P, Li X, Jiang D. Analysis of immunogenic cell death in ascending thoracic aortic aneurysms based on single-cell sequencing data. Front Immunol. 2023;14:1087978. 10.3389/fimmu.2023.1087978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao T, Lum JJ. Methionine cycle-dependent regulation of T cells in cancer immunity. Front Oncol. 2022;12:969563. 10.3389/fonc.2022.969563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C, Gui G, Zhang L, Qin A, Zhou C, Zha X. Overview of methionine adenosyltransferase 2A (MAT2A) as an Anticancer Target: structure, function, and inhibitors. J Med Chem. 2022;65:9531–47. 10.1021/acs.jmedchem.2c00395 [DOI] [PubMed] [Google Scholar]
- 40.Guo DC, Gong L, Regalado ES, Santos-Cortez RL, Zhao R, Cai B, Veeraraghavan S, Prakash SK, Johnson RJ, Muilenburg A, Willing M, Jondeau G, Boileau C, Pannu H, Moran R, Debacker J, GenTac Investigators NHL, Blood Institute Go Exome, Sequencing P, Montalcino Aortic C, Bamshad MJ, Shendure J, Nickerson DA, Leal SM, Raman CS, Swindell EC, Milewicz DM. MAT2A mutations predispose individuals to thoracic aortic aneurysms. Am J Hum Genet. 2015;96:170–7. 10.1016/j.ajhg.2014.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajabian N, Ikhapoh I, Shahini S, Choudhury D, Thiyagarajan R, Shahini A, Kulczyk J, Breed K, Saha S, Mohamed MA, Udin SB, Stablewski A, Seldeen K, Troen BR, Personius K, Andreadis ST. Methionine adenosyltransferase2A inhibition restores metabolism to improve regenerative capacity and strength of aged skeletal muscle. Nat Commun. 2023;14:886. 10.1038/s41467-023-36483-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao Y, Zhang Y, Wang M, Li X, Su D, Qiu J, Li D, Yang Y, Xia M, Ling W. Plasma S-adenosylhomocysteine is associated with the risk of cardiovascular events in patients undergoing coronary angiography: a cohort study. Am J Clin Nutr. 2013;98:1162–9. 10.3945/ajcn.113.058727 [DOI] [PubMed] [Google Scholar]
- 43.Luo X, Xiao Y, Song F, Yang Y, Xia M, Ling W. Increased plasma S-adenosyl-homocysteine levels induce the proliferation and migration of VSMCs through an oxidative stress-ERK1/2 pathway in apoE(-/-) mice. Cardiovasc Res. 2012;95:241–50. 10.1093/cvr/cvs130 [DOI] [PubMed] [Google Scholar]
- 44.Xiao Y, Xia J, Cheng J, Huang H, Zhou Y, Yang X, Su X, Ke Y, Ling W. Inhibition of S-Adenosylhomocysteine hydrolase induces endothelial dysfunction via epigenetic regulation of p66shc-Mediated oxidative stress pathway. Circulation. 2019;139:2260–77. 10.1161/CIRCULATIONAHA.118.036336 [DOI] [PubMed] [Google Scholar]
- 45.Xiao Y, Huang W, Zhang J, Peng C, Xia M, Ling W. Increased plasma S-adenosylhomocysteine-accelerated atherosclerosis is associated with epigenetic regulation of endoplasmic reticulum stress in apoE-/- mice. Arterioscler Thromb Vasc Biol. 2015;35:60–70. 10.1161/ATVBAHA.114.303817 [DOI] [PubMed] [Google Scholar]
- 46.Pfalzer AC, Choi SW, Tammen SA, Park LK, Bottiglieri T, Parnell LD, Lamon-Fava S. S-adenosylmethionine mediates inhibition of inflammatory response and changes in DNA methylation in human macrophages. Physiol Genomics. 2014;46:617–23. 10.1152/physiolgenomics.00056.2014 [DOI] [PubMed] [Google Scholar]
- 47.Hobbs CA, Cleves MA, Melnyk S, Zhao W, James SJ. Congenital heart defects and abnormal maternal biomarkers of methionine and homocysteine metabolism. Am J Clin Nutr. 2005;81:147–53. 10.1093/ajcn/81.1.147 [DOI] [PubMed] [Google Scholar]
- 48.Liu S, Liao R, Dai X, Guo H, Wang D, Xia M, Ling W, Xiao Y. Association between plasma S-adenosylmethionine and risk of mortality in patients with coronary artery disease: a cohort study. Am J Clin Nutr. 2021;114:1360–70. 10.1093/ajcn/nqab210 [DOI] [PubMed] [Google Scholar]
- 49.Becker A, Henry RM, Kostense PJ, Jakobs C, Teerlink T, Zweegman S, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Smulders YM, Stehouwer CD. Plasma homocysteine and S-adenosylmethionine in erythrocytes as determinants of carotid intima-media thickness: different effects in diabetic and non-diabetic individuals. Hoorn Study Atherosclerosis. 2003;169:323–30. 10.1016/S0021-9150(03)00199-0 [DOI] [PubMed] [Google Scholar]
- 50.Lim S, Moon MK, Shin H, Kim TH, Cho BJ, Kim M, Park HS, Choi SH, Ko SH, Chung MH, Lee IK, Jang HC, Kim YB, Park KS. Effect of S-adenosylmethionine on neointimal formation after balloon injury in obese diabetic rats. Cardiovasc Res. 2011;90:383–93. 10.1093/cvr/cvr009 [DOI] [PubMed] [Google Scholar]
