Abstract
Purpose
Implanted devices used in metastatic spine tumor surgery (MSTS) include pedicle screws, fixation plates, fixation rods, and interbody devices. A material to be used to fabricate any of these devices should possess an array of properties, which include biocompatibility, no toxicity, bioactivity, low wear rate, low to moderate incidence of artifacts during imaging, tensile strength and modulus that are comparable to those of cortical bone, high fatigue strength/long fatigue life, minimal or no negative impact on radiotherapy (RT) planning and delivery, and high capability for fusion to the contiguous bone. The shortcomings of Ti6Al4V alloy for these applications with respect to these desirable properties are well recognized, opening the field for an investigation about novel biomaterials that could replace the current gold standard. Previously published reviews on this topic have exhibited significant shortcomings in the studies they included, such as a small, heterogenous sample size and the lack of a cost-benefit analysis, extremely useful to understand the practical possibility of applying a novel material on a large scale. Therefore, this review aims to collect information about the clinical performance of these biomaterials from the most recent literature, with the objective of deliberating which could potentially be better than titanium in the future, with particular attention to safety, artifact production and radiotherapy planning interference. The significant promise showed by analyzing the clinical performance of these devices warrants further research through prospective studies with a larger sample size also taking into account each aspect of the production and use of such materials.
Methods
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines were used to improve the reporting of the review. The search was performed from March 2022 to September 2023.
Results
At the end of the screening process, 20 articles were considered eligible for this study. Polyetheretherketone (PEEK), Carbon-fibre reinforced polyetheretherketone (CFR-PEEK), long carbon fiber reinforced polymer (LCFRP), Polymethylmethacrylate (PMMA), and carbon screw and rods were used in the included studies.
Conclusion
CFR-PEEK displays a noninferior safety and efficacy profile to titanium implanted devices. However, it also has other advantages. By decreasing artifact production, it is able to increase detection of local tumor recurrence and decrease radiotherapy dose perturbation, ultimately bettering prognosis for patients necessitating adjuvant treatment. Nonetheless, its drawbacks have not been explored fully and still require further investigation in future studies. This does not exclude the fact that CFR-PEEK could be a valid alternative to titanium in the near future.
Keywords: Spinal tumors, Metastases, Spine, Radiolucent implanted devices, Radiotherapy, Coated implant
Introduction
The lives of cancer patients are significantly impacted by spine metastases, which often result in severe pain and neurological disability [1]. Over 30% of patients who have received a cancer diagnosis will be affected by metastatic spine tumors [2], with the thoracic spine being the most common localization followed by the lumbar and cervical sections [3, 4]. Statistically, up to 10% of these patients will develop a clinically significant lesion [5]. Four of the major treatment goals for spine tumours are globally applicable to all spine disorders: achieving pain control, restoration or maintenance of neurological function, spinal stability, and improvement in health-related quality of life [6].
Although the treatment of spine metastases remains palliative, the introduction of targeted agents and immunotherapy has significantly improved survival times for patients with metastases from a wide range of tumour histologies. Local treatment for spine tumours needs to provide long-term local control and prioritize an early return to systemic therapy [7]. Radiation therapy and surgery are the main treatment options for spine metastases. However, the most significant overall advancement has been the evolution and integration of spine stereotactic body radiotherapy (SBRT), as its capacity to deliver an adequate adjuvant radiation dose has drastically transformed therapeutic approaches [8]. As supportive evidence, recent findings reported the superiority of surgical treatment in combination with radiotherapy (RT), as opposed to the previously established therapeutical approach combining RT with corticosteroids [9, 10]. Compared to more invasive methods previously used to treat spine metastases, separation surgery and percutaneous vertebral body cement augmentation have been employed and decreased operation-related morbidity [1, 11, 12]. In addition, a further decrease in operation-related morbidity is reported with minimal access surgery using percutaneous and fenestrated pedicle screws [1].
The implant of traditional metal devices could produce heavy metal artifacts in MRI and CT scans [13, 14]. These artifacts could alter the density and compositions of the healthy tissue, causing a perturbation of radiation beams. In general, irradiation through metal implanted devices should be avoided, particularly for RT, where the local control of the disease and the preservation of normal tissue may be compromised by distortion of the dose distributions and range uncertainties [15]. This advice may not always be observed when defining plan geometry, though, if the choice of beam direction is constrained by dose restrictions for the organs at risk or by the lack of a gantry. In addition, imaging artefacts can make it difficult to distinguish between target and normal structures and can reduce dosage calculations’ accuracy [15]. In RT, where dose computation is based on Hounsfield Unit to water equivalent path length calibration curve, CT number assignment to tissues within the irradiation volume is a highly critical issue, which can be exacerbated by the presence of artefacts created by implant materials [16].
In Metastatic Spine Tumour Surgery (MSTS), Titanium alloy Ti6Al4V is the gold standard of implant material. Ti6Al4V has replaced stainless steel due to its higher corrosion and fatigue resistance, lower density and lower modulus of elasticity (E) [17]. However, Ti6Al4V has some shortcomings, including a larger E compared to that of cortical bone (respectively 110 GPa vs. 17–21 GPa). Differences in modulus of elasticity are related to a higher risk of fracture of the adjacent segments or risk of subsidence of cage implanted devices [18–21]. Furthermore, Ti6Al4V may be responsible for the diffusion of metal ions to the surrounding tissue, possibly causing metallosis, redox abnormalities and oxidative stress [22]. Moreover, Ti6Al4V causes artifacts in MRI or CT assessment, decreasing the accuracy of post-RT planning [23]. The presence of artifacts with Ti6Al4V could influence the radiotherapy dose interfering with imaging, hindering the contouring of tumours and surrounding organs [15]. Furthermore, identification of tumour relapse on follow-up imaging may be delayed due to scattering [24]. Software algorithms have been developed to overcome the interference of titanium implanted devices with treatment planning but may result in delayed care and overall waste of resources [25].
Implanted devices used in MSTS include pedicle screws, fixation plates, fixation rods, and interbody devices (cages). A material to be used to fabricate any of these devices should possess an array of properties, which include biocompatibility, no toxicity, bioactivity, low wear rate, low to moderate incidence of artifacts during imaging (via, for example, radiography, fluoroscopy, computed tomography (CT), and magnetic resonance imaging (MRI)), tensile strength and modulus that are comparable to those of cortical bone, high fatigue strength/long fatigue life, minimal or no negative impact on RT planning and delivery, and high capability for fusion to the contiguous bone. The shortcomings of Ti6Al4V alloy for these applications with respect to these desirable properties are well recognized, as stated above. As such, in recent times, other materials, such as polyetheretherketone (PEEK), carbon fiber-reinforced PEEK (CFR-PEEK), nano-sized TiO2-reinforced PEEK, nano-sized hydroxyapatite (HAp)-reinforced PEEK, nano-sized TiO2/HAp/carbon fiber-reinforced PEEK, have been used in conjunction with subtractive manufacturing method(s), such as rolling, forging, and extrusion. Additionally, there are reports of use of some of the aforementioned materials and additive manufacturing (AM), although these still require further investigation [26–29]. Initial studies report that the rigidity and elasticity of 3D-printed PEEK are still similar to that of human bone [30, 31]. Due to the continuous development of new materials and the improvements in 3D printing surfaces, in the last years, several articles have been published on this topic, reflecting the potential interest of the international audience.
PEEK can be used for the reconstruction of vertebral bodies and promotes interbody fusion without decreasing the accuracy of CT, MRI and RT [29, 32]. In addition, this material is bioinert and biocompatible, with a Young’s modulus (3.6 GPa) similar to that of bone [18, 33]. Although PEEK has been shown to have a low fusion rate [34], this limit could be increaased by surface modifications using coatings such as hydroxyapatite (HAp). Furthermore, in conditions requiring higher tensile strength while still allowing for proper radiological follow-up and RT planning, CFR-PEEK may be used as an alternative to titanium [35–40]. The latter has shown complication and revision rates of 9.3% and 17.1% respectively [41], thereby representing a safe material for spinal instrumentation in the oncological context. Other reinforcements for PEEK based implant materials, such as TiO2, have been studied. Nano-sized TiO2-reinforced PEEK has been reported to have high thermal stability and antibacterial action against both Gram-positive and Gram-negative bacteria when exposed to simulated body fluid (SBF) [42].
This systematic review provides a detailed analysis of novel radiolucent biomaterials used in spine metastases management compared with Ti6Al4V and investigates their possible role in the context of MSTS. This study aims to collect information about the clinical performance of these biomaterials from the most recent literature, with the objective of deliberating which could potentially be better than titanium in the future, with particular attention to safety, artifact production and radiotherapy planning interference.
Materials and methods
Eligibility criteria and information sources
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines were used to improve the reporting of the review.
