Abstract
Background
Familial concordance of weight status is an emerging field of study that may guide the development of interventions that operate beyond the individual and within the family context. There is a dearth of published data for concordance of weight status within Pakistani households.
Methods
We assessed the associations between weight status of mothers and their children in a nationally representative sample of households in Pakistan using Demographic and Health Survey data from 2017–18. Our analysis included 3465 mother–child dyads, restricting to children under-five years of age with body mass index (BMI) information on their mothers. We used linear regression models to assess the associations between maternal BMI category (underweight, normal weight, overweight, obese) and child’s weight-for-height z-score (WHZ), accounting for socio-demographic characteristics of mothers and children. We assessed these relationships in all children under-five and also stratified by age of children (younger than 2 years and 2 to 5 years).
Results
In all children under-five and in children 2 to 5 years, maternal BMI was positively associated with child’s WHZ. For all children under-five, children of normal weight, overweight, and obese women had WHZ scores that were 0.21 [95% CI (confidence interval): 0.04, 0.37], 0.43 [95% CI: 0.25, 0.62], and 0.51 [95% CI: 0.30, 0.71] units higher than children of underweight women, respectively. For children ages 2 to 5, children of normal weight, overweight, and obese women had WHZ scores that were 0.26 [95% CI: 0.08, 0.44), 0.50 [95% CI: 0.30, 0.71), and 0.61 [95% CI: 0.37, 0.84] units higher than children of underweight women, respectively. There was no association between maternal BMI and child WHZ for children under-two.
Conclusions
The findings indicate that the weight status of mother’s is positively associated with that of their children, particularly after age 2. These associations further strengthen the call for research regarding interventions and policies aimed at healthy weight promotion among mothers and their children collectively, rather than focusing on individuals in isolation.
Keywords: Non-communicable disease risk concordance, Overweight children, Maternal-child health concordance, Pakistan
Background
There is a growing body of literature focusing on concordance of cardiometabolic disease risk factors and outcomes within families and households, suggesting that noncommunicable disease (NCD) prevention programs may be more effective if the family – rather than the individual – was targeted for intervention. Studies show increased likelihood of adults living with someone with a chronic condition, such as obesity, diabetes, hypertension, hyperlipidemia, and other cardiometabolic conditions, having that same condition. This includes concordance between parent–child [1, 2], sibling [3, 4], spousal dyads [5, 6], and between any co-residing members of households [7, 8]. These studies investigate the genetic predisposition to disease [7], as can be seen in strictly familial relationships, and also the effect of the shared environment [9] and health behavior influences [10, 11], such as in co-residing studies.
Intergenerational concordance within households – and specifically that between parents and children – may reflect a composite of genetic, epigenetic, and shared environmental factors. The magnitude of intergenerational concordance of NCD risk within households in Pakistan is largely unknown. Studies focusing on parent–child concordance have been primarily done in high-income countries, such as in the United Kingdom and Australia [12–17]. A systematic review and meta-analysis looking at high-, middle-, and low-income countries establishes the parent–child concordance of obesity as well, but this study did not include Pakistan and did not analyze continuous child WHZ [18]. There is some additional evidence from low- and middle-income countries (LMICs) regarding concordance of biomarkers such as hemoglobin A1c and C-reactive protein [2]. Specifically in South Asia, studies show statistically significant associations between child under-five obesity and maternal weight, but do not further stratify these relationships by child age [19–21].
Within Pakistan, there is particular importance of the parent–child dyad. Pakistan has one of the highest average household sizes and percentage of multigenerational households in the world, suggesting extended sharing of environment between generations. Moreover, Pakistan is following a global trend of increasing life expectancy, signaling a large population of individuals at higher risk of developing NCDs that the Pakistani healthcare system will have to manage in the future [22]. Additionally, children in Pakistan are becoming increasingly vulnerable to different forms of malnutrition: in the 2018 Pakistan National Nutrition Survey (PNNS), wasting was noted in 17.7% of children, the highest in Pakistan’s history, and prevalence of overweight children under-five almost doubled from 5% in 2011 to 9.5% in 2018. Similarly, women ages 15–49 experienced an increase in overweight and obesity increasing from 28% to 37.8% from 2011 to 2018 [23]. This parallel increase in excess weight across a wide range of Pakistani age demographics may have important implications for cardiometabolic disease at the population level. Thus, there is a need to study Pakistani household concordance of NCD risk factors to further understand the extent, if present, of household concordance in Pakistan, especially with an additional focus on parent–child concordance to advocate for interventions that involve the full household, and not only prevention for adults.
In the present study, we analyzed the concordance of maternal and child weight status, a critical risk factor for NCDs. We further investigate the dynamics of these associations across different age groups of children under 5.
Methods
Data sources and participants
Pakistan’s Demographic and Health Survey, Round VII (PDHS-7) survey was conducted in 2017–18 by the National Institute of Population Studies and funded by the United States Agency for International Development (USAID). PDHS-7 was the 7th round of a nationally representative survey, with information about household, demographic, and maternal and child health indicators. The survey design and sample size calculations were formulated to provide reliable information at the national level and subnational levels, as well as for urban and rural areas separately, with data collected from the four provinces of Punjab, Sindh, Khyber Pakhtunkhwa, and Balochistan, the regions of Azad Jammu and Kashmir and Gilgit Baltistan, the Islamabad Capital Territory, and the Federally Administered Tribal Areas. The sampling design used a two-stage stratified approach and, because of non-proportional sample allocation, the sample weights were generated by the PDHS team. Participants were recruited and data was collected from November 2017 to April 2018.
