Abstract
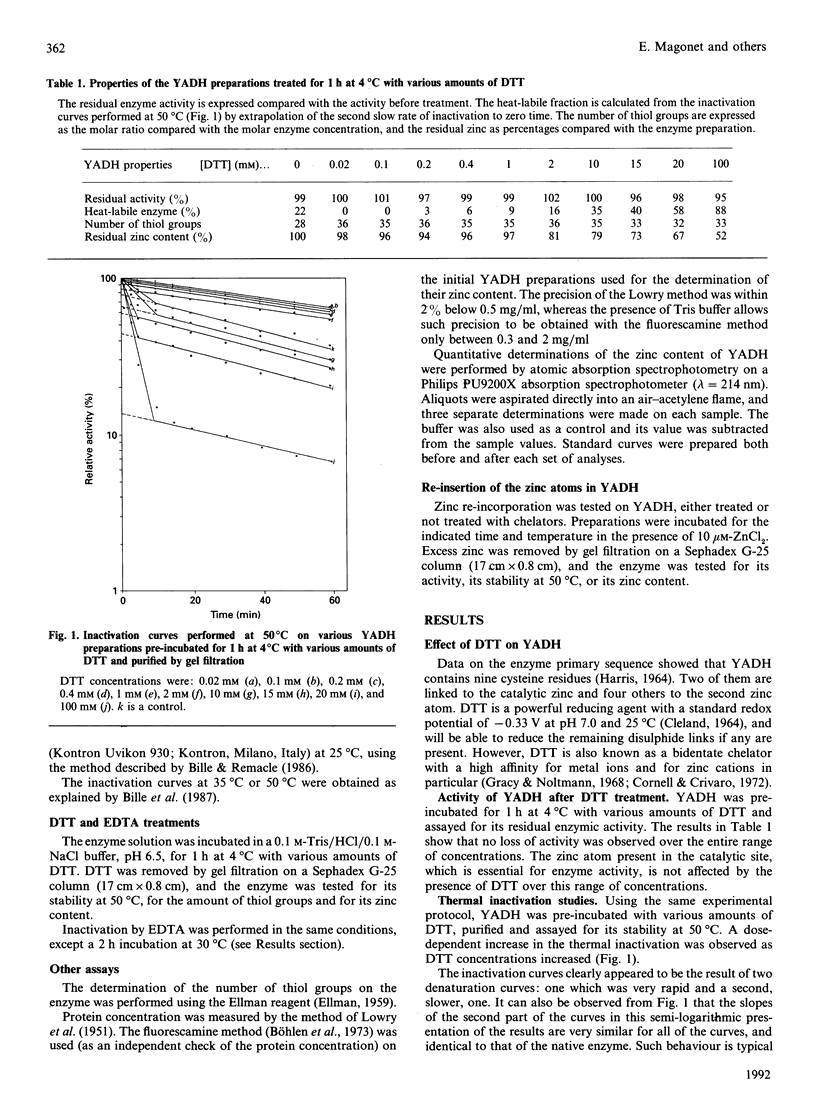
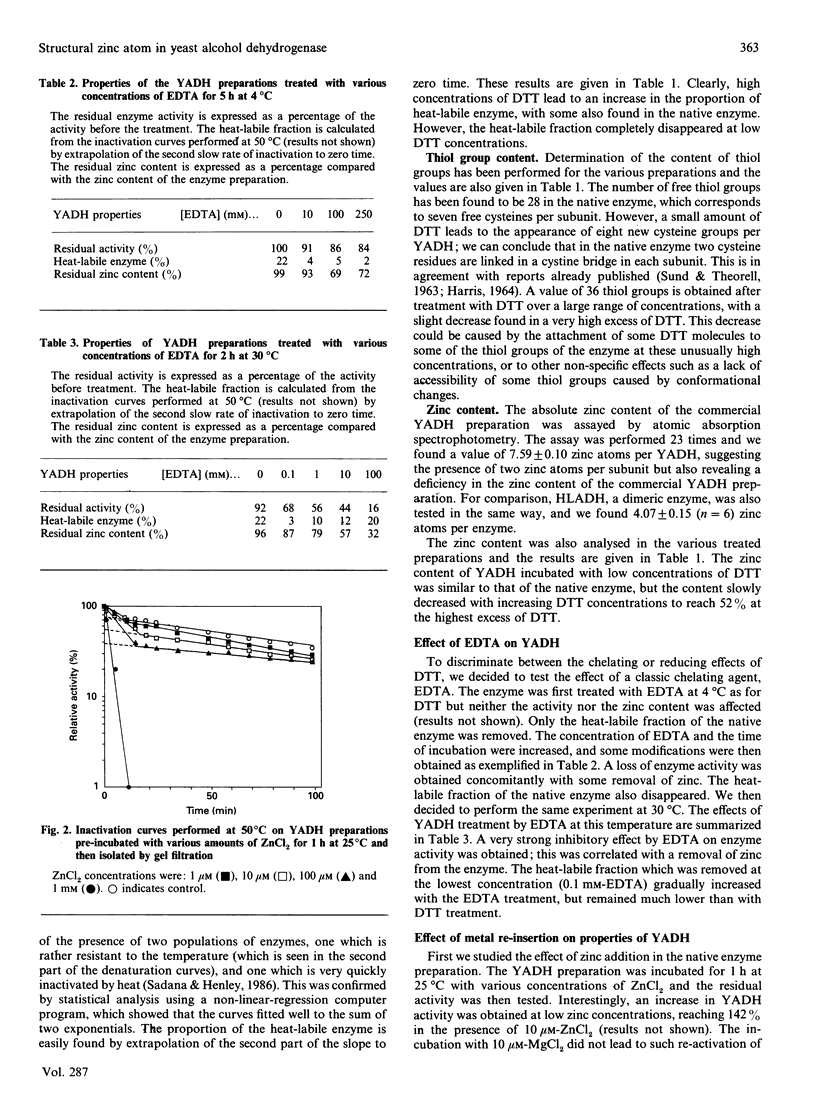
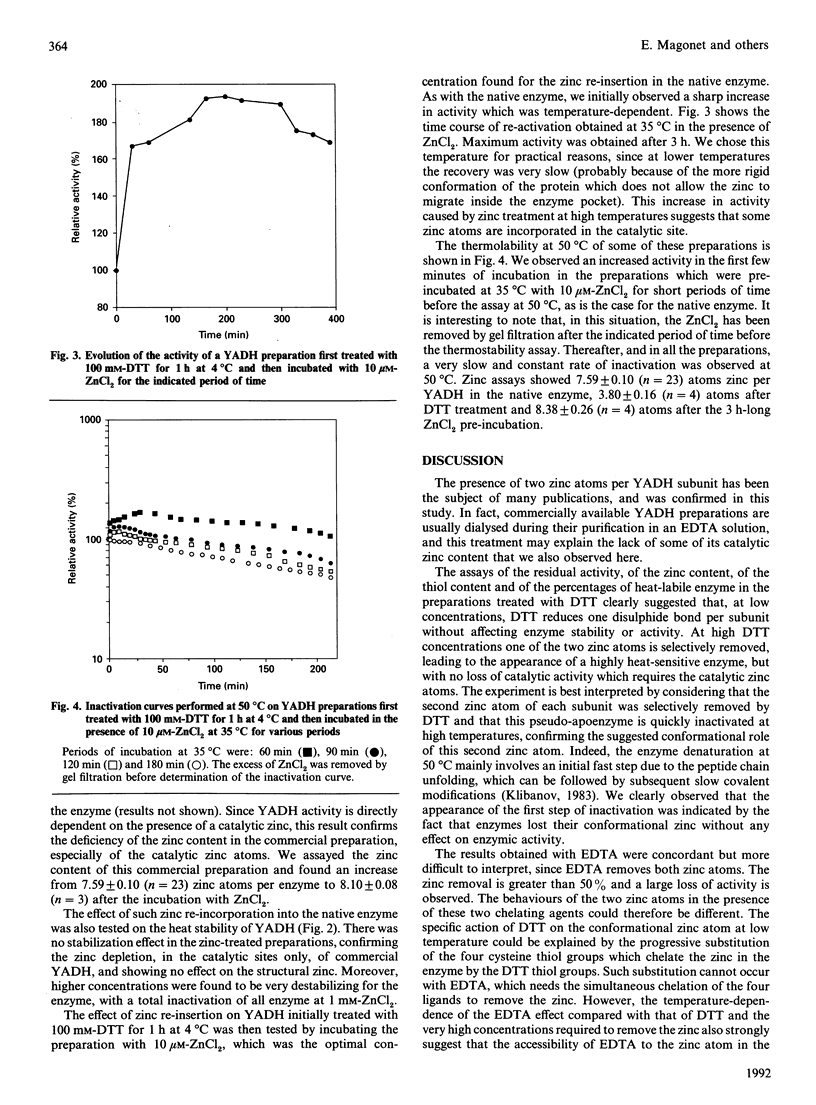
Yeast alcohol dehydrogenase is a tetrameric enzyme containing zinc. Initially we confirmed the presence of two zinc atoms per subunit. Incubation of the enzyme with increasing concentrations of dithiothreitol, a method for partial chelation, allowed first the reduction of four disulphide bridges per enzyme, but eventually was sufficient to chelate the structural zinc atom without having any effect on the zinc located in the active site. The enzyme activity was not affected but the enzyme became very sensitive to heat denaturation. Chelation by EDTA was also performed. Given its location at an external position in the globular protein, protected in each subunit by one disulphide bridge, the results establish that the second zinc atom present on each enzymic subunit plays a prominent conformational role, probably by stabilizing the tertiary structure of yeast alcohol dehydrogenase. Recovery experiments were performed by incubation of the native enzyme, or the dithiothreitol-treated enzyme, with a small amount of Zn2+. A stabilization effect was found when the structural zinc was re-incorporated after its removal by dithiothreitol. In all cases a large increase in activity was also observed, which was much greater than that expected based on the amount of re-incorporated zinc atom, suggesting the re-activation of some inactive commercial enzyme which had lost some of its original catalytic zinc atoms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bille