The research question was formulated using the PICOS approach: Patient (P); Intervention (I); Comparator (C); Outcome (O), and Study design (S). The present study selects articles that include patients with primary spine tumours and/or metastases (P), treated by standard implanted devices of Ti6Al4V (I), comparing them with patients treated with radiolucent materials (C). The aim was to assess the resistance properties of the materials, the grade of osteointegration, the complications rate and the possible advantages in radiotherapy usage and imaging follow-up. Moreover, the goals included: describing the evolution of novel materials in spinal surgery for tumour management, their properties and limitations as an alternative to Ti6Al4V, the current strategies to optimize their usage, the rate of artifacts and possible advantages in RT protocols (O). For this purpose, randomized controlled trials (RCTs), non-randomized controlled trials (NRCTs), prospective observational studies (POSs), case-series (CS), retrospective clinical studies (RCSs), case reports (CRs) and retrospective cohort studies (RCoSs) were included.
A comprehensive search of Medline, Cochrane, Scopus, CINAHL, and Embase was conducted from the databases’ inception to September 2023.
Search strategy
The following keywords were used: “spinal neoplasms”, “metastatic spine tumour surgery”, “spine surgery”, “spine tumours”, “spine oncology”, “spinal metastases”, ‘‘spinal neoplasms”, ‘‘metastatic spine tumour surgery”, ‘‘spine surgery”, “Ti6Al4V”, “titanium dioxide,”, “implant”, “screw”, “rods”, “system”, “polyetheretherketone”, “PEEK,”, “PAEK”, “PMMA”, “silicon nitride”, “HA coated PEEK”, “hydroxyapatite coated“, “titanium PEEK”, “Ti35Nb4Sn”, “carbon”, “carbon fiber” “carbon fiber PEEK”, ‘‘stainless steel”, ‘‘polyetheretherketone”, ‘‘hydroxyapatite”, “radiolucent”, “porosity”, “elastic modulus,”, “weight-bearing”, “corrosion”, “rigidity” “fatigue strength”, “bioactivity”, “intraoperative contouring”, “intra-operative cutting”, “stress shielding”, ‘‘osseointegration”, ‘‘artifacts”, ‘‘radiotherapy”, ‘‘magnetic resonance imaging”, ‘‘computed tomography”. Keywords were used both isolated and combined to their Mesh terms. In addition, more studies were searched among the references of the selected papers. The final search string was: ((((((((((spinal neoplasms) OR (metastatic spine tumor surgery)) OR (spine surgery)) OR (spine tumors)) OR (spine oncology)) OR (spinal metastases)) OR (spinal neoplasms)) OR (spine surgery)) AND (((Ti6Al4V) OR (titanium dioxide)) OR ((((implant) OR (screw)) OR (rods)) OR (system)))) AND ((((((((((((((((polyetheretherketone) OR (PEEK)) OR (PAEK)) OR (PMMA)) OR (silicon nitride)) OR (HA coated PEEK)) OR (hydroxyapatite coated)) OR (titanium PEEK)) OR (Ti35Nb4Sn)) OR (carbon)) OR (carbon fiber)) OR (carbon fiber PEEK)) OR (stainless steel)) OR (polyetheretherketone)) OR (hydroxyapatite)) OR (radiolucent))) AND ((((((radiotherapy) OR (magnetic resonance imaging)) OR (computed tomography)) OR (radiotherapy)) OR (magnetic resonance imaging)) OR (computed tomography)).
Inclusion criteria
Only articles published in English were included, and screening was limited to clinical studies. Articles reporting patients who underwent surgery for primary spinal tumours or metastases were considered. All types of primary tumours were included.
Exclusion criteria
The following exclusion criteria were applied: studies not published in English, technical notes, in vitro, biomechanical, finite elements, animal and cadaver studies.
In addition, papers were discarded if subjects were affected by osteoporotic vertebral fractures, degenerative conditions, rheumatic conditions, infective diseases, and pre-existing vertebral fractures.
Selection process and data collection
The search was performed from March 2022 to September 2023. The initial search of the articles was conducted by two independent authors (GC and GZ) using the previously described search protocol. The chosen research order consisted of screening titles first and abstracts and then full articles. Articles were considered possibly relevant, and their full texts were reviewed if both independent reviewers found they could not be excluded based on their title and abstracts. In case of disagreement, a third reviewer was consulted (SDS). After the full-text assessment, the references of the articles included were screened. Articles were screened using the CADIMA software [43]. The number of articles included or excluded was registered and reported in a PRISMA flowchart. Rules reported by Page et al. were followed in designing the PRISMA chart (44).
Data items and synthesis methods
The extracted study characteristics were: author, year, journal, country, type of study and level of evidence (LOE), sample size, mean age, type of tumour, location of the tumour, histology, type of treatment, implant material and proprieties, postoperative radiotherapy, follow-up, local recurrence rate (defined as the number of patients who experienced a recurrence during the study period), fusion rate, complications and possible advantages of new materials. Furthermore, the Visual Analog Scale (VAS), Oswestry Disability Index (ODI) [44] and the American Spinal Cord Injury Association (ASIA) impairment scale values were reported when available.
Categorical data were summarized as frequencies with percentages. However, the data obtained from the studies were too different and heterogeneous to perform a meta-analysis.
Assessment of risk-of-bias studies
The Risk of Bias (RoB 2) tool for randomized trials and The Methodological Index for Non-Randomized Studies (MINORS) were used to assess the quality of the included studies [45, 46]. However, no randomized controlled trials were found. The MINORS score consists of 12 items: clearly stated aim; inclusion of consecutive patients; prospective data collection; endpoints appropriate to study aim; unbiased assessment of study endpoint; follow-up period appropriate to study aim; <5% lost to follow-up; prospective calculation of study size; adequate control group; contemporary groups; baseline equivalence of groups; and adequate statistical analyses [45]. The reviewers individually evaluated all these items. The MINORS items were scored 0 if not reported, 1 when reported but inadequate, and 2 when reported and adequate. The ideal global score was 20 for NRCTs. The simplicity of MINORS comprising only 12 items, makes this item readily usable by both readers and researchers. The reliability of this score has already been documented [45].
Using the MINORS tool, two authors (GC and GZ) independently assessed the potential risk of bias in the selected studies. If no consensus was obtained between the two reviewers, the opinion of a third independent reviewer (SDS) was decisive.
Results
Study selection
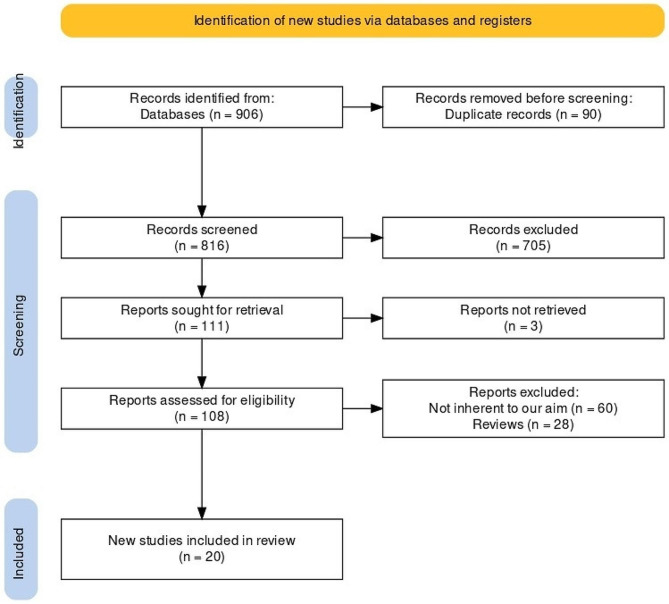
Following the PRISMA protocol, a flowchart diagram displaying the study selection process was reported (Fig. 1). Initially, 906 studies were found (studies from grey literature have not contributed to the research, and unpublished studies were not retrieved). 465 studies were from Medline; 213 from Scopus; 184 from Embase, 39 from CINAHL and 5 from Cochrane. A total of 816 studies were maintained following duplicate removal. Of those, 705 were excluded after the title and abstract screening. Of the 111 remaining reports, 3 were not retrieved. Then, 108 full-text articles were extracted. Among these, 88 were excluded: not inherent to our research (n = 60); no full-text found (n = 28). At the end of the screening process, 20 articles were considered eligible for this study.
Fig. 1.
Prisma flowchart of the included studies
Patients and study characteristics
No RCTs were considered eligible for this study. The selected articles included 20 NRCTs: 13 case-series [21, 24, 47–57], 2 prospective observational studies [35, 58], 2 retrospective cohort studies [59, 60], 2 case reports [37, 61] and 1 retrospective clinical study [62]. The studies ranged from level II to level IV. Studies were published between 2000 [53] and 2021 [50–52, 56, 57]. 823 patients from 41.4 to 86 years were included in this systematic review. The follow-up ranged from 3 to a maximum of 53 months. Thirteen studies (65%) were performed in European countries, three studies in the United States (15%), one study in Japan (5%), one study in China (5%), one study in Korea (5%) and one was a multicentre study (5%). All the characteristics of the patients included were reported in Table 1.
