Abstract
The relationship between amyloid beta (Aβ) and oxidative stress (OS), both prominent factors in Alzheimer’s disease-related neural degeneration, is deeply interconnected. The cleavage of the extracellular domain of Amyloid precursor protein (APP) and phosphorylating different substrates, respectively, the β-site amyloid precursor protein cleaving enzyme-1 (BACE-1) and Glycogen synthase kinase-3-beta (GSK-3β) enzymes initiate the synthesis of Aβ, which causes cognitive deficits in AD. This study aimed to explore the protective potential of Coenzyme Q10 (CoQ10). It also sought to uncover any synergistic effects when combined with donepezil, an acetylcholinesterase inhibitor, in treating Alzheimer’s disease in male albino rats, focusing on the modulation of the BACE-1/GSK-3β pathway. The experiment involved 70 rats categorized into different groups: control, donepezil alone, CoQ10 alone, AD-model, donepezil co-treatment, CoQ10 co-treatment, and CoQ10 + donepezil combination. Various assessments, such as cholinesterase activity, oxidative stress, serum iron profile, Brain Derived Neurotrophic Factor (BDNF), Tau protein, β-site amyloid precursor protein cleaving enzyme-1 (BACE-1), phosphatase and tensin homolog (Pten), and Glycogen synthase kinase-3-beta (GSK-3β), were conducted on behavioral and biochemical aspects. CoQ10 treatment demonstrated memory improvement, enhanced locomotion, and increased neuronal differentiation, mainly through the inhibition of the dual BACE-1/GSK-3β. These findings were substantiated by histological and immunohistological examinations of the hippocampus.
Highlights
Alzheimer’s disease (AD) led to the alteration of BACE-1/GSK-3β.
Coenzyme Q10 (CoQ10) alleviated D-Gal and AlCl3-induced passive avoidance memory deficits in rats.
CoQ10 counteracts Alzheimer’s disease by inhibiting acetylcholine esterase.
CoQ10 significantly increases levels of BDNF and diminishes Tau burden.
CoQ10 acts as a dual BACE1/GSK3β inhibitor.
The combination of CoQ10 treatment and donepezil demonstrated potential as a therapeutic approach.
Keywords: Alzheimer’s disease, coenzyme Q10, BACE-1/GSK-3β, donepezil, tau protein, cholinesterase
Graphical Abstract
Graphical Abstract.
Introduction
Alzheimer’s disease (AD) is a widely prevalent cause of dementia globally, but effective treatments to slow down or halt its progression have been limited until recently.1 Two hallmark features of AD are the accumulation of amyloid beta (Aβ) plaques and neurofibrillary tangles.2 Aβ plaques contribute to neurodegeneration, disrupting the transmission of signals between neurons, while tau tangles impede essential chemical transport within neurons. Despite gaps in our understanding of AD’s pathophysiology, Aβ aggregates have been linked to oxidative stress (OS).3
In AD, the concentrations of neurotrophic factors, such as Brain Derived Neurotrophic Factor (BDNF), might fluctuate over time. In response to neural activity, neurons release BDNF, which is essential for learning and memory in adults as well as for the establishment of adequate synaptic connections during embryonic development.4 Currently, the United States Food and Drug Administration (US FDA) have approved only four drugs for AD: acetylcholinesterase (AChE) inhibitors (donepezil, galantamine, and rivastigmine) and N-methyl-D-aspartate (NMDA) antagonists or glutamate receptors (memantine).5 Due to the complex pathological pathways of AD, combination therapies have emerged, offering novel approaches for various intricate and degenerative diseases like cancer, diabetes, and cardiovascular conditions.6
Iron imbalance, associated with several neurodegenerative disorders, including AD, has been researched.7 Cellular iron imbalances promote excessive lipid peroxidation, leading to ferroptosis, a form of cell death distinct from conventional apoptosis, or autophagy.8 Glycogen synthase kinase 3β (GSK-3β) is a known activator of tau phosphorylation.9 GSK-3β plays a role in the two primary AD-associated pathologies, Aβ toxicity mediated by enhanced β-amyloid cleaving enzyme-1 (BACE1) and presenilin-1 (PS1), as well as tau hyperphosphorylation, synaptic plasticity impairment, and spatial memory deficits, along with promoting inflammation.10 GSK-3β activity is typically inhibited by AKT, which phosphorylates GSK-3β at Serine residue 9 in resting cells.11 According to earlier research, there is a biochemical connection between Aβ and tau, and there is a cross-talk between BACE-1 and GSK-3β.12 According to Lorens-Marítin et al.,13 there is a suggestion that inhibiting GSK-3β kinase reduces the generation of Aβ and the neurotoxicity caused by it. Both BACE-1 and GSK-3β are well-known therapeutic medication targets, and research has shown that their pathways interact with one another. Therefore, it makes sense to construct dual inhibitors for these two targets as they could offer useful treatment approaches for AD.14
Coenzyme Q10, an endogenous proenzyme located in the inner mitochondrial membrane of human cells, emerges as a potential neuroprotective candidate. Coenzyme Q10 serves as an antioxidant, safeguarding against damage from free radicals and lipid oxidation in the mitochondrial membrane. It also plays a vital role in cellular energy supply by transferring electrons from mitochondrial respiratory chain complexes I and II to complex III.15 Supplementation with CoQ10 has shown beneficial anti-oxidative effects, improved mitochondrial function, and prevention of ATP depletion, as demonstrated in animal studies.16 In addition to being an antioxidant, CoQ10 also possesses some anti-inflammatory qualities,17 participates as a cofactor in the synthesis of pyrimidines, which is a prerequisite for DNA replication and RNA repair. Oral CoQ10 therapy has been observed to elevate brain mitochondrial levels, enhance cognition, and reduce oxidative damage markers in the cerebral cortex and hippocampus of rats subjected to oxidative injuries.18 Additionally, according to Wear et al.,19 CoQ10 can decrease the toxicity of typical Aβ aggregates by preventing their buildup. Dumont et al. showed that oral CoQ10 supplementation reversed AD pathologies through the reduction of oxidative stress and Aβ42 levels, while improving cognitive performance.16 CoQ10 can reduce apolipoprotein E (ApoE) level and mitigate the harm caused by Aβ production and phosphorylated Tau aggregation throughout the developing stage of the brain. According to Yang et al.,20 the mechanism may be connected to the energy supply of CoQ10 and its mitochondrial protection, which can lower brain oxidative stress and prevent aberrant tau protein phosphorylation, the buildup of Aβ1-42, and abnormal high expression of ApoE in the brain. Furthermore, CoQ10 supplementation has been found to have favorable anti-ferroptotic effects by regulating iron overload, as indicated by Lazourgui et al.21 According to Abuelezz and Hendawy,22 CoQ10 has neuroprotective effects in AD. It also inhibits oxidative stress, amyloid deposition, and modulates the PI3K/Akt/GSK-3β/CREB/BDNF/TrKB pathway, respectively. Owing to its possible benefits, it is taken into consideration as a dietary supplement in several nervous system disorders, such as multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), Parkinson’s diseases, and Friedreich’s ataxia.23 The objective of this study was to evaluate the protective role of Coenzyme Q10 (CoQ10) as potent anti-oxidant, anti-inflammatory, and anti-ferroptotic agents and to clarify how they work in concert with donepezil (Don), an acetylcholinesterase inhibitor, to prevent brain injury in rats caused by toxicity of D-galactose and AlCl3 (AD model) by altering the BACE-1/GSK-3β pathway.