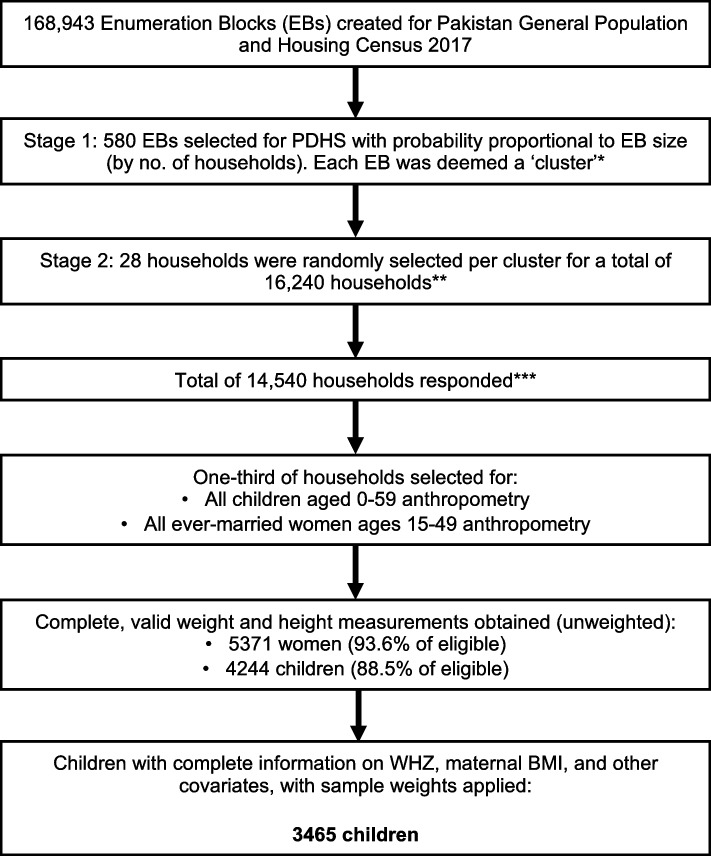
The respondents included all ever-married women aged 15–49 in all selected households. In one-third of the selected households, height and weight were directly measured for ever-married women 15–49 and children under-five years of age. For this study, we included all children under-five who had WHZ information and BMI information on their mothers, leading to a sampling-weight adjusted sample size of 3465. Figure 1 depicts the sampling scheme and relevant response rates for PDHS-7 [24].
Fig. 1.
Flow diagram of household and participant selection for the 2017-18 Pakistan Demographic and Health Survey. *If an EB was greater than 300 households, then the EB was segmented (determined by the field team before household listing) and only one segment was selected to form the cluster (probability of selection was proportional to the household size). **No replacements or changes of pre-selected households were allowed during the implementing stage. ***Survey successfully administered in 561 out of 580 EBs, 19 clusters dropped due to security issues during the fieldwork
Study measures
Child weight status: Weight-for-height z-score (WHZ)
Child weight and height measurements were taken during a single visit. Weight measurements were taken using Seca scales (model no. Seca 878U) and height measurements using a Shorr Board (recumbent if child’s age was less than 24 months and standing if older). Anthropometry data in PDHS-7 was collected by two female enumerators in each field team (total of 44 enumerators) who jointly took measurements. All enumerators were trained beforehand to standardize procedures for anthropometry, including hands-on training to measure ten children twice to assess accuracy and precision of measurements and further training for those enumerators who were out of range more three or more times [24].
The dependent variable was weight-for-height z-score (WHZ) for children under-five per the 2006 Child Growth Standards released by the World Health Organization, reliably shown to describe both overweight (WHZ > 2) and wasting (WHZ < -2) [25]. Every one unit in z-score represents one standard deviation from the mean weight-for-height from a study of more than eight thousand children recruited from Brazil, Ghana, India, Norway, Oman, and the United States of America. For example, a WHZ of -2 indicates that the child’s weight is two standard deviations below the mean for their height and sex [26]. Notably, children contributing data to the development of the growth standards were exclusively or predominantly breastfed for at least the first four months, introduced to complementary foods by six months of age, and partially breastfed for at least 12 months. Consequently, growth standards were derived based on breastfed children [27–29].
Maternal weight status: Body Mass Index (BMI)
The primary exposure of interest for this study was maternal BMI. BMI was measured in the same way as child WHZ, with SECA 878U scales and Shorr Boards (standing height measurement). Maternal BMI was coded as obese (30 or greater), overweight (25 to less than 30), healthy weight (18.5 to less than 25), and underweight (less than 18.5), which was the reference category ([ref]).
Covariates
Several other covariates were also considered in the analysis. A covariate was included in the statistical models if it had evidence of correlation with child weight outcome and if there was sufficient variation in the covariate for it to add information to the model, e.g. if a factor has 99% ‘yes’ response rate and 1% ‘no’ rate, then its use was avoided because it would not meaningfully explain variation in the outcome. Through this process, the following nine covariates were selected for analysis: urban/ rural specific wealth index [20, 25, 30–32], sex of child [25, 30], mother’s employment status [30, 31, 33], type of place of residence [32, 34–36], mother’s youngest child under-five [32, 37, 38], mother’s oldest child under-five [32, 37, 38], child age in months, child breastfeeding status [39–41], and maternal age in years [33, 37, 38].
Household wealth status was measured as quintiles of assets as provided by the PDHS-7 survey. Wealth index quintile categories were labeled as richest, rich, middle, poor, poorest[ref]. Child sex was coded as male or female[ref]. Type of place of residence was coded as urban or rural[ref]. Child breastfeeding status was coded as never breastfed, ever breastfed but not currently breastfeeding, and still breastfeeding[ref]. Mother’s employment status, youngest child under-five and oldest child under-five were all coded as no or yes[ref].