Table 1.
Patient and study characteristics
| Author | Year | Country | Type of study and Level of Evidence (LOE) | Sample size | Age (y) | |||
|---|---|---|---|---|---|---|---|---|
| M | F | M | F | |||||
| Shen, et al. | 2022 | Multicenter | Retrospective, multicenter cohort study, LOE III | 7 | 6 | 59.4 ± 8.8 | 53.8 ± 17.7 | |
| Wagner, et al. | 2021 | Germany | Case series, LOE IV | 23 | 28 | - | - | |
| Trungu, et al. | 2021 | Italy | Case series, LOE IV | 8 | 9 | - | - | |
| Neal, et al. | 2021 | United States | Case series, LOE IV | 14 | 14 | - | - | |
| Müther, et al. | 2021 | Germany | Case series, LOE IV | 3 | 4 | 43 ± 17.6 | 40.3 ± 14.9 | |
| Cofano, et al. | 2020 | Italy | Prospective observational study, LOE II | 48 | 30 | - | - | |
| 23 | 13 | |||||||
| 25 | 17 | |||||||
| Boriani, et al. | 2020 | Italy | Case series, LOE IV | 3 | 3 | - | - | |
| Pipola, et al. | 2020 | Italy | Case series, LOE IV | 1 | 0 | 42 | - | |
| Sakaura, et al. | 2019 | Japan | Retrospective cohort study, LOE III | 63 | 65 | - | - | |
| 19 | 17 | |||||||
| 44 | 48 | |||||||
| Laux, et al. | 2018 | Switzerland | Case series, LOE IV | 1 | 0 | 77 | - | |
| Boriani, et al. | 2018 | Italy | Case series, LOE IV | 18 | 16 | - | - | |
| Ringel, et al. | 2017 | Germany | Case series, LOE IV | 15 | 20 | 61.9 ± 14.1 | 65 ± 10.1 | |
| 7 | 10 | 61 ± 16.3 | 64.6 ± 8.5 | |||||
| 7 | 10 | 60.7 ± 12.7 | 65.3 ± 11.9 | |||||
| 1 | 0 | 76 | - | |||||
| Tedesco, et al. | 2017 | Italy | Case series, LOE IV | 18 | 10 | - | - | |
| Tan, et al. | 2013 | China | Case series, LOE IV | 16 | 12 | - | - | |
| Anselmetti, et al. | 2013 | Italy | Prospective observational study, LOE II | 22 | 18 | - | - | |
| Rajpal, et al. | 2012 | United States | Case series, LOE IV | 37 | 20 | - | - | |
| 14 | 13 | |||||||
| 2 | 3 | |||||||
| 4 | 1 | |||||||
| Disch, et al. | 2011 | Germany | Retrospective clinical study, LOE III | 9 | 11 | - | - | |
| Burkett, et al. | 2012 | United States of America | Case series, LOE IV | 20 | 9 | - | - | |
| Jang, et al. | 2002 | Korea | Case series, LOE IV | 5 | 5 | 50 ± 18.7 | 60.4 ± 9.9 | |
| Schulte, et al. | 2000 | Germany | Case series, LOE IV | 2 | 3 | 56 ± 14.1 | 54 ± 19.7 | |
Type of implanted devices
CFR-PEEK was the most frequent material used (15 studies), vertebral augmentation with Polymethylmethacrylate (PMMA) was adopted in 5 studies, metal implanted devices with titanium screws were used in 4 studies, PEEK devices were employed used in 2 studies, long carbon fiber reinforced polymer (LCFRP) was used in 1 study, carbon clamps were reported in 1 study (Table 2).
Table 2.
Type of treatment and tumour characteristics
| Author | Year | Type of treatment | Type of tumor | Histology | Location |
|---|---|---|---|---|---|
| Shen, et al. | 2022 | Corpectomy and AR with CFR-PEEK cage + PSD with CFR-PEEK screw-rod system and/or | Primary (NS) | Chordoma, (8) Metastatic Colon, (1) Metastatic renal cell | L1, (1) T11, (1) T12, (2) L3, (1) L5, (2) L2, (1) T1, (2) T6, (1) T9, (1) T11-12 (1) |
| anterior CFR-PEEK plate | Metastases (NS) | carcinoma, (1) Metastatic breast, (1) Metastatic pancreatic, (1) Melanoma (1) | |||
| Wagner, et al. | 2021 | PSD | Primary (NS) | Breast, (13) Nonesmall cel lung, (8) Prostate, (7) Unknown primary, (5) Sarcoma, (3) Uterus, (2) Renal cell, (2) Myeloma, (2) Duodenal, (1) Adrenal, (1) Sinus, (1) Oropharynx, (1) Thyroid, (1) Melanoma, (1) Urothelial, (1) Pharynx, (1) Colorectal (1) | Thoracic, (17) Thoracolumbar, (12) Lumbar (22) |
| Trungu, et al. | 2021 | Anterior corpectomy and plating with CFR-PEEK | Metastases (NS) | Lung3 (17.6%), Kidney 2 (11.8%), Colon 1 (5.9%), Prostate 5 (29.4%), Breast 6 (35.3%) | C3 1 (5.9%), C4 8 (47.1%), C5 5 (29.4%), C6 2 (11.8%), C7 1 (5.9%) |
| Müther, et al. | 2021 | PSD CFR-PEEK | Primary (7) | Hemangiopericytoma, (1) Schwannoma, (5) Cavernous hemangioma (1) | T12-L1, (2) L2-L3, (3) L3-L4 (2) |
| Neal, et al. | 2021 | PSD CFR-PEEK |
Primary (5) Metastases (23) |
Breast, (8) Multiple myeloma, (2) Lung, (3) Pancreas, (2) Prostate, (2) Bile duct, (1) Cervical, (1) Colon, (1) Melanoma, (1) Thyroid, (1) Adenoma (NS), unknown origin, (1) Chondrosarcoma, (3) Ewing sarcoma (2) | Cervical, (2) Thoracic, (19) Thoracolumbar, (4) Lumbar or lumbosacral (3) |
| Cofano, et al. | 2020 | PSD for Thoracic or lumbar locations | Primary (NS) | 12 Lung NSCLC (33.3%), 6 Mieloma (16.7%), 4 Breast (11.1%), 4 Prostate (11.1%), 3 Renal Cell (8.3%), 3 Colon (8.3%), 2 Melanoma (5.6%), 2 Non-Hodgkin Lymphoma (5.6%) | Cervical 1 (2.9%), Thoracic 30 (83.3%), Lumbar 5 (13.8%) |
| AR for cervical lesions | |||||
| PSD | 11 Lung NSCLC (26.2%), 7 Mieloma (16.7%), 7 Breast (16.7%), 6 Prostate (14.3%), 4 Melanoma (9.5%),3 Colon (7.1%), 2 Renal Cell (4.7%), 1 Hepatocellular carcinoma (2.4%), 1 Non-Hodgkin Lymphoma (2.4%) | Cervical 2 (4.7%), Thoracic 32 (76.2%), Lumbar 8 (19.1%) | |||
| Boriani, et al. | 2020 | En bloc (2) | Primary (NS) | Epithelioid sclerosing fibrosarcoma, (1) Giant cell tumor, (2) Chordoma, (1) Meningioma, (1) Ewing’s sarcoma (1) | C3-C4-C5-C6-C7-T1 |
| Intralesional excision (4) | |||||
| + PSD and AR (4) | |||||
| + PSD (2) | |||||
| Pipola, et al. | 2020 | PSD | Primary | Sclerosing Epithelioid Fibrosarcoma | C5-C6-C7 |
| Sakaura, et al. | 2019 | PSD with CBT screw + cages filled with local bone | NS | NS | L1 to L2, (1) L2 to L3, (1) L3 to L4, (19) L4 to L5 (99), L5 to L6, (1) L5 to S1 (7) |
| Boriani, et al. | 2018 | PSD (9); |
Primary (20) Metastases (14) |
NS | Thoracic, (5) Thoraco-lumbar, (19) Lumbar, (2) Lumbo-sacral (8) |
| PSD + intralesional excision (21); | |||||
| PSD + en bloc resection (4). | |||||
| PSD + AR (15) | |||||
| Laux, et al. | 2018 | Dorsal instrumentation and fusion + iliac crest autograft and demineralized bone matrix | Multiple Myeloma | Osteolytic metastasis with vertebral wall involvement | T12 |
| Ringel, et al. | 2017 | PSD CFR-PEEK | Hematologic malignancies (3) | Breast, (5) Chordoma, (1) Esophageal, (1) Fibrous dysplasia, (1) Gastrointestinal, (2) Hepatocarcinoma, (1) Lymphoma, (2) Melanoma, (2) Myeloma, (1) Prostate, (3) Rectum, (1) Rhabdomyosarc (2) | T10 (3); T11 (3); T2 + 3 (1); T3 (1); T4 (1); T4 + 5 (1); T5 (1); T5 + 6 (2); T6 (1); T6 + 7 + 8 (1); T6 + 8 (1); T7 (1); T7 + 9 + 10 (1); T8 + 12 (1); T8 + 9 (2); T9 (2) |
| Primary (2) | L1 (1); L1 + 2 + 4 (1); L2 (1); L3 (2); L3 + 4 (1); L4 (4); L5 (1) | ||||
| Metastases (30) | |||||
