Abstract
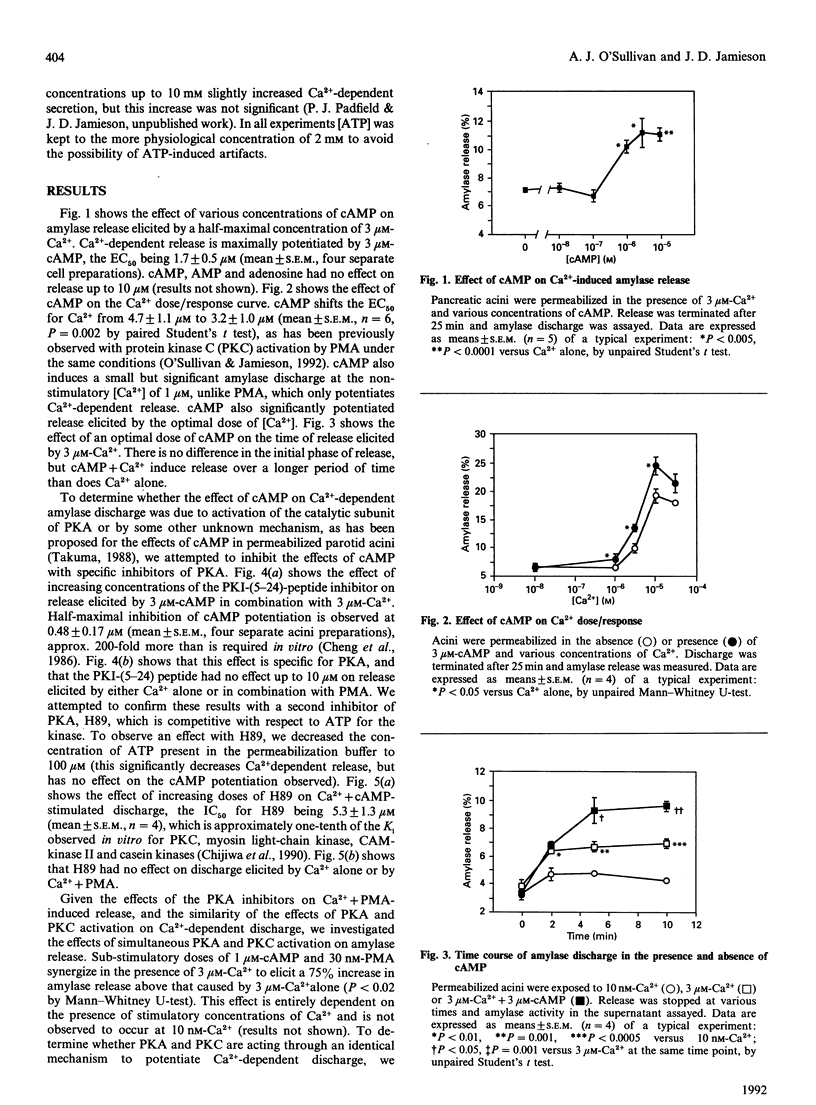
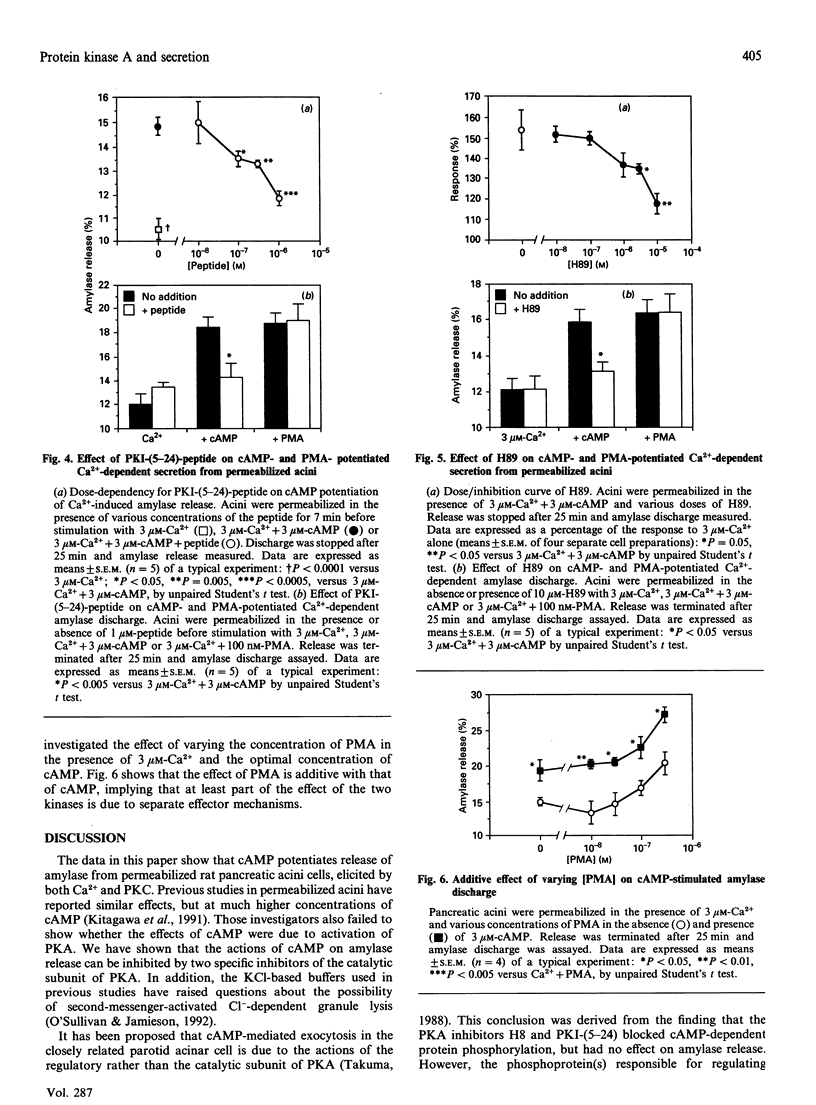
The role of protein kinase A (PKA) in the release of amylase from permeabilized pancreatic acini was investigated. Addition of cyclic AMP (cAMP) to permeabilized acini resulted in a potentiation of Ca(2+)-dependent amylase release, shifting the Ca2+ dose/response curve leftwards. As with protein kinase C (PKC) activation, this is due to an increase in the time of active discharge. The effect of cAMP was shown to be blocked by two inhibitors of PKA, H89 and the PKI-(5-24)-peptide. At low concentration, cAMP synergizes from phorbol 12-myristate 13-acetate (PMA), while at optimal concentrations cAMP and PMA are additive. PKA and PKC appear to work via similar, but not identical mechanisms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruzzone R., Halban P. A., Gjinovci A., Trimble E. R. A new, rapid, method for preparation of dispersed pancreatic acini. Biochem J. 1985 Mar 1;226(2):621–624. doi: 10.1042/bj2260621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Chijiwa T., Mishima A., Hagiwara M., Sano M., Hayashi K., Inoue T., Naito K., Toshioka T., Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990 Mar 25;265(9):5267–5272. [PubMed] [Google Scholar]
- Ederveen A. G., van der Leest J. V., van Emst-de Vries S. E., de Pont J. J. Phosphorylation of low molecular mass cytosolic proteins by protein kinase C and protein kinase A in the rabbit exocrine pancreas. Eur J Biochem. 1989 Nov 6;185(2):461–468. doi: 10.1111/j.1432-1033.1989.tb15137.x. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Gardner J. D., Jensen R. T. Receptors and cell activation associated with pancreatic enzyme secretion. Annu Rev Physiol. 1986;48:103–117. doi: 10.1146/annurev.ph.48.030186.000535. [DOI] [PubMed] [Google Scholar]