Analysis
A dyadic dataset of mothers and their children was created by merging anthropometric and demographic characteristics of mothers and children per DHS guidance for analysis. Mother’s characteristics were treated as exposures for the child [42]. Furthermore, in studying cardiometabolic risk concordance between younger children and their mothers, it is important to consider the impact of children being born small for gestational age (SGA) can have on anthropometric concordance. This is particularly important consideration in Pakistan, where the prevalence of SGA births is > 45% [43]. As approximately 85% of children born SGA have experienced sufficient catch up growth by age 2 [44–46], stratifying analysis by this age point in relation to concordance which, to our knowledge, has not been done before, could yield important findings regarding the ‘unmasking’ of parent–child concordance. Thus, we stratified children by two years of age in our analysis and modeling.
All analysis and modeling accounted for the complex survey design [42]. All data restructuring and analysis was done using IBM SPSS Statistics v29.0.0.0 with Complex Samples v29. The code used for restructuring and analysis can be found via the following link: 10.5281/zenodo.7794384.
Descriptive statistics
Reported statistics (sampling weight-adjusted) included background characteristics of the participants which include average age of mother (in years) and children (in months), mean BMI of mothers, and mean child WHZ with 95% confidence intervals. Other reported adjusted population descriptive statistics were percentage of dyads that were urban vs rural; percentage of male and female children; percentage of mothers currently employed; percentage of children who were never breastfed, previously breastfed but not currently breastfeeding, or currently breastfeeding; percentage of children being the youngest child; percentage of children being the oldest under-five; and the percentage of children in each wealth index quintile. The mean child WHZ with 95% confidence interval and sampling weight-adjusted sample size was also reported for each factor level.
Modeling associations between maternal and child weight status
We estimated linear regression models to investigate concordance between maternal BMI and child WHZ, measured continuously. Three linear models were generated: 1) all children under-five years, 2) all children under-two years, 3) all children ages 2 to 5, with the models adjusted for the 9 covariates. We reported the R2 value and the Wald F for each model and reported the parameter estimates with 95% confidence interval and t-test value for each categorical level. For each model, the statistically significant variables were remodeled including only these variables and an additional term for each two-variable combination to assess for interaction between the statistically significant variables. Cases with missing values for any variables were treated as invalid. Associations for all models were considered statistically significant when p < 0.05.
Further considerations for analysis
We assessed multicollinearity between the independent variables using the variance inflation factor (VIF) calculation. We used a conservative cutoff of 3, as an indicator to further investigate relationships [47]. Using this cutoff, no multicollinearity was observed among the independent variables. To account for multiple comparisons and counter the increased risk of Type I error, the Holm-Bonferroni method was used to adjust the P-value of 0.05 accordingly [48, 49]. Regarding outliers, the DHS data is screened before dissemination for plausibility of Z-scores. For children who had WHZ that were below -5 standard deviations or above + 5 standard deviations were flagged and their WHZ was not reported. Similarly, women who had BMI below 12 or above 60 were also flagged and did not have their BMI reported [42]. Further consideration of outliers was not made because of these adjustments by the PDHS data processing team.
Results
For the analysis, out of 4671 sample unweighted cases, 4130 had complete variable information (with 541 missing) and were included, which led to a sampling-weight adjusted sample size of 3465. With cases that had information on maternal BMI and child WHZ, only two did not have complete information on the other covariates.
Summary statistics
Of the overall sampling weight-adjusted sample of 3465 children (Fig. 1), 1406 (40.6%) children were less than 2 years old, and 2059 (59.4%) children were ages 2 to 5. The mean age of mothers was 29.0 years (95% CI [28.6, 29.4]) and children under-five years was 28.8 months (95% CI [28.0, 29.5]). The mean maternal BMI was 24.6 (95% CI [24.2, 25.1]). The mean child WHZ for the entire sample was -0.30 (95% CI [-0.36, -0.24]), mean child WHZ for children under-two was -0.41 (95% CI [-0.50, -0.31]), and mean child WHZ for children ages 2 to 5 was -0.23 (95% CI [-0.29, -0.16]). The greatest mean child WHZ was in children who had never been breastfed at 0.07 (95% CI [-0.22, 0.36]), and the category with the lowest mean child WHZ was children with mothers who were underweight at -0.66 (95% CI [-0.80, -0.51]). Table 1 summarizes maternal, child, and household characteristics of the analyzed maternal-child dyads and the mean child WHZ for each factor level is summarized in Table 2.
Table 1.
