Abstract
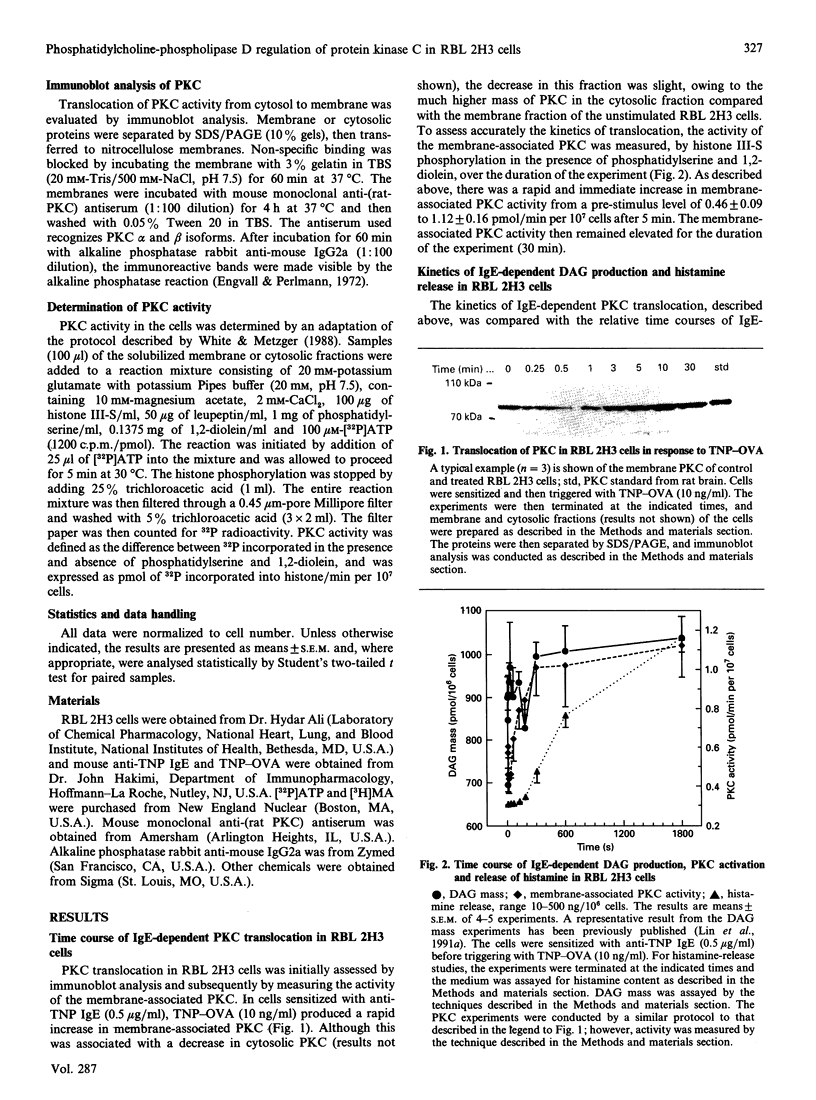
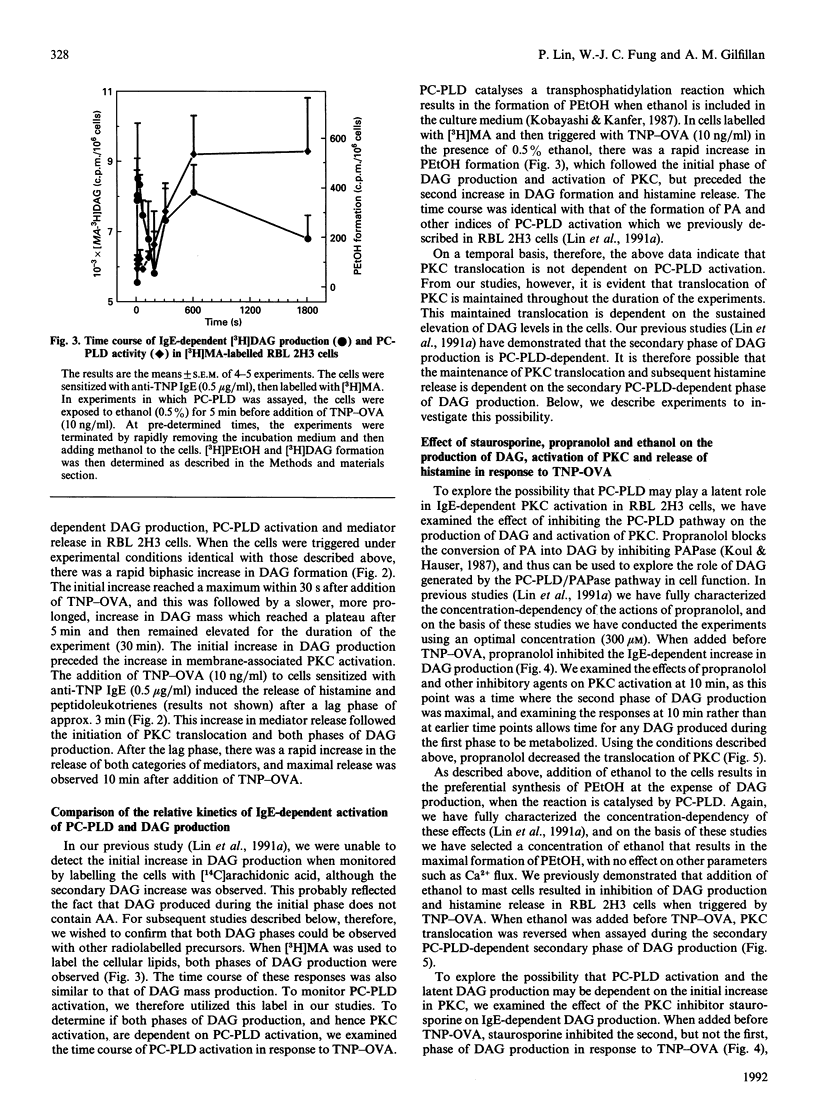
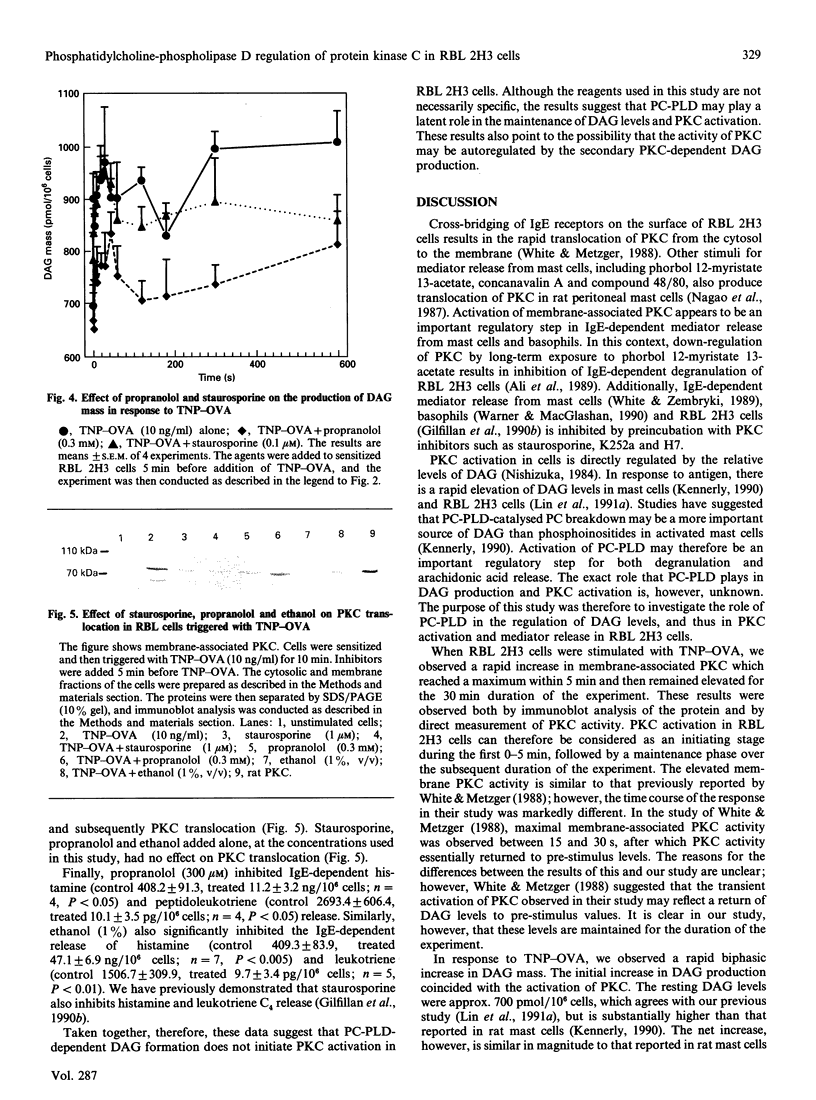
We examined the role of phosphatidylcholine-specific phospholipase D (PC-PLD) in the IgE-dependent activation of protein kinase C (PKC) in RBL 2H3 cells (a model for mast-cell function). Cells were sensitized with mouse monoclonal anti-trinitrophenol (TNP) IgE (0.5 micrograms/ml) and were then triggered with an optimal concentration (10 ng/ml) of TNP-ovalbumin conjugate (TNP-OVA). This resulted in an immediate biphasic increase in the production of 1,2-diacylglycerol (DAG) and activation of PKC. The initial increase in DAG production reached a peak within 30 s, and the second phase reached a plateau within 5 min after stimulation. TNP-OVA-induced PC-PLD activation followed the initial increase in DAG formation in response to IgE-receptor cross-bridging, but coincided with the second peak. Phosphatidic acid (PA), derived from the PC-PLD pathway, is metabolized to DAG by the action of PA phosphohydrolase (PAPase). Propranolol (0.3 mM), which inhibits PAPase, blocked the IgE-dependent increase in DAG, activation of PKC, and subsequently degranulation. The PKC inhibitor staurosporine (0.1 microM) inhibited the second, but not first, peak of DAG accumulation, reversed PKC translocation after 10 min and inhibited subsequent mediator release. Taken together, these results demonstrate that PC-PLD does not initiate, but may play a latent role in, IgE-dependent DAG production, PKC activation and mediator release from RBL 2H3 cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali H., Collado-Escobar D. M., Beaven M. A. The rise in concentration of free Ca2+ and of pH provides sequential, synergistic signals for secretion in antigen-stimulated rat basophilic leukemia (RBL-2H3) cells. J Immunol. 1989 Oct 15;143(8):2626–2633. [PubMed] [Google Scholar]
- Anthes J. C., Eckel S., Siegel M. I., Egan R. W., Billah M. M. Phospholipase D in homogenates from HL-60 granulocytes: implications of calcium and G protein control. Biochem Biophys Res Commun. 1989 Aug 30;163(1):657–664. doi: 10.1016/0006-291x(89)92187-6. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Beaven M. A., Cunha-Melo J. R. Membrane phosphoinositide-activated signals in mast cells and basophils. Prog Allergy. 