V., Plainchamp D., Remacle J. Affinity and stability modifications of immobilized alcohol dehydrogenase through multipoint copolymerization. Biochim Biophys Acta. 1987 Oct 15;915(3):393–398. doi: 10.1016/0167-4838(87)90025-2. [DOI] [PubMed] [Google Scholar]
- Bille V., Remacle J. Simple-kinetic descriptions of alcohol dehydrogenase after immobilization on tresyl-chloride-activated agarose. Eur J Biochem. 1986 Oct 15;160(2):343–348. doi: 10.1111/j.1432-1033.1986.tb09977.x. [DOI] [PubMed] [Google Scholar]
- Block C., Lohmann R. D., Beyersmann D. Probing of active site residues of the zinc enzyme 5-aminolevulinate dehydratase by spin and fluorescence labels. Biol Chem Hoppe Seyler. 1990 Dec;371(12):1145–1152. doi: 10.1515/bchm3.1990.371.2.1145. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- Coleman P. L., Weiner H. Growth, isolation, and characterization of a yeast manganese alcohol dehydrogenase. Biochemistry. 1973 Aug 28;12(18):3466–3472. doi: 10.1021/bi00742a017. [DOI] [PubMed] [Google Scholar]
- Cornell N. W., Crivaro K. E. Stability constant for the zinc-dithiothreitol complex. Anal Biochem. 1972 May;47(1):203–208. doi: 10.1016/0003-2697(72)90293-x. [DOI] [PubMed] [Google Scholar]
- Dent A. J., Beyersmann D., Block C., Hasnain S. S. Two different zinc sites in bovine 5-aminolevulinate dehydratase distinguished by extended X-ray absorption fine structure. Biochemistry. 1990 Aug 28;29(34):7822–7828. doi: 10.1021/bi00486a007. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Gracy R. W., Noltmann E. A. Studies on phosphomannose isomerase. II. Characterization as a zinc metalloenzyme. J Biol Chem. 1968 Aug 10;243(15):4109–4116. [PubMed] [Google Scholar]