Percentage of sample in each factor level and mean values of continuous variables (n = 3465), with 95% confidence interval from PDHS-7 dataset used in analysis
| Variable | All | Children under 2 | Children 2 to 5 years |
|---|---|---|---|
| Maternal Characteristics | |||
| Percent (95% CI) | |||
| Maternal BMI Category | |||
| 30 + | 15.7 (13.1 – 18.8) | 13.1 (10.4 – 16.4) | 17.5 (14.5 – 20.9) |
| 25 to < 30 | 27.6 (24.8 – 30.6) | 27.9 (24.5 – 31.7) | 27.4 (24.3 – 30.7) |
| 18.5 to < 25 | 46.2 (42.9 – 49.6) | 46.7 (42.6 – 50.8) | 45.9 (42.1 – 49.8) |
| < 18.5 | 10.4 (8.5 – 12.7) | 12.3 (9.9 – 15.1) | 9.2 (7.2 – 11.7) |
| Mother currently employed | |||
| No | 86.7 (83.9 – 89.0) | 34.6 (30.1 – 39.4) | 31.4 (27.6 – 35.5) |
| Yes | 13.3 (11.0 – 16.1) | 65.4 (60.6 – 69.9) | 68.6 (64.5 – 72.4) |
| Mean (95% CI) | |||
| Maternal BMI | 24.6 (24.2, 25.1) | 24.3 (23.8, 24.8) | 24.9 (24.4, 25.4) |
| Maternal Age (yrs.) | 29.0 (28.6, 29.4) | 27.4 (26.9, 27.9) | 30.3 (29.6, 30.5) |
| Child Characteristics | |||
| Percent (95% CI) | |||
| Youngest child | |||
| No | 34.3 (32.8 – 35.9) | 5.5 (4.2 – 7.2) | 54.0 (51.3 – 56.7) |
| Yes | 65.7 (64.1 – 67.2) | 94.5 (92.8 – 95.8) | 46.0 (43.3 – 48.7) |
| Oldest child under-five | |||
| No | 32.5 (30.9 – 34.1) | 56.4 (52.9 – 59.8) | 16.2 (14.3 – 18.2) |
| Yes | 67.5 (65.9 – 69.1) | 43.6 (40.2 – 47.1) | 83.8 (81.8 – 85.7) |
| Sex of child | |||
| Male | 51.1 (49.1 – 53.1) | 51.2 (47.3 – 55.0) | 51.0 (48.7 – 53.3) |
| Female | 48.9 (46.9 – 50.9) | 48.8 (45.0 – 52.7) | 49.0 (46.7 – 51.3) |
| Breastfeeding status | |||
| Never breastfed | 4.4 (3.3 – 5.8) | 4.1 (2.9 – 5.8) | 4.6 (3.2 – 6.4) |
| Previously breastfed but not currently breastfeeding | 63.4 (61.3 – 65.5) | 24.9 (22.1 – 28.0) | 90.1 (88.0 – 91.9) |
| Currently breastfeeding | 32.2 (30.2 – 34.2) | 70.9 (67.7 – 74.0) | 5.3 (4.2 – 6.7) |
| Mean (95% CI) | |||
| Child WHZ | -0.30 (-0.36, -0.24) | -0.41 (-0.50, -0.31) | -0.23 (-0.29, -0.16) |
| Child Age (mos.) | 28.8 (28.0, 29.5) | 11.0 (10.5, 11.5) | 40.9 (40.4, 41.5) |
| Household Characteristics | |||
| Percent (95% CI) | |||
| Area | |||
| Urban | 32.7 (29.1 – 36.6) | 34.6 (30.1 – 39.4) | 31.4 (27.6 – 35.5) |
| Rural | 67.3 (63.4 – 70.9) | 65.4 (60.6 – 69.9) | 68.6 (65.4 – 72.4) |
| Wealth index (urban/ rural specific) | |||
| Richest | 18.3 (14.7 – 22.5) | 19.1 (14.7 – 24.5) | 17.7 (14.1 – 21.9) |
| Richer | 20.3 (17.0 – 24.0) | 21.9 (18.0 – 26.3) | 19.1 (15.7 – 23.1) |
| Middle | 20.1 (17.4 – 23.0) | 20.2 (17.0 – 23.9) | 19.9 (17.0 – 23.2) |
| Poor | 21.4 (18.4 – 24.8) | 20.2 (16.8 – 24.1) | 22.3 (18.8 – 26.1) |
| Poorest | 20.0 (16.5 – 24.0) | 18.6 (15.1 – 22.5) | 21.0 (16.8 – 25.8) |
Table 2.
Mean Child WHZ and sampling weight-adjusted sample size (Adj. N) of analyzed sample, stratified by level of selected factors
| Variable | Adj. N | Mean child WHZ (95% CI) |
|---|---|---|
| All | 3465 | -0.30 (-0.36, -0.24) |
| Maternal BMI Category | ||
| 30 + | 545 | -0.02 (-0.16, 0.11) |
| 25 to < 30 | 956 | -0.14 (-0.25, -0.03) |
| 18.5 to < 25 | 1602 | -0.41 (-0.51, -0.31) |
| < 18.5 | 361 | -0.66 (-0.80, -0.51) |
| Mother currently employed | ||
| No | 3003 | -0.29 (-0.36, -0.23) |
| Yes | 462 | -0.35 (-0.52, -0.19) |
| Youngest child | ||
| No | 1189 | -0.21 (-0.29, -0.13) |
| Yes | 2276 | -0.35 (-0.42, -0.28) |
| Oldest child under-five | ||
| No | 1126 | -0.40 (-0.51, -0.28) |
| Yes | 2339 | -0.26 (-0.32, -0.20) |
| Sex of child | ||
| Male | 1770 | -0.32 (-0.39, -0.24) |
| Female | 1695 | -0.29 (-0.36, -0.21) |
| Area | ||
| Urban | 1134 | -0.31 (-0.40, -0.21) |
| Rural | 2331 | -0.30 (-0.37, -0.22) |
| Breastfeeding status | ||
| Never breastfed | 150 | 0.07 (-0.22, 0.36) |
| Previously breastfed but not currently breastfeeding | 2201 | -0.25 (-0.31, -0.18) |
| Currently breastfeeding | 1111 | -0.46 (-0.56, -0.36) |
| Wealth index (urban/ rural specific) | ||
| Richest | 633 | 0.04 (-0.09, 0.17) |
| Richer | 702 | -0.17 (-0.29, -0.06) |
| Middle | 695 | -0.36 (-0.50, -0.22) |
| Poor | 743 | -0.52 (-0.64, -0.40) |
| Poorest | 693 | -0.45 (-0.58, -0.31) |
Associations between mother and child weight status
Maternal BMI was positively associated with child WHZ for all children among children under-five. Children of normal weight, overweight, and obese women had WHZ scores that were 0.21 [95% CI: 0.04, 0.37], 0.43 [95% CI: 0.25, 0.62], and 0.51 [95% CI: 0.30, 0.71] units higher than children of underweight women, respectively. Maternal BMI was also positively associated with child WHZ among children ages 2 to 5, where children of normal weight, overweight, and obese women had WHZ scores that were 0.26 [95% CI: 0.08, 0.44), 0.50 [95% CI: 0.30, 0.71), and 0.61 [95% CI: 0.37, 0.84] units higher than children of underweight women, respectively.