- Holz R. W., Bittner M. A., Peppers S. C., Senter R. A., Eberhard D. A. MgATP-independent and MgATP-dependent exocytosis. Evidence that MgATP primes adrenal chromaffin cells to undergo exocytosis. J Biol Chem. 1989 Apr 5;264(10):5412–5419. [PubMed] [Google Scholar]
- Kitagawa M., Williams J. A., De Lisle R. C. Interactions of intracellular mediators of amylase secretion in permeabilized pancreatic acini. Biochim Biophys Acta. 1991 Jan 23;1073(1):129–135. doi: 10.1016/0304-4165(91)90192-j. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A. J., Jamieson J. D. Activation of protein kinase C is not an absolute requirement for amylase release from permeabilized rat pancreatic acini. Biochem J. 1992 Jul 15;285(Pt 2):597–601. doi: 10.1042/bj2850597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padfield P. J., Ding T. G., Jamieson J. D. Ca2(+)-dependent amylase secretion from pancreatic acinar cells occurs without activation of phospholipase C linked G-proteins. Biochem Biophys Res Commun. 1991 Jan 31;174(2):536–541. doi: 10.1016/0006-291x(91)91450-q. [DOI] [PubMed] [Google Scholar]
- Schulz I., Stolze H. H. The exocrine pancreas: the role of secretagogues, cyclic nucleotides, and calcium in enzyme secretion. Annu Rev Physiol. 1980;42:127–156. doi: 10.1146/annurev.ph.42.030180.001015. [DOI] [PubMed] [Google Scholar]
- Takuma T. Evidence against direct involvement of cyclic AMP-dependent protein phosphorylation in the exocytosis of amylase. Biochem J. 1988 Dec 15;256(3):867–871. doi: 10.1042/bj2560867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieseniss E., Plattner H. Synchronous exocytosis in Paramecium cells involves very rapid (less than or equal to 1 s), reversible dephosphorylation of a 65-kD phosphoprotein in exocytosis-competent strains. J Cell Biol. 1985 Dec;101(6):2028–2035. doi: 10.1083/jcb.101.6.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]