Materials and methods
Chemicals and drugs
D-galactose (D-gal) and AlCl3 were obtained from Sigma-Aldrich, USA. Coenzyme Q10 (CoQ10) tablets, manufactured by PURITAN’S PRIDE, INC. of Hotbrook, NY 11741, U.S.A. donepezil tablet was obtained from Delta pharma (DP) Co., (Egypt).
Animals
This study adhered to ethical guidelines set by the Faculty of Science, Tanta University under Code No. IACUC-SCI-TU-0267. A total of 70 mature male albino rats (aged 7–8 weeks and weighing between 180–200 grams), were acquired from Alexandria University’s Faculty of Pharmacy. The rats were acclimatized in wire mesh cages for one week. They were provided with standard commercial food and unrestricted access to water. The rats were subjected to a 12:12-hour light–dark cycle at a temperature of approximately 25 ± 2 °C.
Experimental design
The rats were randomly divided into seven groups, each consisting of ten rats: Control group (G1): rats received no treatment, donepezil group (G2): rats were orally administered donepezil at a dose of 10 mg/kg body weight, five times per week for four weeks.24 CoQ10 group (G3): rats were orally administered CoQ10 at a dose of 1,200 mg/kg daily for four weeks.25 AD Model group (G4): rats were injected with D-galactose at a dose of 120 mg/kg body weight daily for 30 days and received daily oral administration of AlCl3 at a dose of 50 mg/kg body weight for four weeks to induce an Alzheimer’s disease model.26 donepezil Co-Treated group (G5): rats received oral donepezil at a dose of 10 mg/kg body weight, five times per week for four weeks, concurrently with D-Gal and AlCl3. CoQ10 Co-Treated group (G6): rats received oral CoQ10 at a dose of 1,200 mg/kg daily for four weeks, concurrently with D-Gal and AlCl3. CoQ10 + donepezil combination group (G7): Rats were administered both CoQ10 and donepezil concurrently with D-Gal and AlCl3 at recommended doses for four consecutive weeks.
The classic labyrinth test for neurobehavioral evaluation in rats
The cognitive function of all rat groups was assessed using the classic labyrinth test (CLT) maze before and after the administration of CoQ10 and donepezil. The CLT maze consists of a wooden enclosure measuring 125 × 125 × 40 cm, featuring labyrinth pathways of equal width and height (25 × 35 cm) but varying lengths.26 Video analysis was employed to collect and analyze the data. Following the study’s conclusion, all rats were euthanized, weighed, and subjected to a thorough dissection. Overnight-fasted rats had blood samples drawn into anticoagulant-coated tubes for iron profile analysis. Hippocampus tissues were collected for histological, immunohistopathological, biochemical, and molecular investigations.
Evaluation of biochemical parameters
L-malondialdehyde (MDA) was estimated using the MDA colorimetric kit (Solarbio Cat. No BC 0020; Beijing, China). Catalase (CAT) was estimated using CAT colorimetric kit (Solarbio Cat. No BC 0205; Beijing, China). The level of reduced glutathione (GSH) was assayed as described by Goldberg and Spooner.27 The glutathione peroxidase (GPX) activity was assayed as described by Paglia and Valentine.28 Acetylcholine (ACH) and acetyl cholinesterase activity (Ache) were measured using ELISA kit following the manufacturer protocol (My BioSource Cat. No MBS8820077; San Diego, USA) and (Bio vision Cat. No: K764-100; Milpitas, CA), respectively. Serum Transferrin was estimated via the ELISA-kit (elabscience Cat. No. E-EL-R3028; Houston, Texas, USA). Serum total iron was assayed as described by Dreux.29 Tau Protein and Brain Derived Neurotrophin Factor (BDNF) were estimated via ELISA-kit (Novus bio Cat. No NBP2-81164; Centennial, USA), (My BioSource Cat. No MBS9139053; San Diego, USA), respectively.
Molecular investigation of) BACE-1, Pten, and GSK-3β)
Pure RNA was extracted of the hippocampus tissue according to the protocol described by Thermo Scientific (Fermentas, Cat No. # K0731). The quality and quantity of Total RNA were assessed by reading absorbance at 260/280 nm using spectrophotometer (Implen NanoPhotometer®). The mRNA was converted into complementary DNA (cDNA) using reverse transcription kits (Thermo Scientific, Fermentas, #EP0451). The isolated cDNA was amplified using 2X Maxima SYBR Green/ROX qPCR Master Mix, following the manufacturer’s instructions, to measure the mRNA expression of target genes (BACE-1, Pten, and GSK-3β) with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the housekeeping reference gene. Relative mRNA levels were calculated by the 2−△△Ct method. The primer sequences of different genes are as follows: GAPDH, reverse primer: 5′- CATGGTGCAGCGATGCTTTA-3′ and forward primer: 5′- CTCCTCGAAGTACCCTGTGC-3′; BACE-1, reverse primer: 5′- AGCAAGCCTTAGCCCTACCT-3′ and forward primer: 5′- GCGAATTGGCTTTGCTGTCA-3′; Pten, reverse primer: 5′- AAGAAAACACACCCTCCCACC-3′ and forward primer: 5′- AGAGCGTGCGGATAATGACAA-3′; GSK-3β, reverse primer: 5′- CCCGAGAGATGGTGACAGTT-3′ and forward primer: 5′- CTGCAGTAACCGAGAAGCAA-3′.
Histopathological investigation (HE staining)
Hippocampus excised and fixed in 4% paraformaldehyde in phosphate buffered saline (0.1 M, pH 7.4 PBS) for 24 h at 4 °C. Fixed hippocampus were dehydrated through a graded series of ethanol and embedded in paraffin according to standard procedures. Paraffin sections (5 μm thick) were mounted on gelatin chromalum-coated glass slides and used for haematoxylin-eosin stains.30
Immunohistochemical screening of glial fibrillary acidic protein (GFAP) and Synaptophysin (Syn)
The detection of Glial Fibrillary Acidic Protein (GFAP) and synaptophysin (Syn) with dilution 1:200 & 1:100 respectively (DAKO Japan Co, Tokyo, Japan) after the methods of Tousson et al.31 and Gudi et al.,32 respectively.
Statistical analysis:
Data were presented as means with standard deviations and analyzed using one-way ANOVA t-tests with p-values calculated using GraphPad instat, Version 3.06 (GraphPad Software Inc., San-Diego, California, USA).
Results
Behavioral studies
Rats subjected to daily injections of (D-gal) and (AlCl3) for a four-week period experienced a significant decrease in cognitive response and memory compared to control animals (P < 0.001). The classic labyrinth test revealed a considerable increase in the time taken to navigate the maze following D-gal and AlCl3 injections (AD-model, G4) when compared to normal control animals (G1) and all treated groups. Significant differences were observed (P < 0.01) between G1 and the treated groups (G5 and G6), with a notable decrease (P < 0.01) in G4 if compared to G5 and G6. Furthermore, a significant decrease (P < 0.001) was noted in G4 compared to rats receiving combined treatment (G7) (P > 0.05, Table 1).
Table 1.