For all children under-five, household wealth index was positively associated with child WHZ (Richest: 0.34 [95% CI: 0.16, 0.51]; Rich: 0.18 [95% CI: 0.00, 0.35]; Middle: 0.00 [95% CI: -0.18, 0.18]; Poor: -0.13 [95% CI: -0.31, 0.05]; Poorest [ref.]). Household wealth was similarly positively associated with child WHZ for children under 2 (Richest: 0.42 [95% CI: 0.12, 0.72]; Rich: 0.20 [95% CI: -0.09, 0.49]; Middle -0.32 [95% CI: -0.59, -0.05]; Poor: -0.15 [95% CI: -0.46, 0.17]; Poorest: [ref]) and for children aged two to five (Richest: 0.29 [95% CI: 0.09, 0.49]; Rich: 0.16 [95% CI: -0.03, 0.34]; Middle 0.22 [95% CI: 0.02, 0.42]; Poor -0.11 [95% CI: -0.28, 0.06]; Poorest: [ref.]).
There were no statistically significant interaction effects for any linear model. Table 3 summarizes information for each model.
Table 3.
Linear regression results of the association between maternal BMI and child WHZ, with selected covariates
| Predictor | Analysis I – all children under-five | Analysis II—children under-two | Analysis III—children 2 to 5 years | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (Category) | Coefficient (95% CI) | t-value | P | Coefficient (95% CI) | t-value | P | Coefficient (95% CI) | t-value | P |
| Mother BMI category | (< 0.001) | 0.181 | (< 0.001) | ||||||
| 30 or greater | 0.51(0.30, 0.71) | 4.930 | < .001 | 0.29(-0.06, 0.64) | 1.625 | 0.105 | 0.61(0.37, 0.84) | 5.018 | < .001 |
| 25 to < 30 | 0.43(0.25, 0.62) | 4.613 | < .001 | 0.25(-0.06, 0.56) | 1.597 | 0.111 | 0.50(0.30, 0.71) | 4.826 | < .001 |
| 18.5 to < 25 | 0.21(0.04, 0.37) | 2.502 | 0.13 | 0.06(-0.22, 0.33) | 0.402 | 0.688 | 0.26(0.08, 0.44) | 2.873 | 0.004 |
| less than 18.5 | * | * | * | ||||||
| Mother currently working | 0.369 | 0.845 | 0.183 | ||||||
| No | -0.08(-0.25, 0.09) | -0.899 | 0.369 | 0.03(-0.22, 0.27) | 0.196 | 0.845 | -0.13(-0.32, 0.06) | -1.333 | 0.183 |
| Yes | * | * | * | ||||||
| Youngest child | 0.062 | 0.021 | 0.153 | ||||||
| No | 0.12(-0.01, 0.24) | 1.874 | 0.062 | 0.37(0.06, 0.68) | 2.324 | 0.021 | 0.10(-0.04, 0.24) | 1.431 | 0.153 |
| Yes | * | * | * | ||||||
| Oldest child under-five | 0.570 | 0.637 | 0.678 | ||||||
| No | -0.04(-0.18, 0.10) | -0.569 | 0.57 | -0.05(-0.24, 0.15) | -0.473 | 0.637 | -0.04(-0.24, 0.16) | -0.416 | 0.678 |
| Yes | * | * | * | ||||||
| Sex of child | 0.403 | 0.163 | 0.925 | ||||||
| Male | -0.04(-0.14, 0.06) | -0.838 | 0.403 | -0.13(-0.31, 0.05) | -1.398 | 0.163 | 0.01(-0.10, 0.11) | 0.094 | 0.925 |
| Female | * | * | * | ||||||
| Area | 0.447 | 0.827 | 0.422 | ||||||
| Urban | -0.04(-0.16, 0.07) | -0.761 | 0.447 | -0.02(-0.20, 0.16) | -0.219 | 0.827 | -0.05(-0.19, 0.08) | -0.804 | 0.422 |
| Rural | * | ||||||||
| Breastfeeding status | 0.063 | 0.477 | 0.237 | ||||||
| Never breastfed | 0.35(0.04, 0.65) | 2.255 | 0.025 | 0.3(-0.21, 0.81) | 1.149 | 0.251 | 0.30(-0.17, 0.78) | 1.254 | 0.211 |
| Previously breastfed, not currently breastfeeding | 0.15(-0.02, 0.32) | 1.704 | 0.089 | -0.02(-0.25, 0.20) | -0.188 | 0.851 | 0.09(-0.31, 0.49) | 0.424 | 0.672 |
| Still breastfeeding | * | * | * | ||||||
| Wealth index (urban/ rural specific) | (< 0.001) | (< 0.001) | (< 0.001) | ||||||
| Richest | 0.34(0.16, 0.51) | 3.702 | < .001 | 0.42(0.12, 0.72) | 2.771 | 0.006 | 0.29(0.09, 0.49) | 2.852 | 0.005 |
| Rich | 0.18(0.00, 0.35) | 1.986 | 0.048 | 0.20(-0.09, 0.49) | 1.387 | 0.166 | 0.16(-0.03, 0.34) | 1.679 | 0.094 |
| Middle | 0.00(-0.18, 0.18) | 0.015 | 0.988 | -0.32(-0.59, -0.05) | -2.339 | 0.02 | 0.22(0.02, 0.42) | 2.124 | 0.034 |
| Poor | -0.13(-0.31, 0.05) | -1.432 | 0.153 | -0.15(-0.46, 0.17) | -0.925 | 0.355 | -0.11(-0.28, 0.06) | -1.242 | 0.215 |
| Poorest | * | * | * | ||||||
| Mother age (yrs) | 0.00(-0.01, 0.01) | -0.159 | 0.874 | 0.00(-0.02, 0.02) | 0.171 | 0.865 | 0.00(-0.01, 0.01) | -0.616 | 0.538 |
| Child age (mos) | 0.00(-0.01, 0.00) | -0.549 | 0.583 | 0.01(-0.01, 0.02) | 1.269 | 0.205 | -0.01(-0.02, 0.00) | -2.428 | 0.016 |
| R^2 | 0.053 | 0.062 | 0.066 | ||||||
| Wald F | 7.182 | 3.345 | 7.43 | ||||||
Overall variable p-values in parenthesis denote statistically significant associations (Holm-Bonferroni corrected)
*denotes reference category
Discussion
We found that maternal BMI was positively associated with child WHZ in children under 5. This positive association was present for children ages 2 to 5, even after accounting for several socio-demographic factors, but not for children under-two. The findings suggest that there is intergenerational concordance in weight status in Pakistani mothers and young children which is unmasked with increased age.