1988;42:123–184. [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church M. K., Holgate S. T., Hughes P. J. Adenosine inhibits and potentiates IgE-dependent histamine release from human basophils by an A2-receptor mediated mechanism. Br J Pharmacol. 1983 Dec;80(4):719–726. doi: 10.1111/j.1476-5381.1983.tb10063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran F. R., Roddick V. L., Connor J. R., Thornburg J. T., Waite M. Regulation of arachidonic acid metabolism in resident and BCG-activated alveolar macrophages: role of lyso(bis)phosphatidic acid. J Immunol. 1987 Mar 15;138(6):1877–1883. [PubMed] [Google Scholar]
- Cunha-Melo J. R., Dean N. M., Moyer J. D., Maeyama K., Beaven M. A. The kinetics of phosphoinositide hydrolysis in rat basophilic leukemia (RBL-2H3) cells varies with the type of IgE receptor cross-linking agent used. J Biol Chem. 1987 Aug 25;262(24):11455–11463. [PubMed] [Google Scholar]
- Eardley D. D., Koshland M. E. Glycosylphosphatidylinositol: a candidate system for interleukin-2 signal transduction. Science. 1991 Jan 4;251(4989):78–81. doi: 10.1126/science.1824727. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Fewtrell C. M., Foreman J. C., Jordan C. C., Oehme P., Renner H., Stewart J. M. The effects of substance P on histamine and 5-hydroxytryptamine release in the rat. J Physiol. 1982 Sep;330:393–411. doi: 10.1113/jphysiol.1982.sp014347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan A. M., Wiggan G. A., Welton A. F. The effects of the protein kinase C inhibitors staurosporine and H7 on the IgE dependent mediator release from RBL 2H3 cells. Agents Actions. 1990 Jun;30(3-4):418–425. doi: 10.1007/BF01966307. [DOI] [PubMed] [Google Scholar]
- Haak-Frendscho M., Arai N., Arai K., Baeza M. L., Finn A., Kaplan A. P. Human recombinant granulocyte-macrophage colony-stimulating factor and interleukin 3 cause basophil histamine release. J Clin Invest. 1988 Jul;82(1):17–20. doi: 10.1172/JCI113567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugli T. E., Müller-Eberhard H. J. Anaphylatoxins: C3a and C5a. Adv Immunol. 1978;26:1–53. doi: 10.1016/s0065-2776(08)60228-x. [DOI] [PubMed] [Google Scholar]