| Tedesco, et al. | 2017 | PSD (3) with CFR-PEEK screws | Primary (NS) | Angioma, (1) Hemangioma, (1) Osteoblastoma, (1) Epithelioid hemangioendothelioma, (1) Chordoma, (9) Chondrosarcoma, (2) Myoepithelioma, (1) Myopericytoma, (1) Osteosarcoma, (2) Dedifferentiated liposarcoma, (1) Fibrosarcoma, (1) Ewing sarcoma (1) | Thoracic and lumbar |
| + Debulking (15); | |||||
| + en bloc resection (4) | |||||
| AR | |||||
| + CFR-PEEK cage (4) | |||||
| + acrylic cement (5) | |||||
| + titanium cage (2) | |||||
| + allograft (1) | |||||
| Tan, et al. | 2013 | Vertebroplasty with | Myeloma (9) | Colon, (2) lung, (8) breast, (7) liver (2) | Thoracic, (10) Lumbar spine (18) |
| PMMA | Metastases (19) | ||||
| Anselmetti, et al. | 2013 | PVA + endovertebral devices | Myeloma (9) | Osteolytic metastasis with vertebral wall involvement | NS |
| Metastasis (3) | |||||
| Rajpal, et al. | 2012 | AR (13) | Metastases (NS) | NS | Thoracic 23 (61.2%), Lumbar 14 (37.8%) |
| PSD (14) | |||||
| AR-PSD (10) | |||||
| Burkett, et al. | 2012 | Anterior cervical corpectomy | Primary (NS) | NS | Cervical (1) |
| Disch, et al. | 2011 | Multilevel (2–5 segments) en-bloc | Primary (15) | Teratoma, (2) Renal cell, (2) Breast, (3) Osteosarcoma (NS), Synovial sarcoma, (4) Chordoma (NS), Osteoblastoma (NS), Giant cell tumor (2) Chondrosarcoma (NS), Neurofibrosarcoma (NS), Solitary plasmocytoma (NS) | Thoracic, (13) Thoracolumbar, (3) lumbar (4) |
| Metastases (5) | |||||
| Jang, et al. | 2002 | Posterior laminectomy and partial corpectomy. Corpectomy, (1) | Primary (NS) | Thyroid, (2) Testicular, (1) lung, (1) rectal, (1) metastasis unknown origin, (1) multiple myeloma, (1) breast, (1) urethral, (1) parotid gland (1) | T10–12 (2 |
| anterior PMMA-augmented screw fixation with | T12–L1 (2) | ||||
| Z-plate | T6–8, (1) T1–2, (1) T5–7, (1) T12–L2, (1) T9–L1, (1) T10–L1 (1) | ||||
| Schulte, et al. | 2000 | Intralesional excision + discectomy and anterior longitudinal ligament excision | Primary (NS) | Breast, (3) Kidney (2) | L1-L4 |
| Metastases (NS) |
AR: anterior reconstruction; CBT: cortical bone trajectory; CFR-PEEK: carbon fiber reinforced poly-ether-ether-ketone; NS: not specified; NSCLC: non-small-cell lung cancer; PMMA: polymethylmethacrylate; PVA: Percutaneous vertebral augmentation; PSD: Posterior Stabilization and decompression
Type of tumours and type of implanted device
Several authors did not report the incidence of primary or secondary tumors. Among the data reported, 94 patients were affected by metastases; while 49 with primary tumors. RT was used in 6 studies, adjuvant RT for a variety of duration was used in 6 studies, VAS, ODI, and/or ASIA scores were given for each of the studies, incidence of local recurrence was given in 13 studies, and fusion rate was given in only 1 of the studies [59] (Tables 3, 4 and 5).
Table 3.
Implant properties and radiotherapy management
| Author | Year | Implant proprieties | Regimen of RT post-surgery | Follow-up (months) |
|---|---|---|---|---|
| Duration of RT post-surgery | ||||
| Shen, et al. | 2022 | CFR-PEEK | 8 | 7 ± 5.9 |
| Müther, et al. | 2021 | CFR-PEEK | NA | 9.5 |
| Neal, et al. | 2021 | CFR-PEEK carboclear | 18 | 6,5 |
| Wagner, et al. | 2021 | Fenestrated CFR-PEEK pedicle screw system, PMMA | NA | 9.8 ± 8.6 |
| Trungu, et al. | 2021 | CFR-PEEK mesh cage with carbon composite. CFR-PEEK anterior cervical plate | 17 | 12.9 ± 4.0 |
| Cofano, et al. | 2020 | CFR-PEEK fixation system and Titanium | NA | 12,6 |
| Boriani, et al. | 2020 | Two composite CFR-PEEK rods, polyester (polyethylene-terephthalate) clamps and titanium clamps | 03-giu | 33 ± 4,42 |
| Thoracic composite CFR-PEEK screws | ||||
| Sakaura, et al. | 2019 | Titanium-coated PEEK and CFR-PEEK cage | NA | - |
| Boriani, et al. | 2018 | CFR-PEEK fixation system | NA | Not specified |
| Ringel, et al. | 2017 | CFR-PEEK and Pedicle screws coated with titanium | NA | Not specified |
| Tedesco, et al. | 2017 | CFR-PEEK | 19 | 10 |
| Anselmetti, et al. | 2013 | PMMA | NA | 10.0 ± 3 |
| PEEK endovertebral devices | ||||
| Tan, et al. | 2013 | PMMA | NA | 23 (3–44) |
| Rajpal, et al. | 2012 | Metal implants, bone implants, and PMMA | NA | 21,1 |
| Dish, et al. | 2011 | LCFRP | NA | 25 |
| Jang, et al. | 2002 | PMMA | NA | 7.8 ± 4 |
| Schulte, et al. | 2000 | CFR-PEEK | 3 | 19.4 ± 14.2 |
CFR-PEEK: Carbon-fibre reinforced polyetheretherketone; LCFRP: long carbon fiber reinforced polymer; NA: not assessed; PMMA: Polymethylmethacrylate
Table 4.
Outcomes
| Author | Year | VAS | ODI | ASIA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | P-value | Pre | Post | P-value | Pre | Post | P-value | ||
| Schulte, et al. | 2000 | - | - | - | - | - | - | |||
| - | - | - | ||||||||
| Jang, et al. | 2002 | 7 ± 1.3 | 2.3 ± 1.1 | 0.005* | - | - | - | - | - | - |
| Disch, et al. | 2011 | - | - | - | - | - | - | - | - | - |
| Rajpal, et al. | 2012 | - | - | - | - | - | - | E: 16 (43%) | All patients either improved or maintained neurologic function at the time of discharge | < 0.05* |
| Anselmetti, et al. | 2013 | 10 (6–10) | 1 (after 1 m, 0–3) | < 0.001* | 82.2% | 4.1% | < 0.001* | - | - | - |
| Tan, et al. | 2013 | 8.7 ± 0.95 | 2.2 ± 1.03 | < 0.001* | 73.80 ± 5.47 | 30 ± 5.46 | < 0.001 |
A:0 B:5 C:14 D:9 E:0 |
All patients had significantly improved neurological function after surgery | < 0.05* |
| Tedesco, et al. | 2017 | 2.7 ± 2.3 (0–8) | 0.3 ± 0.6 (0–2) | - | - | - | - |
A:0 B:1 C:3 D:5 E:25 |
A:0 B:1 C:3 D:5 E:25 |
- |
| Ringel, et al. | 2017 | - | - | - | - | - | - | - | - | - |
| Boriani, et al. | 2018 | 2.7 ± 2.3 | 0.3 ± 0.6 (1w) | - | - | - | - |
A:0 B:1 C:3 D:5 E:25 |
A:0 B:1 C:1 D:6 E:26 |
- |
| Sakaura, et al. | 2019 | - | - | - | - | - | - | - | - | - |
| Boriani, et al. | 2020 | - | - | - | - | - | - | - | - | - |
| Cofano, et al. | 2020 | 8.5 1.5/7–10 | 1.9 1.1/1–3 (last fu) | < 0.01* | - | - | - |
A:0 B:0 C:3 D:9 E:24 |
A:0 B:1 C:0 D:4 E:31 |
< 0.01* |
| 8.4 1.4/7–9 | 2.1 1.0/1–3 | < 0.01* | - | - | - |
A:0 B:0 C:5 D:15 E:22 |
A:0 B:1 C:1 D:8 E:32 |
< 0.01* | ||
| Trungu, et al. | 2021 | NDI. 54.4 (12.2–24.2) | NDI. 3.8 (last fu) | < 0.001* | - | - | - | - | - | < 0.001* |
| Neal, et al. | 2021 | - | - | - | - | - | - | - | - | - |
| Müther, et al. | 2021 | - | - | - | - | - | - | - | - | - |
| Shen, et al. | 2022 | - | - | - | - | - | - |
A:0 B:1 C:1 D:0 E:11 |
A:0 B:0 C:0 D:3 E:10 |
- |
ODI: Oswestry Disability Index; VAS: visual analogue scale; *: statistical significant (p < 0.05)
Table 5.