The findings of parent–child concordance of BMI-WHZ for all children under-five are consistent with previous literature which specifically explore parent–child concordance of NCD risk [2, 12–18] and of association studies in South Asia which have observed a positive association between maternal and children under-five weight status [19–21]. However, previously described phenomenon of the double burden of malnutrition at the household level (DBMHL) suggests co-existence of undernourished children and overweight mothers in the same household, but this phenomenon is primarily described for child stunting and, in some cases, underweight [50–58]. Although a recent study by Biswas et al. [59], in which they analyzed the co-existence of overweight mothers with all types of undernourished children under-five (stunted, wasted, and underweight) in South and Southeast Asia from 2006 to 2017 data, described positive relationships between maternal weight status and all three types of child undernutrition, with the greatest co-existence in Pakistan. However, this study did not analyze the relationship between non-overweight or obese mothers and their children, nor did it split the children under-five into two age groups [59]. Another study by Hossain et al. showed a positive association between maternal overweight status and children ages 24 to 59 months overweight status, but this study did not include children from 0 to 24 months due to most classification systems defining child overweight status after 24 months [21]. In our analysis, using WHZ for the child’s weight status provided more flexibility in analyzing a wider age group.
Furthermore, double burden of malnutrition (DBM) at the country or population level is well-established in previous literature [60–63]. DBM prevalence has been increasing in Pakistan as shown by PNNS 2018 results, with wasting noted in 17.7% of children, the highest in Pakistan’s history, prevalence of overweight children under-five almost doubling from 5% in 2011 to 9.5% in 2018, and women ages 15–49 experiencing an increase in overweight and obesity increasing from 28% to 37.8% from 2011 to 2018. Both, DBMHL and DBM, can co-exist with maternal-child BMI-WHZ concordance, as the increasing burden of DBM, with an increasing percentage of overweight children, could be leading to the more recent findings of positives association of maternal-child weight status. Co-existence of these phenomena within the same society highlight the complexity of interventions and approaches required to address malnutrition among LMICs, as different regions and households are experiencing various challenges.
To our knowledge, there are no studies which specifically investigate the relationship between maternal and child concordance under-five stratified by 2 years of age, as we analyzed in this study. These findings, which show a positive association between maternal BMI and child WHZ only in the 2 to 5 age group, potentially indicate that concordance between child WHZ and maternal BMI is ‘unmasked’ after age 2, when the majority of early childhood catch-up growth has occurred [44, 45]. As in, children who may be born SGA may not show concordance of WHZ with maternal BMI during the first two years of life, but as they experience their expected catch up growth and have spent more time in a shared environment with their mothers, their weight status starts to become concordant with their mothers, as seen by our analysis which shows the difference in association between children older than and younger than two. This suggests that children under-two who do not initially appear to have WHZ concordant to their mother’s BMI may develop this positive relationship later in life.
Furthermore, a targeted review on maternal and child overweight and obesity in LMICs shows that junk food, such as potato chips, sponge cakes, sugary biscuits, and sugary drinks are a significant part of child diet by the first two years of life [64], potentially yielding an avenue of intervention and an explanation for the development of mother–child concordance after two years of age. This relationship could also be affected by other dietary aspects such as breastfeeding status and weaning, although in our analysis, breastfeeding history did not show a statistically significant association with weight status. However, it is important to note that the population surveyed showed little variability in breastfeeding, with only 4.4% of the population having been never breastfeed. Furthermore, limitations of the survey prevented us from investigating duration and exclusivity of breastfeeding, which can play a role in the strength of association between breastfeeding and weight status. Prior studies have shown that longer duration of breastfeeding decreases excess weight gain in children [39], and that exclusively breastfed children through 6 months tend to be more likely to grow within the normal limits set by the 2006 WHO Child Growth Standards while also being protective against overweight, obesity, and type 2 diabetes through childhood and adulthood [40, 41, 65]. Overall, this may indicate an avenue for early interventions for weight-related risks in children, where healthier nutritional sources during infancy may lead to a decreased sharing of NCD risk found between very young children and their mothers as children age. This approach is well established within public health, focusing on building public policy that focuses on health in domains beyond simply clinical settings, such as the commitment to health promotion and disease prevention societal domains laid out by experts within declarations such as the Ottawa Charter for Health Promotion and the 2030 Agenda for Sustainable Development Goals (SDGs) [66–68].
Within Pakistan specifically, although there have been attempts to establish a national policy to implement health promotion strategies for prevention and reduction of NCDs, there is room for improvement and further work. For example, the National Action Plan for Non-Communicable Disease Prevention, Control and Health Promotion was introduced in 2003 as a means to develop a robust NCD surveillance system, with a focus on policy measures and communications focusing on diet, activity, and accessibility of healthier foods [69]; however, due to government changes, the policies and plans were not implemented. Similarly, in 2009, a public–private partnership dubbed the National Commission for Prevention of NCDs was proposed by the Pakistan Ministry of Health, but implementation was halted due to legal concerns [70]. Consequentially, despite calls from experts in Pakistan [71], there remains little in terms of surveillance systems in Pakistan to track the progress of NCDs, the country’s progress in achieving NCD-related SDGs, or even understanding the role of household/ family intervention to mitigate risks of a rapidly growing burden [72, 73].