Memory-related behavioral tests, Total iron, and transferrin in the different groups.
| Treatment | Memory related behavioral tests (sec) | Total iron (μg/dL) | Transferrin (mg/dL) |
|---|---|---|---|
| Normal control (G1) | 45.38 ± 14.22 | 151.03 ± 12.66 | 20.66 ± 11.76 |
| donepezil Control (G2) | 55.32 ± 17.17 | 168.18 ± 19.51 | 19.19 ± 12.44 |
| CoQ10 control (G3) | 47.55 ± 16.08 | 153.91 ± 12.01 | 21.23 ± 13.98 |
| AD model (G4) | 177.14 ± 23.55a | 391.22 ± 23.03a | 7.93 ± 11.63a |
| AD + donepezil (G5) | 111.28 ± 21.60b,c | 282.12 ± 20.37b,d | 13.31 ± 9.54d,e |
| AD + CoQ10 (G6) | 101.85 ± 19.39b,c | 232.99 ± 14.54d,e | 15.76 ± 12.09d,e |
| AD + donepezil+CoQ10 (G7) | 67.55 ± 13.95d,e | 185.15 ± 18.62d | 17.98 ± 12.23d |
Data are presented as mean ± S.D, n = 10 per group.
aSignificant VS. Control group at P < 0.001.
bSignificant VS. Control group at P < 0.01.
cSignificant VS. AD model at P < 0.01.
dSignificant VS. AD model at P < 0.001.
eSignificant VS. Control group at P < 0.05.
Biochemical investigations—total iron and transferrin
In the serum of D-gal and AlCl3-treated rats (G4), there was a substantial (P < 0.001) increase in total iron compared the normal control (G1). Treatment with CoQ10 alone or in combination with donepezil resulted in a significant decrease (P < 0.001) in total iron compared to G4. Detailed numerical results are provided in (Table 1). Similarly, the concentration of transferrin was significantly declined (P < 0.001) in transferrin compared to G4. Treatment with CoQ10 alone or in combination with donepezil led to a significant (P < 0.001) increase in transferrin compared to G4.
Oxidative stress markers—MDA, CAT, GPx, and GSH
Biochemical studies showed a significant (P < 0.001) increase in MDA level in the brain homogenates of D-gal and AlCl3-treated rats (G4) compared to normal control (G1). Treatment with CoQ10 alone or in combination with donepezil resulted in a significant (P < 0.001) reduction in MDA compared to G4. Specific numerical data can be found in (Table 2). Moreover, there was a substantial (P < 0.001) increase in the levels of CAT, GPx, and GSH observed in all treated rats (G5, G6, and G7). The use of CoQ10 either alone or in conjunction with donepezil led to a significant (P < 0.001) decrease in CAT, GPx, and GSH compared to G4 (Table 2).
Table 2.
Oxidative stress and antioxidant enzymes in different groups.
| Parameters | ||||
|---|---|---|---|---|
| Groups | MDA, nmol/g. tissue | CAT, U/g. tissue | GPx, U/g. tissue | GSH, mg/g. tissue |
| G1 | 15.12 ± 5.22 | 35.86 ± 2.06 | 214.25 ± 1 4.68 | 18.93 ± 1.3 |
| G2 | 20.46 ± 6.05 | 35.03 ± 1.63 | 210.31 ± 16.80 | 16.14 ± 1.30 |
| G3 | 16.88 ± 6.32 | 36.72 ± 4.95 | 216.65 ± 17.91 | 19.01 ± 2.01 |
| G4 | 35.34 ± 1.72a | 7.26 ± 2.17a | 78.27 ± 13.70a | 6.96 ± 1.46a |
| G5 | 28.45 ± 3.40b,c | 19.29 ± 2.56b,d | 114.42 ± 15.49b,c | 10.47 ± 1.70b,d |
| G6 | 26.10 ± 4.27b,d | 20.46 ± 3.52b,d | 126.77 ± 2.98b,c | 11.40 ± 1.77b,d |
| G7 | 19.70 ± 3.94d | 28.43 ± 1.68d | 187.75 ± 1.47d | 13.91 ± 2.20d |
Data are presented as mean ± S.D, n = 10 per group.
aSignificant VS. Control group at P < 0.001.
bSignificant VS. Control group at P < 0.05.
cSignificant VS. AD model at P < 0.01.
dSignificant VS. AD model at P < 0.001.
eSignificant VS. Control group at P < 0.01.
fSignificant VS. AD model at P < 0.05.
Neurotransmitter parameters, tau protein and hippocampus BDNF levels
Neurotransmitter results showed that rats injected with D-gal and AlCl3 (G4) exhibited a significant increase in acetylcholinesterase (Ache) activity and a significant decrease in acetylcholine (Ach) compared to normal control (G1). Treatment with CoQ10 alone or in combination with donepezil resulted in a significant (P < 0.001) reduction in Ache activity and an enhancement in Ach compared to G4 (Table 3).
Table 3.
Hippocampus neurotransmitters levels, tau protein, and BDNF level in the different groups.
| Parameters | ||||
|---|---|---|---|---|
| Groups | Ach, U/g.tissue | Ache, nmol/min/g.tissue | Tau protein, pg/g.tissue | BDNF, pg/g.tissue |
| G1 | 19.61 ± 2.21 | 0.15 ± 0.001 | 40.39 ± 0.76 | 101.38 ± 1.55 |
| G2 | 17.55 ± 2.04 | 0.14 ± 0.001 | 40.13 ± 0.81 | 100.10 ± 4.53 |
| G3 | 18.23 ± 3.08 | 0.16 ± 0.003 | 38.7 ± 1.47 | 102.24 ± 2.87 |
| G4 | 5.82 ± 1.22a | 0.59 ± 0.002a | 97.15 ± 3.98a | 20.10 ± 1.18a |
| G5 | 12.24 ± 0.53b | 0.21 ± 0.001b,c | 79.29 ± 0.41b,c | 51.03 ± 9.12b,c |
| G6 | 10.50 ± 0.60b,c | 0.24 ± 0.002b,c | 69.22 ± 0.75b,c | 57.40 ± 0.38b,c |
| G7 | 13.84 ± 1.60b | 0.18 ± 0.002b | 58.32 ± 1.93b | 81.48 ± 2.07b |
Data are presented as mean ± S.D, n = 10 per group.
aSignificant VS. Control group at P < 0.001.
bSignificant VS. AD model at P < 0.001.
cSignificant VS. Control group at P < 0.05.
dSignificant VS. Control group at P < 0.01.
eSignificant VS. AD model at P < 0.01.
fSignificant VS. AD model at P < 0.05.
There was a significant (P < 0.001) reduction in the level of Tau protein in treated animals (G5, G6, and G7) compared to G4. Conversely, there was a significant decrease (P ≤ 0.001) in hippocampus BDNF levels following D-gal and AlCl₃ treatment (G4) in comparison to G1 and treated animals. However, a significant (P < 0.001) elevation in hippocampus BDNF levels was observed in treated animals (G5, G6, and G7) compared to G4. Detailed numerical values can be found in (Table 3).
Molecular investigations
Molecular investigations revealed a significant (P < 0.001) up-regulation in BACE-1, Pten, and GSK-3β in the brain homogenates of D-gal and AlCl3-treated rats (G4) compared to normal control (G1). Treatment with CoQ10 and donepezil resulted in a significant (P < 0.001) down-regulation of BACE-1, Pten, and GSK-3β compared to the ad model (G4) (Table 4).
Table 4.