Summary of rate of local recurrence and complications
| Author | Year | Local recurrence | Fusion rate | Sample size | Complications | |
|---|---|---|---|---|---|---|
| Types | Rate | |||||
| Shen, et al. | 2022 | 1/13 | - | 1/13 | Asymptomatic pseudomeningocele, Distal Junctional kyphosis, Chyle leak, Posterior construct revised, DVT/PE Pleural | 0.35 |
| Müther, et al. | 2021 | 0/7 | - | 0/7 | - | - |
| Neal, et al. | 2021 | 3/28 | - | 3/28 | 11 death (39%) | 0.39 |
| Trungu, et al. | 2021 | 0/51 | - | 0/51 | Postoperative dysphagia | 1 (5.9%) |
| Boriani, et al. | 2020 | 2/6 | - | 2/6 | - | - |
| Cofano, et al. | 2020 | 2/36 | - | 2/36 | 1 CSF leakage (2.7%) | 0.02 |
| 1 Wound Dehiscence (2.7%) | ||||||
| 0 Breakage of screws, rods or plates | ||||||
| 5/42 | - | 5/42 | 1 CSF leakage (2.2%) | 0.06 | ||
| 1 Neurological worsening (2.2%) | ||||||
| 2 Wound Dehiscence (4.4%) | ||||||
| 1 Infections (2.2%) | ||||||
| 1 Screw Loosening (2.2%) | ||||||
| Sakaura, et al. | 2019 | - | 103 (80.5%) | - | - | - |
| Pipola, et al. | 2020 | 0/1 | 0/1 | 0/1 | 0 | 0 |
| Tan, et al. | 2013 | 0/28 | - | 0/28 | 3 Deep venous thrombosis | 0.18 |
| 1 wound drainage | ||||||
| 1 decrease in renal function | ||||||
| Anselmetti, et al. | 2013 | 2/40 | - | 2/40 | Death: 3 (after 3 m); 3 after 6 m) | Leakage: (16.3%) |
| New vertebral fracture: 3 | ||||||
| CSF Leakage: 7/43 | ||||||
| Boriani, et al. | 2018 | 6/34 | - | 6/34 | Screw breakage (1) | 0.03 |
| Loosening of sacral screw (2) after 9–12 months | ||||||
| Laux, et al. | 2018 | 0/1 | 0/1 | 0/1 | 0 | 0 |
| Ringel, et al. | 2017 | - | - | - | - | |
| Tedesco, et al. | 2017 | 7/17 | - | 7/17 | - | - |
| Rajpal, et al. | 2012 | - | - | - | 5 deep venous thrombosis | 0.432 |
| 5 pneumonias | ||||||
| 3 mental status changes, | ||||||
| 1 postoperative ileus, urinary tract infection, sepsis, thrombocytopenia, respiratory failure, pneumothorax, and pulmonary edema | ||||||
| Burkett, et al. | 2012 | 0/29 | - | 0/29 | Retropharyngeal hematoma | 0.03 |
| Disch, et al. | 2011 | 1/20 | - | 1/20 | Major complications: 2 chylus fistula, 1 postoperative ileus, 1 pancreatitis, 1 Dural sac compressing hematoma 1 persistent neurologic deficit | - |
| Minor complications: 3 healing disturbances, 1 CSF leakage, 2 temporary neurologic deficits, 1 hematoma | ||||||
| Jang, et al. | 2002 | - | - | - | - | - |
| Schulte, et al. | 2000 | 0/5 | - | 0/5 | 0 | 0 |
Fusion Rate: the percentage of patients with successful spinal fusion at follow-up; rate: the percentage of patients reporting said complications
ASIA scale: Grade A: completed SCI; Grade B: sensory incomplete; Grade C: motor incomplete; Grade D: Motor incomplete state with a muscle grade of less than three and at least half (or more) of the major muscle functions below the single neurological level of injury; Grade E: normal patient.
Risk of bias in studies
The MINORS tool was used to assess the risk of bias in NRCTs. The computed MINORS scores for the studies found 11 of them [35, 47, 48, 50–53, 55, 56, 59, 62] (55%) had a low risk of bias (total MINORS score ≥ 9), and 9 [21, 24, 37, 49, 54, 57, 58, 60, 61] (45%) had an intermediate risk of bias (total MINORS score < 10) (Table 6).
Table 6.
MINORS
| Author | Clearly stated aim | Inclusion of consecutive patients | Prospective data collection | Endpoints appropriate to study aim | Unbiased assessment of study endpoint | Follow-up period appropriate to study aim | < 5% lost to follow-up | Prospective calculation of study size | Adequate control group | Contemporary groups | Baseline equivalence of groups | Adequate statistical analyses | Total score (…/16) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NON COMPARATIVE STUDIES | ||||||||||||||
| Schulte | 2000 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | 11 |
| Jang | 2002 | 1 | 0 | 0 | 1 | 0 | 2 | 1 | 0 | - | - | - | - | 5 |
| Disch | 2011 | 1 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | 9 |
| Rajpal | 2012 | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | 11 |
| Burkett | 2012 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | - | - | - | - | 6 |
| Anselmetti | 2013 | 1 | 2 | 0 | 2 | 0 | 1 | 2 | 0 | - | - | - | - | 8 |
| Tan | 2013 | 1 | 2 | 0 | 2 | 0 | 1 | 2 | 0 | - | - | - | - | 8 |
| Anselmetti | 2013 | 1 | 2 | 0 | 2 | 0 | 1 | 2 | 0 | - | - | - | - | 8 |
| Ringel | 2017 | 1 | 1 | 0 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | 8 |
| Tedesco | 2017 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | 12 |
| Boriani | 2018 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | 12 |
| Laux | 2018 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | - | - | - | - | 6 |
| Boriani | 2020 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | 12 |
| Pipola | 2020 | 2 | 0 | 1 | 1 | 0 | 2 | 2 | 0 | - | - | - | - | 8 |
| Wagner | 2021 | 1 | 1 | 0 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | 8 |
| Neal | 2021 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | 10 |
| Trungu | 2021 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | 12 |
| Shen | 2022 | 2 | 1 | 0 | 2 | 0 | 1 | 2 | 0 | - | - | - | - | 8 |
| COMPARATIVE STUDIES | Total score (…/24) | |||||||||||||
| Sakaura | 2019 | 1 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 1 | 2 | 17 |
| Cofano | 2020 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 2 | 1 | 2 | 2 | 17 |
The MINORS items were scored 0 if not reported, 1 when reported but inadequate, and 2 when reported and adequate. The ideal global score was 20 for NRCTs.
Results of individual studies
Ti6Al4V pedicle screws (gold standard)
One study [35] compared the effectiveness of CFR-PEEK and Ti6Al4V pedicle screws. The results are reported in Tables 3, 4 and 5.
Vertebral augmentation + PMMA
PMMA was employed in five studies [49, 52, 54, 57, 58]. The results are reported in Tables 3, 4 and 5.
Carbon screws + CFR-PEEK
CFR-PEEK was the most used radiolucent material in the studies included. Thirteen studies [24, 35, 47, 48, 50, 51, 53, 55–57, 59, 60, 62] used CFR-PEEK pedicle screws. The results are reported in Tables 3, 4 and 5.
Carbon screws + titanium coated PEEK
Titanium-coated PEEK (Ti-PEEK) was used in two studies [24, 59]. The results are reported in Tables 3, 4 and 5.
PEEK pedicle screws
Only one study [58] used PEEK endovertebral devices without coating or composite material. The results are reported in Tables 3, 4 and 5.
Discussion
This systematic review offers a contemporary assessment of radiolucent biomaterials in spinal surgery, with a particular focus on their attributes and practical implications. The principal finding of this review underscores these materials as potentially dependable solutions for individuals with primary and metastatic spinal lesions. Nonetheless, it’s crucial to acknowledge that the field of application of radiolucent implanted devices in metastatic patients remains underexplored. The present review is the only one among recent literature to have performed a qualitative analysis of the included articles, reviewing the most recent literature as well. This study was the one that included the most articles, as an exhaustive literature analysis was carried out. The aim is to underscore the importance of three crucial aspects when deliberating over implanted devices, namely, safety, artifact production, and radiotherapy dose perturbation.