Regarding other covariates which were analyzed, we showed wealth index as a statistically significant positive factor across all regression analyses. This association existing in Pakistan is well established in the literature [74–79]. Increasing wealth quintile has been suggested to correlate with access to nutrient rich foods and increased sedentary behaviors [20, 80–83].
Considering strengths of this study, the analysis was done on a nationally representative dataset which involved a complex sampling process to obtain the best possible demographic snapshot of Pakistan. The analysis also included a significant number of covariates to unveil potential confounders and used rigid levels of correction and validation regarding significance and multi-collinearity. Limitations of the study included the use of cross-sectional data, whereas a cohort study could provide more in-depth data on trends in specific households.
Conclusions
We found that maternal-child concordance of weight status among children ages 2 to 5, but not among children under 2. Future work should involve more detailed data collection of health behaviors and environmental factors relevant to obesity and cardiometabolic disease early in life within the household. Such information may elucidate pathways for NCD risk concordance between mothers and children, and possibly all family members, to develop evidence or family-wide health promotion interventions for healthy weight across the life course.
Acknowledgements
We thank the DHS Program supported by USAID, the National Institute of Population Studies, and ICF for the data used in this study. We also thank the Emory Global Diabetes Research Center and Global Health Equity Scholars NIH FIC TW010540.
Abbreviations
- adj
Adjusted
- BMI
Body Mass Index
- CI
Confidence interval
- DBM
Double burden of malnutrition
- DBMHL
Double burden of malnutrition at the household level
- LMICs
Low- and middle- income countries
- mos.
Months
- NCD
Noncommunicable disease
- PDHS-7
Pakistan’s Demographic and Health Survey, Round VII
- PNNS
Pakistan National Nutrition Survey
- SGA
Small for Gestational Age
- USAID
United States Agency for International Development
- ref.
Reference
- VIF
Variance Inflation Factor
- WHO
World Health Organization
- WHZ
Weight-for-height z-score
- yrs
Years
Authors’ contributions
The concept was drafted by F.A. and R.I. F.A. and S.A.P. contributed to the analysis plan. M.K.A., R.I., and S.A.P. advised on paper conceptualization. F.A. was the primary writer of the article with comprehensive feedback from M.K.A., S.A.P., and R.I. All authors read and approved the final manuscript.
Funding
No grants or other funding sources were provided for the analysis and writing of this manuscript. Regarding survey design and data collection, financial support was provided by the United States Agency for International Development.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available with DHS program. The data can be requested at: https://dhsprogram.com/data/available-datasets.cfm
Declarations
Ethics approval and consent to participate
The PDHS-7 survey was reviewed and approved by the National Bioethics Committee of the Pakistan Health Research Council and the Inner City Fund/ ICF Institutional Review Board. Adult participants were consented both, for themselves and for their children (24). Prior to obtaining access to and performing analysis on the PDHS-7 data, the researchers of the present study submitted a concept note, gained data access, and obtained permission from USAID. The data were de-identified and anonymized by the DHS program prior to being shared, therefore not considered human subjects research by an author-affiliated institution (Emory University, Atlanta, GA, USA) and not requiring further ethical clearance.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Park HS, Park JY, Cho SI. Familial aggregation of the metabolic syndrome in Korean families with adolescents. Atherosclerosis. 2006;186(1):215–21. 10.1016/j.atherosclerosis.2005.07.019 [DOI] [PubMed] [Google Scholar]
- 2.Dong F, Howard AG, Herring AH, Adair LS, Thompson AL, Popkin BM, et al. Concordance of haemoglobin A1c, blood pressure and C-reactive protein between children and their parents in Chinese households. Pediatr Obes. 2017;12(5):422–30. 10.1111/ijpo.12160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng Y, Zang T, Xu X, Xu X. Familial aggregation of metabolic syndrome and its components in a large Chinese Population. Obesity. 2008;16(1):125–9. 10.1038/oby.2007.22 [DOI] [PubMed] [Google Scholar]
- 4.Gupta S, Kapoor S. Genetic and environmental influences on blood pressure in an urban indian population. J Biosoc Sci. 2013;45(1):1–11. 10.1017/S0021932012000478 [DOI] [PubMed] [Google Scholar]
- 5.Kim HC, Kang DR, Choi KS, Nam CM, Thomas GN, Suh I. Spousal concordance of metabolic syndrome in 3141 Korean Couples: a nationwide survey. Ann Epidemiol. 2006;16(4):292–8. 10.1016/j.annepidem.2005.07.052 [DOI] [PubMed] [Google Scholar]
- 6.Nielsen J, Shivashankar R, Cunningham SA, Prabhakaran D, Tandon N, Mohan V, et al. Couple concordance in diabetes, hypertension and dyslipidaemia in urban India and Pakistan and associated socioeconomic and household characteristics and modifiable risk factors. J Epidemiol Community Health. 2023;77(5):336–42. 10.1136/jech-2022-219979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee MH, Kim HC, Thomas GN, Ahn SV, Hur NW, Choi DP, et al. Familial concordance of metabolic syndrome in Korean population—Korean National Health and Nutrition Examination Survey 2005. Diabetes Res Clin Pract. 2011;93(3):430–6. 10.1016/j.diabres.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 8.Patel SA, Dhillon PK, Kondal D, Jeemon P, Kahol K, Manimunda SP, et al. Chronic disease concordance within Indian households: a cross-sectional study. PLoS Med. 2017;14(9):e1002395. 10.1371/journal.pmed.1002395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franco M, Bilal U, Diez-Roux AV. Preventing non-communicable diseases through structural changes in urban environments. J Epidemiol Community Health. 2015;69(6):509–11. 10.1136/jech-2014-203865 [DOI] [PubMed] [Google Scholar]