Relative gene expression of BACE-1, Pten, and GSK-3β in hippocampus tissues of the different groups.
| Groups | BACE-1 expression by fold change | Pten expression by fold change | GSK- 3β expression by fold change |
|---|---|---|---|
| G1 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| G2 | 1.51 ± 0.01a,b | 1.13 ± 0.01b | 1.29 ± 0.05b |
| G3 | 1.40 ± 0.01a,b | 1.19 ± 0.02b | 1.21 ± 0.01b |
| G4 | 6.90 ± 0.26c | 5.97 ± 0.24c | 6.39 ± 0.34c |
| G5 | 1.88 ± 0.02a,b | 2.15 ± 0.02a,b | 2.44 ± 0.01a,b |
| G6 | 1.53 ± 0.04a,b | 2.01 ± 0.01a,b | 2.00 ± 0.02a,b |
| G7 | 1.10 ± 0.03b | 1.15 ± 0.02b | 1.21 ± 0.02b |
Data are presented as mean ± S.D, n = 10 per group.
aSignificant VS. Control group at P < 0.05.
bSignificant VS. AD model at P < 0.001.
cSignificant VS. Control group at P < 0.001.
dSignificant VS. AD model at P < 0.01.
Histopathological findings of hippocampus tissue in different groups
Figure 1 illustrated that; the microstructure of hippocampus in coronal sections in the different groups under study that were stained with Haematoxylin and Eosin. No histological changes were observed in the three regions of hippocampus of control and treated rats with CoQ10 and donepezil groups (Fig. 1A–C). In contrast, hippocampus sections in treated rats with D-galactose and AlCl3 (AD model) group revealed a large number of damaged neurons with moderate atrophy, vacuolated astrocytes, apoptotic, marked diffuse vacuolar degeneration, and decrease and distortion of the pyramidal cells (Fig. 1D and E). On the other hand, a co-treatment of AD with donepezil and co-treatment of AD with CoQ10 exhibits good improvement degree (Fig. 1F and G). Hippocampus sections in co-treated AD with donepezil and CoQ10 revealed increased in the number of pyramidal cells, mild atrophy, mild diffuse vacuolar degeneration were detected (Fig. 1H).
Fig. 1.
Photomicrograph of the rat stained with haematoxylin and eosin. A–C) G1, G2, and G3 revealed the normal structure of pyramidal cells, astrocytes, and fibers. D and E) Hippocampus sections in G4 revealed a large number of damages neurons, degenerated and decreased in pyramidal cells numbers (arrows) and vacuolated astrocytes. F and G) Hippocampus sections in G5 and G6 revealed mild improvements in damaged neurons with a mild increased in pyramidal cell numbers. H) Hippocampus sections in G7 revealed increased in the number of pyramidal cells, mild atrophy, and mild diffuse vacuolar degeneration.
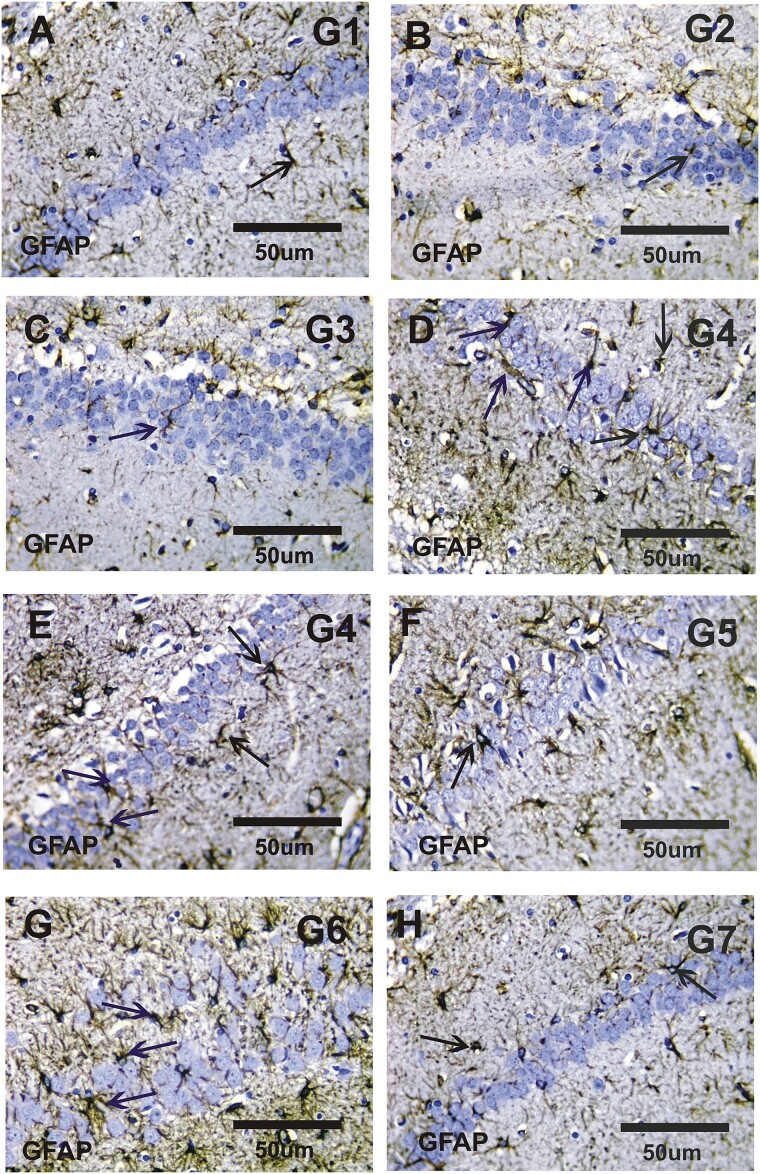
Immunohistological detection of glial fibrillary acid protein (GFAP) and Synaptophysin (Syn) immunoreactivity findings of hippocampus tissue in different groups
Figure 2 illustrated that; a negative or faint positive reactions of GFAP immunoreactivity (GFAP-ir) in the hippocampus sections in the control groups (Fig. 2A–C). In contrast, moderate to strong positive (GFAP-ir) were observed in the hippocampus sections of the AD group (Fig. 2D and E). There were mild positive (GFAP-ir) observed in the hippocampus sections in the co-treated groups (Fig. 2F and G). While, there was a faint positive reaction to GFAP expression was detected in the combined group (Fig. 2H).
Fig. 2.
Photomicrograph of the rat hippocampus stained with GFAP immunoreactivity. A–C) Faint positive reactions for GFAP immunoreactivity (GFAP-ir) in the hippocampus sections of G1, G2, and G3. D and E) Moderate to strong positive GFAP expressions in the hippocampus sections in G4. F and G) Mild positive for GFAP expressions in hippocampus sections in G5 and G6. H) Faint positive reactions for GFAP immunoreactivity in hippocampus sections in G7.
Figure 3 illustrated that; a strong positive reactions of synaptophysin immunoreactivity (Syn-ir) in the hippocampus sections in the control groups (Fig. 3A–C). In contrast, negative or faint positive (Syn-ir) were observed in the hippocampus sections of the AD group (Fig. 3D and E). There were mild positive (Syn-ir) observed in the hippocampus sections in the co-treated groups (Fig. 3F and G). While, there was a moderate to strong positive reaction of Syn expression was detected in combined group (Fig. 3H).
Fig. 3.