- 51.Kim SY, Hong SW, Kim MO, Kim HS, Jang JE, Leem J, Park IS, Lee KU, Koh EH. S-adenosyl methionine prevents endothelial dysfunction by inducing heme oxygenase-1 in vascular endothelial cells. Mol Cells. 2013;36:376–84. 10.1007/s10059-013-0210-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kerins DM, Koury MJ, Capdevila A, Rana S, Wagner C. Plasma S-adenosylhomocysteine is a more sensitive indicator of cardiovascular disease than plasma homocysteine. Am J Clin Nutr. 2001;74:723–9. 10.1093/ajcn/74.6.723 [DOI] [PubMed] [Google Scholar]
- 53.Castro R, Rivera I, Struys EA, Jansen EE, Ravasco P, Camilo ME, Blom HJ. Jakobs C and Tavares De Almeida I. increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin Chem. 2003;49:1292–6. 10.1373/49.8.1292 [DOI] [PubMed] [Google Scholar]
- 54.Shen X, Xie X, Wu Q, Shi F, Chen Y, Yuan S, Xing K, Li X, Zhu Q, Li B, Wang Z. S-adenosylmethionine attenuates angiotensin II-induced aortic dissection formation by inhibiting vascular smooth muscle cell phenotypic switch and autophagy. Biochem Pharmacol. 2024;219:115967. 10.1016/j.bcp.2023.115967 [DOI] [PubMed] [Google Scholar]
- 55.Ailawadi G, Moehle CW, Pei H, Walton SP, Yang Z, Kron IL, Lau CL, Owens GK. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J Thorac Cardiovasc Surg. 2009;138:1392–9. 10.1016/j.jtcvs.2009.07.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petsophonsakul P, Furmanik M, Forsythe R, Dweck M, Schurink GW, Natour E, Reutelingsperger C, Jacobs M, Mees B, Schurgers L. Role of vascular smooth muscle cell phenotypic switching and calcification in aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2019;39:1351–68. 10.1161/ATVBAHA.119.312787 [DOI] [PubMed] [Google Scholar]
- 57.DeVorkin L, Pavey N, Carleton G, Comber A, Ho C, Lim J, McNamara E, Huang H, Kim P, Zacharias LG, Mizushima N, Saitoh T, Akira S, Beckham W, Lorzadeh A, Moksa M, Cao Q, Murthy A, Hirst M, DeBerardinis RJ, Lum JJ. Autophagy regulation of metabolism is required for CD8(+) T cell anti-tumor immunity. Cell Rep. 2019;27:502–13. e5. 10.1016/j.celrep.2019.03.037 [DOI] [PubMed] [Google Scholar]
- 58.Huang Y, Wang S, Ding X, Wu C, Chen J, Hu Z, Du X, Wang G. Inhibition of S-adenosyl-L-homocysteine hydrolase alleviates alloimmune response by down-regulating CD4(+) T-cell activation in a mouse heart transplantation model. Ann Transl Med. 2020;8:1582. 10.21037/atm-20-2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng J, Lu S, Ding Y, Zheng M, Wang X. Homocysteine activates T cells by enhancing endoplasmic reticulum-mitochondria coupling and increasing mitochondrial respiration. Protein Cell. 2016;7:391–402. 10.1007/s13238-016-0245-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fu YF, Zhu YN, Ni J, Zhong XG, Tang W, Re YD, Shi LP, Wan J, Yang YF, Yuan C, Nan FJ. Lawson BR and Zuo JP. A reversible S-adenosyl-L-homocysteine hydrolase inhibitor ameliorates experimental autoimmune encephalomyelitis by inhibiting T cell activation. J Pharmacol Exp Ther. 2006;319:799–808. 10.1124/jpet.106.107185 [DOI] [PubMed] [Google Scholar]
- 61.Wolos JA, Frondorf KA, Babcock GF, Stripp SA, Bowlin TL. Immunomodulation by an inhibitor of S-adenosyl-L-homocysteine hydrolase: inhibition of in vitro and in vivo allogeneic responses. Cell Immunol. 1993;149:402–8. 10.1006/cimm.1993.1165 [DOI] [PubMed] [Google Scholar]
- 62.Wolos JA, Frondorf KA, Esser RE. Immunosuppression mediated by an inhibitor of S-adenosyl-L-homocysteine hydrolase. Prevention and treatment of collagen-induced arthritis. J Immunol. 1993;151:526–34. 10.4049/jimmunol.151.1.526 [DOI] [PubMed] [Google Scholar]
- 63.Song Z, Barve S, Chen T, Nelson W, Uriarte S, Hill D, McClain. C. S-adenosylmethionine (AdoMet) modulates endotoxin stimulated interleukin-10 production in monocytes. Am J Physiol Gastrointest Liver Physiol. 2003;284:G949–55. 10.1152/ajpgi.00426.2002 [DOI] [PubMed] [Google Scholar]
- 64.Watson WH, Zhao Y, Chawla RK. S-adenosylmethionine attenuates the lipopolysaccharide-induced expression of the gene for tumour necrosis factor alpha. Biochem J. 1999;342(Pt 1):21–5. 10.1042/bj3420021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.