An aspect that is emphasized by numerous publications is the significance of utilizing multiple fields in radiotherapy (RT) to augment dosimetric accuracy, especially when patients possess metal implanted devices [34]. These radiolucent implanted devices were created especially for oncology and they are typically advised for patients eligible for radiation therapy. Treatment plan dosimetric accuracy and robustness can be increased by reducing image artefacts and the contouring uncertainties that result and by significantly reducing dose perturbation effects [64].
Most of the reviewed studies have demonstrated favorable outcomes when using PEEK. The mechanical properties of this material, which closely resemble those of cortical bone, facilitate load distribution in the anterior column and reduce stress shielding at the bone-to-screw interface [65]. Moreover, lower artifact volume, imaging scattering, and lower Hounsfield Unit (HU) variation between implanted devices and adjacent tissues [47, 61, 66] may allow operators to allocate their time and resources better during treatment planning.
Composite implanted devices: combining carbon fiber or titanium with PEEK to overcome its limitations
Limitations of PEEK implanted devices include low integration with surrounding tissues due to their bioinertness [67]. Furthermore, they are semi-rigid and do not achieve immediate postoperative spinal stability [68]. Many studies showed that the limitations of PEEK implanted devices can be mitigated by creating composite, surface-coated pedicle screws materials like carbon fiber [47, 55]. CFR-PEEK has been shown to be a reliable composite material, combining the advantages of carbon fiber stiffness while maintaining PEEK’s radiolucent properties [56]. Moreover, most included articles have confirmed that CFR-PEEK is able to stimulate osteointegration [24], enhancing the secondary stability of implanted screws. The challenge of intraoperative visualization with non-metal biomaterials has also been addressed. Ringel et al. [24] conducted a study including 35 patients to analyze intraoperative fluoroscopic visualization of vertebral screws. In this context, CFR-PEEK implanted devices were coated with titanium around the pedicle area. This technique has demonstrated efficacy in improving the visualization of screws during surgery. Only one suboptimal placement and one intraoperative screw breakage were recorded due to sclerotic bone due to prostate cancer metastasis. However, using four or more screws still impairs spinal canal visualization on postoperative MRI due to artifact from the titanium screw head.
Additionally, as shown in the study by Sakaura et al. [59], the integration of titanium with PEEK materials can enhance tissue integration and osteoconductivity. Titanium compensates for PEEK’s semi-rigidity by strengthening fixation [18]. It is important to note that this study concluded there was no statistically significant difference in patient survival between titanium and CFR-PEEK implanted devices. However, CFR-PEEK devices may be preferable for better postoperative management and follow-up, especially in the context of primary spinal tumors that require precise radiotherapy planning and radiographic monitoring [54, 58]. It has to be noted that this study used a homogenous sample size, with all patients undergoing the same procedure.
Safety of CFR-PEEK in metastatic spine tumor surgery
The consensus among the authors of the reported studies is that radiolucent implanted devices exhibit low implant failure rates and are reliable alternatives to traditional systems in the treatment of vertebral tumors. Specifically, Boriani and colleagues [47] described only 1 case of intraoperative screw breakage that occurred in 232 radiolucent screw placements in a short-term period. In the long-term postoperative setting (mean follow-up of 13 months), only two patients displayed a loosening of the sacral screw.
In direct comparison with titanium implanted devices, CFR-PEEK demonstrates a non-inferior profile concerning intraoperative and postoperative complications, as well as functional recovery [35]. This has been reported in a study by Cofano et al. [35], in which thirty-six patients who received CFR-PEEK implanted devices for anterior spinal reconstruction were compared with 42 patients who had previously received titanium implanted devices. No screw breakage was observed in either of the groups. The latter study exhibits similar limitations to the previously cited one by Boriani, meaning it has a small sample size and heterogenous instrumentation, though also lacks a follow-up long enough to draw any conclusions on the oncological benefits of CFR-PEEK.
In another retrieved study, conducted by Anselmetti et al. [58], it was stated that CFR-PEEK implanted devices show promise in reducing the risk of PMMA extravasation as compared to conventional vertebral augmentation techniques. This due to the unique design of these novel composite implanted materials. However, it has to be stated that the studies in question did show some shortcomings, such as a small and heterogenous sample size and the use of different materials in the reconstruction of the anterior column, and also lacked a cost-benefit analysis regarding the production of the implanted devices. Nevertheless, the safety and effectiveness of this novel device warrant further exploration through prospective studies with larger patient cohorts to ensure its reliability in the context of metastatic spine tumors.
Advantages of CFR-PEEK in imaging and radiotherapy
The benefits of CFR-PEEK composite implanted devices in imaging definition and radiotherapy in the postoperative setting have been emphasized in most of the reviewed studies. PEEK’s radiolucency and excellent imaging properties bring significant advantages in postoperative radiotherapy, allowing for precise administration of the maximum dose on the target lesion while minimizing radiotoxicity in surrounding tissues [24, 47–57, 69]. Boriani et al. [48] have studied the benefits of CFR-PEEK composite implanted devices in imaging definition and radiotherapy in the postoperative setting. These granted excellent imaging definition, with no scattering effects and minimal artifact production. The therapeutic approach to primary tumours often involves radiation therapy, a notably artifact-sensitive technique [24, 55]. Titanium produces higher HU variation between the screw and surrounding tissue, influencing dosimetry. Furthermore, during RT, titanium screws cause 60–80% dose reduction behind the screws, while CFR-PEEK is associated with a 10% reduction [15].
Another study, conducted by Laux et al. [61], showed different though favorable results, with fewer artifacts on both CT and MRI. Furthermore, CFR-PEEK showed minimal dose alteration, with an attenuation of approximately 5%.
However, findings by this and other cited studies should be validated by further studies analyzing the long-term stability of these implanted devices in a different population with longer-term follow up.
PMMA vertebral augmentation
Several authors used PMMA for vertebral augmentation with titanium and radiolucent screws. No differences were reported in term of complications or advantages, other than the potential reduction of PMMA extravasation when used in conjunction with CFR-PEEK [58]. Therefore, it may be assumed that PMMA augmentation could be used also with new radiolucent implanted devices [70, 71].
Limitations
The present review has six limitations. First, the study did not include randomized control trials or low-quality studies. Second, the meta-analysis of results could not be performed due to the heterogeneity of the collected data. Third, English-language restraint limited the number of eligible articles. Fourth, only one study reported the fusion rate, and few reported an influence on RT management. Fifth, the time and the type of recurrence was not reported. Sixth, different treatments and tumours were included, making it impossible to compare data. Further trials are needed to determine the applicability of each material in different contexts. These should employ homogenous instrumentation in all patients, grouping them based on different types of tumors, then comparing them with a control group. Cost-benefit analyses should also be performed in future studies, so as to properly understand the economic implications of employing new materials in this context.
Conclusions
The studies exhibited the potential benefits of utilizing radiolucent materials, such as CFR-PEEK, in the context of MSTS. These studies presented compelling evidence regarding the reduction of artifact production, making postoperative management more effective and enhancing the compatibility with adjuvant radiotherapy.
However, it is important to recognize certain limitations in the reviewed studies. Some studies had relatively small sample sizes, limiting the generalizability of their findings to a broader patient population. Additionally, long-term follow-up data were often lacking, making it challenging to evaluate the durability and longevity of the implanted devices, especially in the context of potential implant failures over extended periods. Furthermore, the cost implications associated with using complex materials like CFR-PEEK were not comprehensively addressed in the studies, which could be a crucial consideration in real-world clinical settings.
Among the reviewed studies, CFR-PEEK emerged as the material demonstrating the best clinical performance. The advantages of CFR-PEEK, including its noninferior safety profile compared to titanium, minimized artifact production, and reduced dose perturbation effects during adjuvant radiotherapy, make it a promising candidate for replacing traditional titanium alloys in MSTS.
To advance the understanding of radiolucent implanted devices and improve future clinical studies, three recommendations are made. First, future research should prioritize large-scale, well-designed clinical trials with a homogenous patient sample affected by similar types of tumors, utilizing standardized instrumentation for spine reconstruction. Second, long-term follow-up should be integral to these trials to assess the durability and potential complications of the implanted devices. Third, cost-benefit analyses should be incorporated into future studies to evaluate the economic feasibility of implementing these complex materials in routine clinical practice. By addressing these considerations, future research can provide a more comprehensive understanding of the clinical performance and applicability of radiolucent implanted devices in the realm of spine tumor surgery.
Acknowledgements
None.