Photomicrograph of rat hippocampus stained with Syn immunoreactivity. A–C) Strong positive reactions for Synaptophysin immunoreactivity (Syn-ir) in the hippocampus sections of G1, G2 and G3. D and E) Weak positive GFAP expressions in the hippocampus sections in G4. F and G) Mild positive for Syn expressions in hippocampus sections in G5 and G6. H) Moderate to strong positive reactions for Syn immunoreactivity in hippocampus sections in G7.
Discussion
Alzheimer disease (AD) is a particularly prevalent cause of dementia worldwide, yet recently, effective treatments to halt or slow down the disease’s development were mostly lacking.1 The current investigation sought to determine whether CoQ10 and donepezil had a synergistic impact in a rat of AD model brought on by D-galactose and AlCl3. Our observations from classic labyrinth test (CLT) indicated that oral administration of CoQ10 could ameliorate memory impairment and behavioral changes in the AD model, revealing significant differences among the control, AD-model, and protective groups of rats. Notably, the AD model rats (G4) exhibited a significantly prolonged maze-running time, approximately 3.90 times longer than the normal control (G1) group, which aligns with prior studies.26 Rats in groups G5, G6, and G7 demonstrated significantly reduced running times, approximately 0.62, 0.57, and 0.38 times shorter than the AD model rats (G4). Our findings were supported by previous research demonstrating CoQ10’s ability to enhance memory and cognitive function.33
Neurodegenerative diseases, including Alzheimer’s disease, have been associated with the accumulation of iron in specific brain regions responsible for mobility and cognitive function.34 Our results indicated a significant increase in total iron content in the hippocampus due to beta-amyloid buildup in the AD model group, consistent with the findings of Viktorinova and Durfinova.35 Prolonged exposure to D-galactose was shown to lead to excessive iron accumulation, elevating oxidative stress, and impairing antioxidant mechanisms, thereby accelerating age-related degenerative changes in the brain.36 However, the combined therapy of donepezil and CoQ10 restored normal iron levels in G4, demonstrating their iron-chelating properties and protective effects against oxidative stress-induced brain damage. Our findings align with Lazourgui et al.,21 who reported that CoQ10 supplementation exerts favorable anti-ferroptotic effects by regulating iron levels. Furthermore, compared to G1 rats, G4 rats exhibited a significantly reduced serum transferrin level, approximately 0.3 times lower. Co-treatment with CoQ10 and donepezil significantly increased serum transferrin levels due to CoQ10’s ability to prevent ferroptosis, in accordance with the study by Squitti et al.37 Elevated hippocampal levels of malondialdehyde (MDA) and reduced antioxidant activity in G4 indicated oxidative stress-induced damage, which was exacerbated in this group. CoQ10 and donepezil exhibited antioxidant effects against hippocampal damage induced by D-galactose and AlCl3, as evident from the restoration of antioxidant enzyme activities and reduction in MDA levels.26 Additionally, findings from38 demonstrated that CoQ10 has potent antioxidant effects in the inner mitochondrial layer. α-tocopherol is generated from α-tocopheroxyl radicals or it scavenges reactive oxygen species (ROS) directly to prevent lipid peroxidation and inhibits free radical generation. This anti-oxidant property is only evident when ubiquinol is reduced. Following dietary absorption, the oxidized form (ubiquinone) is easily converted to ubiquinol.39 A transgenic mouse model of AD treated with CoQ10 demonstrated decreased oxidative stress and amyloid pathology as well as enhanced behavioural performance in the mice in an in vivo research. Treatment with CoQ10 lowered levels of protein carbonyls in the brain, a sign of oxidative stress.40 The main medication used to treat AD and boost cerebral cholinergic neurotransmission is donepezil. The compound donepezil, which has several functions including raising Ach levels and acting as an antioxidant, will be beneficial for AD. One crucial component of neuroprotection is the capacity of cholinesterase inhibitors to function as antioxidants.41 These findings suggest that this is due to the synergistic effect of these compounds.
The loss of the cholinergic neurotransmitter acetylcholine (ACh) due to ongoing acetylcholinesterase (AChE) activity is a significant contributor to cognitive impairment in Alzheimer’s disease.42 In our study, hippocampal ACh levels were significantly reduced in D-galactose and AlCl3-treated rats (G4) compared to G1, while AChE activity was significantly elevated in G4. These findings are consistent with Haider et al.,43 who reported decreased ACh levels in both the hippocampus and cortex in AD-like model rats. However, co-treatment with CoQ10 and donepezil normalized neurotransmitter parameters in the AD group.44 Brain Derived Neurotrophic Factor (BDNF) plays critical roles in the development, maintenance, differentiation, and repair of neurons in the brain via regulating neuronal survival and neuroplasticity.45 In our investigation, the administration of AlCl3 and D-galactose induced oxidative stress, leading to reduced BDNF levels in the rats’ hippocampi. Nevertheless, CoQ10-treated AD rats exhibited restored BDNF levels.46
The morphology and function of neurons are significantly influenced by the microtubule-associated Tau protein. Our study revealed a substantial difference in a number of brain biochemical markers between the AD group (G4) and the healthy control group (G1). Tau protein levels were significantly elevated, approximately 2.42 times higher in G4 compared to G1. These results are compatible with Beltagy et al.47 who reported that higher hippocampus Tau protein levels by knowing normal, may be linked with AD pathophysiology. However, hippocampal Tau protein levels showed improvement in treated groups G5, G6, and G7 compared to G4. These results were consistent with several studies.20,48 Again; these findings suggest that this is due to the synergistic effect of these compounds.
BACE-1 concentrations and activity rates are elevated in AD brains and body fluids, playing a crucial role in AD pathophysiology.49 Our study found that BACE-1 expression was significantly up-regulated in the AD model group compared to the healthy control group. Other research supported our findings and showed up-regulation in BACE-1 expression in the AD model group.47 However, co-treatment with CoQ10 and donepezil reduced BACE-1 expression, suggesting their ability to reduce oxidative stress, amyloid pathology, and plaque formation in the hippocampus.16 According to Taylor and Abdel-Wahab,50 Phosphatase and tensin homologue (Pten) is a phosphatase that inhibits the PI3K/AKT pathway, hence acting as a tumor suppressor. It has been demonstrated that the PI3K/AKT pathway is essential for neuroprotection and for preventing apoptosis by upregulating superoxide dismutases (SODs) expression. The PI3K/AKT/Pten pathway, which is mediated by intracellular ROS generation, is a crucial element in cell fate with reference to cell death and senescence.51 Our data indicated an upregulation in Pten gene expression in the AD model, approximately 6.06 times higher than in G1. However, CoQ10 administration significantly downregulated Pten gene expression, lowering it to approximately 0.19 times that of the G4 group. This can be attributed to CoQ10’s promotion of wound healing via the Cav-1/PI3K/Akt signaling pathway.52 GSK-3β, regulated by the PI3K/Akt and Wnt/β-catenin pathways, plays a key role in controlling Tau phosphorylation, axonal transport, hippocampal neurodegeneration, and learning impairment when overexpressed or over activated.53 Our study demonstrated an upregulation of GSK-3β gene expression in the AD model, approximately 6.06 times higher than in G1. However, the expression of the GSK-3β gene was significantly downregulated following CoQ10 and donepezil administration. In comparison to the G4 group, gene expression in groups G5, G6, and G7 improved by approximately 0.38, 0.31, and 0.18 times, respectively. These findings are in line with various research studies.22,54 These findings suggest that this is due to the synergistic effect of these compounds.