- Jörnvall H. Differences between alcohol dehydrogenases. Structural properties and evolutionary aspects. Eur J Biochem. 1977 Feb;72(3):443–452. doi: 10.1111/j.1432-1033.1977.tb11268.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Eklund H., Brändén C. I. Subunit conformation of yeast alcohol dehydrogenase. J Biol Chem. 1978 Dec 10;253(23):8414–8419. [PubMed] [Google Scholar]
- Klibanov A. M. Immobilized enzymes and cells as practical catalysts. Science. 1983 Feb 11;219(4585):722–727. doi: 10.1126/science.219.4585.722. [DOI] [PubMed] [Google Scholar]
- Klinman J. P., Welsh K. The zinc content of yeast alcohol dehydrogenase. Biochem Biophys Res Commun. 1976 Jun 7;70(3):878–884. doi: 10.1016/0006-291x(76)90673-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Sadana A., Henley J. P. Influence of chemical modification on enzyme inactivation kinetics and stability. Biotechnol Adv. 1986;4(1):27–74. doi: 10.1016/0734-9750(86)90004-2. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Short and long spacer sequences and other structural features of zinc binding sites in zinc enzymes. FEBS Lett. 1989 Oct 23;257(1):138–140. doi: 10.1016/0014-5793(89)81805-8. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990 Jun 19;29(24):5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- Veillon C., Sytkowski A. J. The intrinsic zinc atoms of yeast alcohol dehydrogenase. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1494–1500. doi: 10.1016/0006-291x(75)90195-3. [DOI] [PubMed] [Google Scholar]