Author contributions
Conceptualization, S.D.S. and U.G.L.; methodology, S.D.S.; software, V.B; validation, F.P.; formal analysis, G.Z.; investigation, G.C.; resources, S.D.S.; data curation, G.C.; writing—original draft preparation, G.C. and G.Z.; writing—review and editing, S.D.S.; visualization, U.G.L.; supervision, V.D.; project administration, U.G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data availability
The datasets used and/or analysed during the present are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Laufer I, Bilsky MH. Advances in the treatment of metastatic spine tumors: the future is not what it used to be. J Neurosurg Spine. 2019;30:299–307. 10.3171/2018.11.SPINE18709 [DOI] [PubMed] [Google Scholar]
- 2.Perez-Roman RJ, et al. The use of carbon fiber-reinforced instrumentation in patients with spinal oncologic tumors: a systematic review of literature and future directions. World Neurosurg. 2023;173:13–22. 10.1016/j.wneu.2023.01.090 [DOI] [PubMed] [Google Scholar]
- 3.Groenen KHJ, et al. The Dutch national guideline on metastases and hematological malignancies localized within the spine; a multidisciplinary collaboration towards timely and proactive management. Cancer Treat Rev. 2018;69:29–38. 10.1016/j.ctrv.2018.05.013 [DOI] [PubMed] [Google Scholar]
- 4.Wewel JT, O’Toole JE. Epidemiology of spinal cord and column tumors. Neurooncol Pract. 2020;7:i5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Farii H, Aoude A, Al Shammasi A, Reynolds J, Weber M. Surgical management of the metastatic spine disease: a review of the literature and proposed algorithm. Global Spine J. 2023;13:486–98. 10.1177/21925682221146741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barzilai O, et al. Predictors of quality of life improvement after surgery for metastatic tumors of the spine: prospective cohort study. Spine J. 2018;18:1109–15. 10.1016/j.spinee.2017.10.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suit H, et al. Proton vs carbon ion beams in the definitive radiation treatment of cancer patients. Radiother Oncol. 2010;95:3–22. 10.1016/j.radonc.2010.01.015 [DOI] [PubMed] [Google Scholar]
- 8.Yamada Y, et al. The impact of histology and delivered dose on local control of spinal metastases treated with stereotactic radiosurgery. Neurosurg Focus. 2017;42:E6. 10.3171/2016.9.FOCUS16369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu JX, et al. Local tumor control for metastatic epidural spinal cord compression following separation surgery with adjuvant cyberknife stereotactic radiotherapy or image-guided intensity-modulated radiotherapy. World Neurosurg. 2020;141:e76–85. 10.1016/j.wneu.2020.04.183 [DOI] [PubMed] [Google Scholar]
- 10.Patchell RA, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–8. 10.1016/S0140-6736(05)66954-1 [DOI] [PubMed] [Google Scholar]
- 11.Di Perna G, et al. Separation surgery for metastatic epidural spinal cord compression: a qualitative review. J Bone Oncol. 2020;25:100320. 10.1016/j.jbo.2020.100320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spratt DE, et al. An integrated multidisciplinary algorithm for the management of spinal metastases: an international spine oncology consortium report. Lancet Oncol. 2017;18:e720–30. 10.1016/S1470-2045(17)30612-5 [DOI] [PubMed] [Google Scholar]
- 13.Kumar N, et al. Evolution of materials for implants in metastatic spine disease till date - have we found an ideal material? Radiother Oncol. 2021;163:93–104. 10.1016/j.radonc.2021.08.007 [DOI] [PubMed] [Google Scholar]
- 14.Soriani A, et al. The advantages of carbon fiber based orthopedic devices in patients who have to undergo radiotherapy. Acta Biomed. 2020;91:e2020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mastella E, et al. Dosimetric characterization of carbon fiber stabilization devices for post-operative particle therapy. Phys Med. 2017;44:18–25. 10.1016/j.ejmp.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 16.Papanastassiou ID, Gerochristou M, Aghayev K, Vrionis FD. Defining the indications, types and biomaterials of corpectomy cages in the thoracolumbar spine. Expert Rev Med Devices. 2013;10:269–79. 10.1586/erd.12.79 [DOI] [PubMed] [Google Scholar]
- 17.Tahal D, Madhavan K, Chieng LO, Ghobrial GM, Wang MY. Metals in spine. World Neurosurg. 2017;100:619–27. 10.1016/j.wneu.2016.12.105 [DOI] [PubMed] [Google Scholar]
- 18.Kumar N, et al. Can polyether ether ketone dethrone titanium as the choice implant material for metastatic spine tumor surgery? World Neurosurg. 2021;148:94–109. 10.1016/j.wneu.2021.01.059 [DOI] [PubMed] [Google Scholar]
- 19.Salvatore G, et al. Biomechanical effects of metastasis in the osteoporotic lumbar spine: a finite element analysis. BMC Musculoskelet Disord. 2018;19:38. 10.1186/s12891-018-1953-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longo UG, Denaro V. in Lancet. (England. 2009), vol. 373, pp. 1947; author reply 1947–1948. [DOI] [PubMed]
- 21.Burkett CJ, Baaj AA, Dakwar E, Uribe JS. Use of titanium expandable vertebral cages in cervical corpectomy. J Clin Neurosci. 2012;19:402–5. 10.1016/j.jocn.2011.07.030 [DOI] [PubMed] [Google Scholar]
- 22.Borys J et al. Exposure to Ti4Al4V titanium alloy leads to redox abnormalities, oxidative stress, and oxidative damage in patients treated for mandible fractures. Oxid Med Cell Longev. 2018;2018:3714725. [DOI] [PMC free article] [PubMed]
- 23.Krätzig T, et al. Carbon fiber-reinforced PEEK versus titanium implants: an in vitro comparison of susceptibility artifacts in CT and MR imaging. Neurosurg Rev. 2021;44:2163–70. 10.1007/s10143-020-01384-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ringel F, et al. Radiolucent carbon fiber-reinforced pedicle screws for treatment of spinal tumors: advantages for radiation planning and follow-up imaging. World Neurosurg. 2017;105:294–301. 10.1016/j.wneu.2017.04.091 [DOI] [PubMed] [Google Scholar]
- 25.Son SH, Kang YN, Ryu MR. The effect of metallic implants on radiation therapy in spinal tumor patients with metallic spinal implants. Med Dosim. 2012;37:98–107. 10.1016/j.meddos.2011.01.007 [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Müller WD, Rumjahn A, Schwitalla A. Parameters influencing the outcome of additive manufacturing of tiny medical devices based on PEEK. Mater (Basel). 2020;13. [DOI] [PMC free article] [PubMed]
- 27.Verma R, Virk S, Qureshi S. Interbody fusions in the lumbar spine: a review. HSS J. 2020;16:162–7. 10.1007/s11420-019-09737-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar N, et al. Novel 3D printable PEEK-HA-Mg. Eur Spine J. 2023;32:2255–65. 10.1007/s00586-023-07734-0 [DOI] [PubMed] [Google Scholar]
- 29.Trungu S, et al. Percutaneous carbon-PEEK instrumentation for spine tumors: a prospective observational study. J Neurosurg Sci. 2023;67:303–10. 10.23736/S0390-5616.21.05153-5 [DOI] [PubMed] [Google Scholar]
- 30.Chen J, et al. Implant materials for anterior column reconstruction of cervical spine tumor. Orthop Surg. 2023;15:1219–27. 10.1111/os.13702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landy BC, Vangordon SB, McFetridge PS, Sikavitsas VI, Jarman-Smith M. Mechanical and in vitro investigation of a porous PEEK foam for medical device implants. J Appl Biomater Funct Mater. 2013;11:e35–44. [DOI] [PubMed] [Google Scholar]
- 32.Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28:4845–69. 10.1016/j.biomaterials.2007.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xin-ye N, Xiao-bin T, Chang-ran G, Da C. The prospect of carbon fiber implants in radiotherapy. J Appl Clin Med Phys. 2012;13:3821. 10.1120/jacmp.v13i4.3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takayanagi A, et al. Radiolucent carbon fiber-reinforced implants for treatment of spinal tumors-clinical, radiographic, and dosimetric considerations. World Neurosurg. 2021;152:61–70. 10.1016/j.wneu.2021.05.100 [DOI] [PubMed] [Google Scholar]
- 35.Cofano F, et al. Carbon fiber reinforced vs titanium implants for fixation in spinal metastases: a comparative clinical study about safety and effectiveness of the new carbon-strategy. J Clin Neurosci. 2020;75:106–11. 10.1016/j.jocn.2020.03.013 [DOI] [PubMed] [Google Scholar]