On the level of histopathology changes, our results indicated a harmful alteration with distortion in the hippocampus regions (CA1, CA2 and CA3) of AD rats. No histological alterations were noted in the CA1, CA2 and CA3 sections of the hippocampus of control and treated rats with CoQ10 and donepezil (G1, G2, and G3). In contrast, hippocampus sections in treated rats with D-galactose and AlCl3 (AD model) revealed a large number of damages neurons with moderate atrophy, vacuolated astrocytes, apoptotic, marked diffuse vacuolar degeneration, and decrease and distortion of the pyramidal cells. Similar findings showed that there were fewer hippocampal neurons arranged heterocentrically in D-galactose and AlCl3, in which the cell body, pycnotic and deeply stained nuclei shrank and the elongated axon could be identified.26 Fortunately, the treatment of CoQ10 and donepezil considerably enhanced these changes, demonstrating well-preserved pyramidal cells in both groups. This finding supports the neuroprotective action of both CoQ10 and donepezil on brain cells by enhancing neurogenesis.25,55
An immunohistochemical study of the hippocampus of the AD model group revealed strong staining for GFAP in the AD group, which may be an astrocyte marker.26 CoQ10 and donepezil co-treatment reduced GFAP levels.56 Additionally, a weak positive expression for Syn was successfully discovered by treatment with D-galactose and AlCl3. This finding aligns with earlier research indicating a reduction in synaptophysin immuno-expression in the initial phases of Alzheimer’s disease.57 CoQ10 is essential for the mitochondrial cell power supply. CoQ10 is an oxidative depressant that moves electrons from complexes I and II to complex III. As a result of the high energy needs of neurons for synaptic transmission, significant neuronal degeneration is frequently caused by mitochondrial malfunction, as is the case in many neurological illnesses.58
Studies have showed that an excess of D-gal and AlCl3 leads to abnormal metabolism due to the excessive oxidative stress due to enhanced lipid peroxidation, decreased of the catalase activity, reduced glutathione (GSH) concentration, induced ferroptosis,47 neuronal damage due to neuroinflammation, increased Tau protein, and caused alteration in hippocampal gene expressions leading to cognitive impairment in Albino rat.59 All the pathological hallmarks were observed in this model. Safe and reliable model. However, the present study has several limitations. First, the amyloid plaques expressed in this model were pathologically different from the senile plaques of human AD and Insulin resistance has been observed after D-galactose treatment that could lead to diabetes. Second, we only used male rat in the study, because it has been previously pointed out that males were more susceptible to Alzheimer’s than females. So, in order to establish a stable cognitive impairment model, the rats used in this experiment were all male.
A number of factors, including incomplete models, the choice of animal species, a lack of variability, and the models’ validity, could be to blame for the discrepancy between preclinical models and clinical trials. A well-designed model is crucial for facilitating a better translation of preclinical AD research into clinical trials. The combination of CoQ10 and donepezil holds promise for treatment of Alzheimer, especially those with overexpression of BACE-1/GSK-3β. Our study has shown potential in AD model, demonstrating the effectiveness of this approach. However, to establish its widespread use in Alzheimer treatment and to translate it into the real-world clinical setting, further comprehensive studies and clinical trials are imperative. These future studies will shed light on the safety, efficacy, and optimal application of this combination across different animal model for Alzheimer disease and stages, paving the way for its integration into routine Alzheimer treatment protocols.
In conclusion, CoQ10 demonstrated beneficial neuroprotective effects in a D-galactose and AlCl3-induced AD in a rat model by improving learning behavior (Classic labyrinth test), decreasing malondialdehyde (MDA) level, increasing Catalase (CAT), glutathione peroxidase (GPx) activities and reduced glutathione (GSH) concentration, reducing Tau burden, decreasing hippocampal acetylcholinesterase activity, increasing acetylcholine and brain-derived neurotrophic factor (BDNF) levels in brain tissue and improving ferroptotic parameters. CoQ10’s antioxidant, anti-ferroptotic effects and BACE-1/GSK-3β inhibitor were a significant if compared to donepezil alone. The synergistic effects of these compounds could potentially provide an effective therapeutic approach for treating AD.
Supplementary Material
Acknowledgments
The authors appreciate the support that Tanta University and Damanhour University provided to complete this work.
Contributor Information
Nagat Fawzy Nawar, Division of Biochemistry, Department of Chemistry, Faculty of Science, Tanta University, 31527, Egypt.
Doha Mohammad Beltagy, Division of Biochemistry, Department of Chemistry, Faculty of Science, Damanhour University, 22514, Egypt.
Tarek Mostafa Mohamed, Division of Biochemistry, Department of Chemistry, Faculty of Science, Tanta University, 31527, Egypt.
Ehab Mostafa Tousson, Department of Zoology, Faculty of Science, Tanta University, 31527, Egypt.
Mai Mahmoud El-Keey, Division of Biochemistry, Department of Chemistry, Faculty of Science, Tanta University, 31527, Egypt.
Author contributions
Methodology, validation, writing – original draft [Nagat F. Nawar].
Conceptualization, formal analysis, Methodology, supervision, visualization [Doha M. Beltagy].
Conceptualization, formal analysis, Methodology, resources, supervision, writing –review & editing [Tarek M. Mohamed].
Data curation, investigation, supervision, formal analysis [Mai M. El-Keey].
Data curation, investigation, Methodology, formal analysis [Ehab Tousson].
Conflict of interest statement
The authors have no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval and consent to participate
The animals were handled and the experiments were done according to the suggested National ethical guidelines for the care of laboratory animals and the Animal Ethics Committee of the Faculty of Science, Tanta University (protocol approval code Serial No. IACUC-SCI-TU-0267).
Consent for publication
The authors approve publication.
Data availability
The data will be available upon request. The data of this article are included within the article and its additional files.