- Kennerly D. A. Diacylglycerol metabolism in mast cells. Analysis of lipid metabolic pathways using molecular species analysis of intermediates. J Biol Chem. 1987 Dec 5;262(34):16305–16313. [PubMed] [Google Scholar]
- Kennerly D. A. Phosphatidylcholine is a quantitatively more important source of increased 1,2-diacylglycerol than is phosphatidylinositol in mast cells. J Immunol. 1990 May 15;144(10):3912–3919. [PubMed] [Google Scholar]
- Kobayashi M., Kanfer J. N. Phosphatidylethanol formation via transphosphatidylation by rat brain synaptosomal phospholipase D. J Neurochem. 1987 May;48(5):1597–1603. doi: 10.1111/j.1471-4159.1987.tb05707.x. [DOI] [PubMed] [Google Scholar]
- Koul O., Hauser G. Modulation of rat brain cytosolic phosphatidate phosphohydrolase: effect of cationic amphiphilic drugs and divalent cations. Arch Biochem Biophys. 1987 Mar;253(2):453–461. doi: 10.1016/0003-9861(87)90199-8. [DOI] [PubMed] [Google Scholar]
- Lin P. Y., Wiggan G. A., Gilfillan A. M. Activation of phospholipase D in a rat mast (RBL 2H3) cell line. A possible unifying mechanism for IgE-dependent degranulation and arachidonic acid metabolite release. J Immunol. 1991 Mar 1;146(5):1609–1616. [PubMed] [Google Scholar]
- Lohse M. J., Maurer K., Gensheimer H. P., Schwabe U. Dual actions of adenosine on rat peritoneal mast cells. Naunyn Schmiedebergs Arch Pharmacol. 1987 May;335(5):555–560. doi: 10.1007/BF00169124. [DOI] [PubMed] [Google Scholar]
- Merida I., Pratt J. C., Gaulton G. N. Regulation of interleukin 2-dependent growth responses by glycosylphosphatidylinositol molecules. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9421–9425. doi: 10.1073/pnas.87.23.9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y., Goto M., Miyamoto T. Effect of interleukin 2 on basophil histamine release. Allergy. 1987 Feb;42(2):104–108. doi: 10.1111/j.1398-9995.1987.tb02367.x. [DOI] [PubMed] [Google Scholar]
- Nagao S., Nagata K., Kohmura Y., Ishizuka T., Nozawa Y. Redistribution of phospholipid/Ca2+-dependent protein kinase in mast cells activated by various agonists. Biochem Biophys Res Commun. 1987 Feb 13;142(3):645–653. doi: 10.1016/0006-291x(87)91463-x. [DOI] [PubMed] [Google Scholar]