- 36.Delaney FT, Denton H, Dodds M, Kavanagh EC. Multimodal imaging of composite carbon fiber-based implants for orthopedic spinal fixation. Skeletal Radiol. 2021;50:1039–45. 10.1007/s00256-020-03622-6 [DOI] [PubMed] [Google Scholar]
- 37.Pipola V, et al. Composite peek/carbon fiber pre-shaped rods and sublaminar bands for posterior stabilization of cervico-thoracic junction: a novel technique. J Clin Neurosci. 2020;72:429–33. 10.1016/j.jocn.2019.12.035 [DOI] [PubMed] [Google Scholar]
- 38.Alvarez-Breckenridge C, et al. Carbon fiber-reinforced polyetheretherketone spinal implants for treatment of spinal tumors: perceived advantages and limitations. Neurospine. 2023;20:317–26. 10.14245/ns.2244920.460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poel R, et al. Assessing the advantages of CFR-PEEK over titanium spinal stabilization implants in proton therapy-a phantom study. Phys Med Biol. 2020;65:245031. 10.1088/1361-6560/ab8ba0 [DOI] [PubMed] [Google Scholar]
- 40.Long JR, Kalani MA, Goulding KA, Ashman JB, Flug JA. Carbon-fiber-reinforced polyetheretherketone orthopedic implants in musculoskeletal and spinal tumors: imaging and clinical features. Skeletal Radiol. 2023;52:393–404. 10.1007/s00256-022-04069-7 [DOI] [PubMed] [Google Scholar]
- 41.Joerger AK et al. CFR-PEEK pedicle screw instrumentation for spinal neoplasms: a single center experience on safety and efficacy. Cancers (Basel). 2022;14. [DOI] [PMC free article] [PubMed]
- 42.Díez-Pascual AM, Díez-Vicente AL. Nano-TiO2 reinforced PEEK/PEI blends as biomaterials for load-bearing implant applications. ACS Appl Mater Interfaces. 2015;7:5561–73. 10.1021/acsami.5b00210 [DOI] [PubMed] [Google Scholar]
- 43.Van der Mierden S, Tsaioun K, Bleich A, Leenaars CHC. Software tools for literature screening in systematic reviews in biomedical research. Altex. 2019;36:508–17. [DOI] [PubMed] [Google Scholar]
- 44.Longo UG, Loppini M, Denaro L, Maffulli N, Denaro V. Rating scales for low back pain. Br Med Bull. 2010;94:81–144. 10.1093/bmb/ldp052 [DOI] [PubMed] [Google Scholar]
- 45.Slim K, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–6. 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 46.Sterne JAC, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 47.Boriani S, et al. Carbon-fiber-reinforced PEEK fixation system in the treatment of spine tumors: a preliminary report. Eur Spine J. 2018;27:874–81. 10.1007/s00586-017-5258-5 [DOI] [PubMed] [Google Scholar]
- 48.Boriani S, et al. Composite PEEK/carbon fiber rods in the treatment for bone tumors of the cervical spine: a case series. Eur Spine J. 2020;29:3229–36. 10.1007/s00586-020-06534-0 [DOI] [PubMed] [Google Scholar]
- 49.Jang JS, Lee SH, Rhee CH. Polymethylmethacrylate-augmented screw fixation for stabilization in metastatic spinal tumors. Technical note. J Neurosurg. 2002;96:131–4. [DOI] [PubMed] [Google Scholar]
- 50.Müther M, Lüthge S, Gerwing M, Stummer W, Schwake M. Management of spinal dumbbell tumors via a minimally invasive posterolateral approach and carbon fiber-reinforced polyether ether ketone instrumentation: technical note and surgical case series. World Neurosurg. 2021;151:277–e283271. 10.1016/j.wneu.2021.04.068 [DOI] [PubMed] [Google Scholar]
- 51.Neal MT, et al. Carbon fiber-reinforced PEEK instrumentation in the spinal oncology population: a retrospective series demonstrating technique, feasibility, and clinical outcomes. Neurosurg Focus. 2021;50:E13. 10.3171/2021.2.FOCUS20995 [DOI] [PubMed] [Google Scholar]
- 52.Rajpal S, Hwang R, Mroz T, Steinmetz MP. Comparing vertebral body reconstruction implants for the treatment of thoracic and lumbar metastatic spinal tumors: a consecutive case series of 37 patients. J Spinal Disord Tech. 2012;25:85–91. 10.1097/BSD.0b013e318214b489 [DOI] [PubMed] [Google Scholar]
- 53.Schulte M, et al. Vertebral body replacement with a bioglass-polyurethane composite in spine metastases–clinical, radiological and biomechanical results. Eur Spine J. 2000;9:437–44. 10.1007/s005860000162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan JW, Shen BH, DU W, Liu JQ, Lu SQ. Bone cement enhanced pedicle screw fixation combined with vertebroplasty for elderly patients with malignant spinal tumors. Chin Med J (Engl). 2013;126:2495–8. 10.3760/cma.j.issn.0366-6999.20120323 [DOI] [PubMed] [Google Scholar]
- 55.Tedesco G, Gasbarrini A, Bandiera S, Ghermandi R, Boriani S. Composite PEEK/Carbon fiber implants can increase the effectiveness of radiotherapy in the management of spine tumors. J Spine Surg. 2017;3:323–9. 10.21037/jss.2017.06.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trungu S et al. Anterior corpectomy and plating with Carbon-PEEK instrumentation for cervical spinal metastases: clinical and radiological outcomes. J Clin Med. 2021;10. [DOI] [PMC free article] [PubMed]
- 57.Wagner A, et al. Cement-augmented carbon fiber-reinforced pedicle screw instrumentation for spinal metastases: safety and efficacy. World Neurosurg. 2021;154:e536–46. 10.1016/j.wneu.2021.07.092 [DOI] [PubMed] [Google Scholar]
- 58.Anselmetti GC, et al. Percutaneous vertebral augmentation assisted by PEEK implant in painful osteolytic vertebral metastasis involving the vertebral wall: experience on 40 patients. Pain Physician. 2013;16:E397–404. 10.36076/ppj.2013/16/E397 [DOI] [PubMed] [Google Scholar]
- 59.Sakaura H, Ohnishi A, Yamagishi A, Ohwada T. Early fusion status after posterior lumbar interbody fusion with cortical bone trajectory screw fixation: a comparison of titanium-coated polyetheretherketone cages and carbon polyetheretherketone cages. Asian Spine J. 2019;13:248–53. 10.31616/asj.2018.0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen FH, et al. Integrated custom composite polyetheretherketone/carbon fiber (PEEK/CF) vertebral body replacement (VBR) in the treatment of bone tumors of the spine: a preliminary report from a multicenter study. Spine (Phila Pa 1976). 2022;47:252–60. 10.1097/BRS.0000000000004177 [DOI] [PubMed] [Google Scholar]
- 61.Laux CJ, Hodel SM, Farshad M, Müller DA. Carbon fibre/polyether ether ketone (CF/PEEK) implants in orthopaedic oncology. World J Surg Oncol. 2018;16:241. 10.1186/s12957-018-1545-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Disch AC, et al. Oncosurgical results of multilevel thoracolumbar en-bloc spondylectomy and reconstruction with a carbon composite vertebral body replacement system. Spine (Phila Pa 1976). 2011;36:E647–655. 10.1097/BRS.0b013e3181f8cb4e [DOI] [PubMed] [Google Scholar]
- 63.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Upasani VV, et al. Pedicle screw surface coatings improve fixation in nonfusion spinal constructs. Spine (Phila Pa 1976). 2009;34:335–43. 10.1097/BRS.0b013e318194878d [DOI] [PubMed] [Google Scholar]
- 65.Niinomi M, Nakai M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Int J Biomater. 2011;2011:836587. [DOI] [PMC free article] [PubMed]
- 66.Nevelsky A, Borzov E, Daniel S, Bar-Deroma R. Perturbation effects of the carbon fiber-PEEK screws on radiotherapy dose distribution. J Appl Clin Med Phys. 2017;18:62–8. 10.1002/acm2.12046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gültan T, Yurtsever M, Gümüşderelioğlu M. NaOH-etched/boron-doped nanohydroxyapatite-coated PEEK implants enhance the proliferation and differentiation of osteogenic cells. Biomed Mater. 2020;15:035019. 10.1088/1748-605X/ab7198 [DOI] [PubMed] [Google Scholar]
- 68.Colangeli S, et al. Polyetheretherketone (PEEK) rods: short-term results in lumbar spine degenerative disease. J Neurosurg Sci. 2015;59:91–6. [PubMed] [Google Scholar]
- 69.Khan HA et al. Carbon fiber-reinforced PEEK spinal implants for primary and metastatic spine tumors: a systematic review on implant complications and radiotherapy benefits. J Neurosurg Spine. 2023:1–14. [DOI] [PubMed]
- 70.Berton A, et al. A 3D finite element model of prophylactic vertebroplasty in the metastatic spine: vertebral stability and stress distribution on adjacent vertebrae. J Spinal Cord Med. 2020;43:39–45. 10.1080/10790268.2018.1432309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garnon J, et al. PMMA bone cement in interventional oncology. Crit Rev Biomed Eng. 2021;49:35–50. 10.1615/CritRevBiomedEng.2021037591 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the present are available from the corresponding author on reasonable request.