Reference
- 1. Self WK, Holtzman DM. Emerging diagnostics and therapeutics for Alzheimer disease. Nat Med. 2023:29(9):2187–2199. [DOI] [PubMed] [Google Scholar]
- 2. DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019:14(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anadozie SO, Effiom BO, Adewale OB, Jude J, Zosela I, Akawa OB, Olayinka JN, Roux S. Hibiscus sabdariffa synthesized gold nanoparticles ameliorate aluminum chloride induced memory deficits through inhibition of COX-2/BACE-1 mRNA expression in rats. Arab J Chem. 2023:16(4):104604. [Google Scholar]
- 4. Li Y, Chen J, Yu H, Ye J, Wang C, Kong L. Serum brain-derived neurotrophic factor as diagnosis clue for Alzheimer's disease: a cross-sectional observational study in the elderly. Front Psychiatry. 2023:14:1127658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cummings J, Lee G, Nahed P, Kambar MEZM, Zhong K, Fonseca J, Taghva K. Alzheimer's disease drug development pipeline: 2022. Alzheimers Dement (NY). 2022:8(1):e12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ekundayo BE, Obafemi TO, Adewale OB, Oyinloye BO. Donepezil-based combination therapy for Alzheimer’s disease and related neuropathies. Comp Clin Pathol. 2023:32(4):699–708. [Google Scholar]
- 7. Ryan SK, Zelic M, Han Y, Teeple E, Chen L, Sadeghi M, Shankara S, Guo L, Li C, Pontarelli F, et al. Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat Neurosci. 2023:26(1):12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mazhar M, Din AU, Ali H, Yang G, Ren W, Wang L, Fan X, Yang S. Implication of ferroptosis in aging. Cell Death Discov. 2021:7(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehta SL, Kim T, Chelluboina B, Vemuganti R. Tau and GSK-3β are critical contributors to α-Synuclein-mediated post-stroke brain damage. NeuroMolecular Med. 2022:25(1):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fuster-Matanzo A, Jurado-Arjona J, Benvegnù S, García E, Martín-Maestro P, Gómez-Sintes R, Hernández F, Ávila J. Glycogen synthase kinase-3beta regulates fractalkine production by altering its trafficking from Golgi to plasma membrane: implications for Alzheimer's disease. Cell Mol Life Sci. 2017:74(6):1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sarawi WS, Alhusaini AM, Fadda LM, Alomar HA, Albaker AB, Aljrboa AS, Alotaibi AM, Hasan IH, Mahmoud AM. Curcumin and Nano-curcumin mitigate copper neurotoxicity by modulating oxidative stress, inflammation, and Akt/GSK-3β Signaling. Molecules. 2021:26(18):5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prati F, De Simone A, Armirotti A, Summa M, Pizzirani D, Scarpelli R, Bertozzi SM, Perez DI, Andrisano V, Perez-Castillo A, et al. 3,4-Dihydro-1,3,5-triazin-2(1H)-ones as the first dual BACE-1/GSK-3β fragment hits against Alzheimer's disease. ACS Chem Neurosci. 2015:6(10):1665–1682. [DOI] [PubMed] [Google Scholar]
- 13. Llorens-Martín M, Jurado J, Hernández F, Avila J. GSK-3β, a pivotal kinase in Alzheimer disease. Front Mol Neurosci. 2014:7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumar A, Srivastava G, Negi AS, Sharma A. Docking, molecular dynamics, binding energy-MM-PBSA studies of naphthofuran derivatives to identify potential dual inhibitors against BACE-1 and GSK-3β. J Biomol Struct Dyn. 2019:37(2):275–290. [DOI] [PubMed] [Google Scholar]
- 15. Mantle D, Heaton RA, Hargreaves IP. Coenzyme Q10, ageing and the nervous system: an overview. Antioxidants (Basel). 2021:11(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dumont M, Kipiani K, Yu F, Wille E, Katz M, Calingasan NY, Gouras GK, Lin MT, Beal MF. Coenzyme Q10 decreases amyloid pathology and improves behavior in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2011:27(1):211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu HT, Cheng SB, Huang YC, Huang YT, Lin PT. Coenzyme Q10 and oxidative stress: inflammation status in hepatocellular carcinoma patients after surgery. Nutrients. 2017:9(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumar P, Singh A, Kumar A, Kumar R, Pal R, Sachan AK, Dixit RK, Nath R. Effect of curcumin and coenzyme Q10 alone and in combination on learning and memory in an animal model of Alzheimer's disease. Biomedicines. 2023:11(5):1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wear D, Vegh C, Sandhu JK, Sikorska M, Cohen J, Pandey S. Ubisol-Q10, a Nanomicellar and water-dispersible formulation of coenzyme-Q10 as a potential treatment for Alzheimer's and Parkinson's disease. Antioxidants (Basel). 2021:10(5):764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang M, Lian N, Yu Y, Wang Y, Xie K, Yu Y. Coenzyme Q10 alleviates sevoflurane-induced neuroinflammation by regulating the levels of apolipoprotein E and phosphorylated tau protein in mouse hippocampal neurons. Mol Med Rep. 2020:22(1):445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lazourgui MA, El-Aoufi S, Labsi M, Maouche B. Coenzyme Q10 supplementation prevents iron overload while improving glycaemic control and antioxidant protection in insulin-resistant Psammomys obesus. Biol Trace Elem Res. 2016:173(1):108–115. [DOI] [PubMed] [Google Scholar]
- 22. Abuelezz SA, Hendawy N. Spotlight on coenzyme Q10 in scopolamine-induced Alzheimer's disease: oxidative stress/PI3K/AKT/GSK 3ß/CREB/BDNF/TrKB. J Pharm Pharmacol. 2023:75(8):1119–1129. [DOI] [PubMed] [Google Scholar]
- 23. Goschorska M, Gutowska I, Baranowska-Bosiacka I, Barczak K, Chlubek D. The use of antioxidants in the treatment of migraine. Antioxidants (Basel). 2020:9(2):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kawashiri T, Shimizu S, Shigematsu N, Kobayashi D, Shimazoe T. Donepezil ameliorates oxaliplatin-induced peripheral neuropathy via a neuroprotective effect. J Pharmacol Sci. 2019:140(3):291–294. [DOI] [PubMed] [Google Scholar]
- 25. Saeed A, Qusti SY, Almarwani RH, Jambi EJ, Alshammari EM, Gusty NF, Balgoon MJ. Effects of aluminum chloride and coenzyme Q10 on the molecular structure of lipids and the morphology of the brain hippocampus cells. RSC Adv. 2021:11(48):29925–29933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beltagy D, Nawar N, Tarek M, Tousson E, EL-Keey M. Beneficial consequences of probiotic on mitochondrial hippocampus In Alzheimer’s disease. J Complement Integr Med. 2021:18(4):761–767. [DOI] [PubMed] [Google Scholar]
- 27. Goldberg DM, Spooner RJ. Assay of glutathione reductase. In: Methods of enzymatic analysis. Vol. 113. 3rd ed. Deerfiled Beach: Verlog Chemie; 1983. pp. 258–265. [Google Scholar]
- 28. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967:70(1):158–169. [PubMed] [Google Scholar]
- 29. Dreux C. Determination of iron in serum. Ann Biol Clin. 1977:35:275. [PubMed] [Google Scholar]
- 30. Tousson E. Histopathological alterations after a growth promoter boldenone injection in rabbits. Toxicol Ind Heath. 2016:32(2):299–305. [DOI] [PubMed] [Google Scholar]
- 31. Tousson E, Beltagy DM, El-Gerbed MS, Gazia MA, Akela MA. The ameliorating role of folic acid in rat hippocampus after propyl thio uracil-induced hypothyroidism. Biomed Aging Pathol. 2012:2(3):104–110. [Google Scholar]
- 32. Gudi V, Gai L, Herder V, Tejedor LS, Kipp M, Amor S, Sühs KW, Hansmann F, Beineke A, Baumgärtner W, et al. Synaptophysin is a reliable marker for axonal damage. J Neuropathol Exp Neurol. 2017:76(2):109–125. [DOI] [PubMed] [Google Scholar]