- Nakashima S., Fujimiya H., Miyata H., Nozawa Y. Antigen-induced biphasic diacylglycerol formation in RBL-2H3 cells: the late sustained phase due to phosphatidylcholine hydrolysis is dependent on protein kinase C. Biochem Biophys Res Commun. 1991 May 31;177(1):336–342. doi: 10.1016/0006-291x(91)91988-o. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ohno I., Tanno Y., Yamauchi K., Takishima T. Gene expression and production of tumour necrosis factor by a rat basophilic leukaemia cell line (RBL-2H3) with IgE receptor triggering. Immunology. 1990 May;70(1):88–93. [PMC free article] [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Schleimer R. P., Gillespie E., Lichtenstein L. M. Release of histamine from human leukocytes stimulated with the tumor-promoting phorbol diesters. I. Characterization of the response. J Immunol. 1981 Feb;126(2):570–574. [PubMed] [Google Scholar]
- Siraganian R. P. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem. 1974 Feb;57(2):383–394. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]
- Subramanian N., Bray M. A. Interleukin 1 releases histamine from human basophils and mast cells in vitro. J Immunol. 1987 Jan 1;138(1):271–275. [PubMed] [Google Scholar]
- Warner J. A., MacGlashan D. W., Jr Signal transduction events in human basophils. A comparative study of the role of protein kinase C in basophils activated by anti-IgE antibody and formyl-methionyl-leucyl-phenylalanine. J Immunol. 1990 Sep 15;145(6):1897–1905. [PubMed] [Google Scholar]
- White J. R., Pluznik D. H., Ishizaka K., Ishizaka T. Antigen-induced increase in protein kinase C activity in plasma membrane of mast cells. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8193–8197. doi: 10.1073/pnas.82.23.8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. R., Zembryki D. Differentiation of second messenger systems in mast cell activation. Agents Actions. 1989 Jun;27(3-4):410–413. doi: 10.1007/BF01972837. [DOI] [PubMed] [Google Scholar]
- White K. N., Metzger H. Translocation of protein kinase C in rat basophilic leukemic cells induced by phorbol ester or by aggregation of IgE receptors. J Immunol. 1988 Aug 1;141(3):942–947. [PubMed] [Google Scholar]
- Wodnar-Filipowicz A., Heusser C. H., Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989 May 11;339(6220):150–152. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]