- 33. Hosseini L, Majdi A, Sadigh E, Farajdokht F, Ziaee M, Aghsan SR, Farzipour M, Mahmoudi J. Coenzyme Q10 ameliorates aging-induced memory deficits via modulation of apoptosis, oxidative stress, and mitophagy in aged rats. Exp Gerontol. 2022:168:111950. [DOI] [PubMed] [Google Scholar]
- 34. Pal A, Cerchiaro G, Rani I, Ventriglia M, Rongioletti M, Longobardi A, Squitti R. Iron in Alzheimer’s disease: from physiology to disease disabilities. Biomol Ther. 2022:12(9):1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Viktorinova A, Durfinova M. Mini-review: is iron-mediated cell death (ferroptosis) an identical factor contributing to the pathogenesis of some neurodegenerative diseases? Neurosci Lett. 2021:745:135627. [DOI] [PubMed] [Google Scholar]
- 36. Lou Q, Meng XE, Wei C, Tong J, Chen Y, Li M, Wang Q, Guo S, Duan JA, Shang EX. Jian-Yan-Ling capsules ameliorate cognitive impairment in mice with D-galactose-induced senescence and inhibit the oxidation-induced apoptosis of HT22 hippocampal cells by regulating the Nrf2-HO1 signaling pathway. J Ethnopharmacol. 2023:310:116356. [DOI] [PubMed] [Google Scholar]
- 37. Squitti R, Sparks DL, Hoogenraad TU, Brewer GJ. Copper status in Alzheimer’s disease and other neurodegenerative disorders: genetics, mechanisms, neurophysiology, and therapies. Int. J Alzheimers Dis. 2011:2011(1):903940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sedaghat A, Samadi M, Shirvani H, Sepandi M, Tahmasebi W. Coenzyme Q10 supplementation and oxidative stress parameters: an updated systematic review and meta-analysis of randomized controlled clinical trials. Asian. J Sports Med. 2022:13:131308. [Google Scholar]
- 39. Drobnic F, Lizarraga MA, Caballero-García A, Cordova A. Coenzyme Q10 supplementation and its impact on exercise and sport performance in humans: a recovery or a performance-enhancing molecule? Nutrients. 2022:14(9):1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ebrahimi A, Kamyab A, Hosseini S, Ebrahimi S, Ashkani-Esfahani S. Involvement of coenzyme Q10 in various neurodegenerative and psychiatric diseases. Biochem Res Int. 2023:2023:5510874–5510811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Munishamappa V, Seethalakshmi, Vijayakumar AE, Rajathilagam T. Evaluation of the antioxidant activity of donepezil - in vitro study. Natl J Physiol Pharm Pharmacol. 2019:9(2):108–110. [Google Scholar]
- 42. Sharma K. Cholinesterase inhibitors as Alzheimer's therapeutics (review). Mol Med Rep. 2019:20:1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haider S, Liaquat L, Ahmad S, Batool Z, Siddiqui RA, Tabassum S, Shahzad S, Rafiq S, Naz N. Naringenin protects AlCl3/D-galactose induced neurotoxicity in rat model of AD via attenuation of acetylcholinesterase levels and inhibition of oxidative stress. PLoS One. 2020:15(1):e0227631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sharma A, Kshetrimayum C, Sadhu HG, Kumar S. Arsenic-induced oxidative stress, cholinesterase activity in the brain of Swiss albino mice, and its amelioration by antioxidants vitamin E and coenzyme Q10. Environ Sci Pollut Res Int. 2018:25(24):23946–23953. [DOI] [PubMed] [Google Scholar]
- 45. Lin CC, Huang TL. Brain-derived neurotrophic factor and mental disorders. Biom J. 2020:43(2):134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sheykhhasan M, Amini R, Soleimani Asl S, Saidijam M, Hashemi SM, Najafi R. Neuroprotective effects of coenzyme Q10-loaded exosomes obtained from adipose-derived stem cells in a rat model of Alzheimer's disease. Biomed Pharmacother. 2022:152:113224. [DOI] [PubMed] [Google Scholar]
- 47. Beltagy DM, Nawar NF, Mohamed TM, Tousson E, El-Keey MM. The synergistic effect of nanocurcumin and donepezil on Alzheimer's via PI3K/AKT/GSK-3β pathway modulating. Prostaglandins Other Lipid Mediat. 2024:170:106791. [DOI] [PubMed] [Google Scholar]
- 48. Guan PP, Cao LL, Wang P. Elevating the levels of calcium ions exacerbate Alzheimer’s disease via inducing the production and aggregation of β-amyloid protein and phosphorylated tau. Int J Mol Sci. 2021:22(11):5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hampel H, Vassar R, De Strooper B, Hardy J, Willem M, Singh N, Zhou J, Yan R, Vanmechelen E, De Vos A, et al. The β-secretase BACE-1 in Alzheimer’s disease. Biol Psychiatry. 2021:89(8):745–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taylor J, Abdel-Wahab O. PTEN isoforms with dual and opposing function. Nat Cell Biol. 2019:21(11):1306–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Matsuda S, Nakagawa Y, Tsuji A, Kitagishi Y, Nakanishi A, Murai T. Implications of PI3K/AKT/PTEN Signaling on superoxide Dismutases expression and in the pathogenesis of Alzheimer's disease. Diseases. 2018:6(2):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kurashiki T, Horikoshi Y, Kamizaki K, Sunaguchi T, Hara K, Morimoto M, Kitagawa Y, Nakaso K, Otsuki A, Matsura T. Molecular mechanisms underlying the promotion of wound repair by coenzyme Q10: PI3K/Akt signal activation via alterations to cell membrane domains. J Clin Biochem Nutr. 2022:3(3):222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Farr SA, Sandoval KE, Niehoff ML, Witt KA, Kumar VB, Morley JE. Peripheral administration of GSK-3beta antisense oligonucleotide improves learning and memory in SAMP8 and Tg2576 mouse models of Alzheimer's disease. J Alzheimers Dis. 2016:54(4):1339–1348. [DOI] [PubMed] [Google Scholar]
- 54. Jiang YJ, Jin J, Nan QY, Ding J, Cui S, Xuan MY, Piao MH, Piao SG, Zheng HL, Jin JZ, et al. Coenzyme Q10 attenuates renal fibrosis by inhibiting RIP1-RIP3-MLKL-mediated necroinflammation via Wnt3α/β-catenin/GSK-3β signaling in unilateral ureteral obstruction. Int Immunopharmacol. 2022:108:108868. [DOI] [PubMed] [Google Scholar]
- 55. Min D, Mao X, Wu K, Cao Y, Guo F, Zhu S, Xie N, Wang L, Chen T, Shaw C, et al. Donepezil attenuates hippocampal neuronal damage and cognitive deficits after global cerebral ischemia in gerbils. Neurosci Lett. 2012:510(1):29–33. [DOI] [PubMed] [Google Scholar]
- 56. Ramezani M, Sahraei Z, Simani L, Heydari K, Shahidi F. Coenzyme Q10 supplementation in acute ischemic stroke: is it beneficial in short-term administration? Nutr Neurosci. 2020:23(8):640–645. [DOI] [PubMed] [Google Scholar]
- 57. Baerends E, Soud K, Folke J, Pedersen AK, Henmar S, Konrad L, Lycas MD, Mori Y, Pakkenberg B, Woldbye DPD, et al. Modeling the early stages of Alzheimer's disease by administering intracerebroventricular injections of human native Aβ oligomers to rats. Acta Neuropathol Commun. 2022:10(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Salama M, Yuan TF, Machado S, Murillo-Rodríguez E, Vega JA, Menéndez-González M, Nardi AE, Arias-Carrión O. Co-enzyme Q10 to treat neurological disorders: basic mechanisms, clinical outcomes, and future research direction. CNS Neurol Disord Drug Targets. 2013:12(5):641–664. [DOI] [PubMed] [Google Scholar]
- 59. Nawar NF, Beltagy DM, Mohamed TM, Tousson E, El-Keey MM. Ameliorative anti-coagulant, anti-oxidative and anti-ferroptotic activities of nanocurcumin and donepezil on coagulation, oxidation and ferroptosis in Alzheimer's disease. Toxicol Res (Camb). 2024:13(2):tfae054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be available upon request. The data of this article are included within the article and its additional files.