- 10.Meyler D, Stimpson JP, Peek MK. Health concordance within couples: A systematic review. Soc Sci Med. 2007;64(11):2297–310. 10.1016/j.socscimed.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 11.Intarakamhang U, Macaskill A. Multi-group causal model of health literacy and behaviors on family wellbeing among Thai adults at risk of Non-Communicable Diseases (NCDs). J Res Health Sci. 2018;18(4):e00429. [PMC free article] [PubMed] [Google Scholar]
- 12.Hawkins SS, Cole TJ, Law C, Group and the MCSCH. An ecological systems approach to examining risk factors for early childhood overweight: findings from the UK millennium cohort study. J Epidemiol Commun Health. 2009;63(2):147–55. 10.1136/jech.2008.077917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330(7504):1357. 10.1136/bmj.38470.670903.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salsberry PJ, Reagan PB. Dynamics of early childhood overweight. Pediatrics. 2005;116(6):1329–38. 10.1542/peds.2004-2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson SM. Preventing childhood obesity: early-life messages from epidemiology. Nutr Bull. 2017;42(3):219–25. 10.1111/nbu.12277 [DOI] [Google Scholar]
- 16.Clifford SA, Gillespie AN, Olds T, Grobler AC, Wake M. Body composition: population epidemiology and concordance in Australian children aged 11–12 years and their parents. BMJ Open. 2019;9(Suppl 3). Available from: https://bmjopen.bmj.com/content/9/Suppl_3/95. Cited 2021 Nov 2. [DOI] [PMC free article] [PubMed]
- 17.Gibson LY, Byrne SM, Davis EA, Blair E, Jacoby P, Zubrick SR. The role of family and maternal factors in childhood obesity. Med J Aust. 2007;186(11):591–5. 10.5694/j.1326-5377.2007.tb01061.x [DOI] [PubMed] [Google Scholar]
- 18.Lee JS, Jin MH, Lee HJ. Global relationship between parent and child obesity: a systematic review and meta-analysis. Clin Exp Pediatr. 2021;65(1):35–46. 10.3345/cep.2020.01620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cesare MD, Bhatti Z, Soofi SB, Fortunato L, Ezzati M, Bhutta ZA. Geographical and socioeconomic inequalities in women and children’s nutritional status in Pakistan in 2011: an analysis of data from a nationally representative survey. Lancet Glob Health. 2015;3(4):e229–39. 10.1016/S2214-109X(15)70001-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bishwajit G, Yaya S. Overweight and obesity among under-five children in South Asia. Child Adolescent Obes. 2020;3(1):105–21. 10.1080/2574254X.2020.1769992 [DOI] [Google Scholar]
- 21.Hossain FB, Shawon MSR, Al-Abid MSU, Mahmood S, Adhikary G, Bulbul MMI. Double burden of malnutrition in children aged 24 to 59 months by socioeconomic status in five South Asian countries: evidence from demographic and health surveys. BMJ Open. 2020;10(3):e032866. 10.1136/bmjopen-2019-032866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2022: Summary of Results. UN DESA/POP/2022/TR/NO. 3. 2022. Cited 4 Dec 2022.
- 23.Government of Pakistan, UNICEF. National Nutrition Survey 2018 - Full Report (3 Volumes) & Key Findings Report. Islamabad, Pakistan: UNICEF; 2020. Available from: https://www.unicef.org/pakistan/reports/national-nutrition-survey-2018-full-report-3-volumes-key-findings-report.
- 24.National Institute of Population Studies (NIPS) [Pakistan], ICF. In: Pakistan Demographic and Health Survey 2017–18. Islamabad, Pakistan and Rockville, Mayland, USA: NIPS and ICF; 2019. Available from: https://www.dhsprogram.com/pubs/pdf/FR354/FR354.pdf. [Google Scholar]
- 25.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. The Lancet. 2013;382(9890):427–51. 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. In: Training Course on Child Growth Assessment. Geneva: WHO; 2008. p. 58. Available from: https://apps.who.int/iris/handle/10665/43601. Cited 14 Jan 2023. [Google Scholar]
- 27.Nutrition and Food Safety WHO Team. In: WHO child growth standards:: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age; methods and development. Geneva: WHO Press; 2006. p. 312 https://www.who.int/publications/i/item/924154693X. (WHO child growth standards). [Google Scholar]
- 28.ICF. Demographic and Health Surveys Standard Recode Manual for DHS7. Rockville: The Demographic and Health Surveys Program. Available from: https://dhsprogram.com/pubs/pdf/DHSG4/Recode7_DHS_10Sep2018_DHSG4.pdf. Cited 2022 Dec 4.
- 29.de Onis M, Garza C, Victora CG, Onyango AW, Frongillo EA, Martines J. The who Multicentre growth reference study: planning, study design, and methodology. Food Nutr Bull. 2004;25(1_suppl_1):S15–26. 10.1177/15648265040251S104 [DOI] [PubMed] [Google Scholar]
- 30.Abera L, Dejene T, Laelago T. Prevalence of malnutrition and associated factors in children aged 6–59 months among rural dwellers of damot gale district, south Ethiopia: community based cross sectional study. International Journal for Equity in Health. 2017;16(1):111. 10.1186/s12939-017-0608-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mushtaq MU, Gull S, Shahid U, Shafique MM, Abdullah HM, Shad MA, et al. Family-based factors associated with overweight and obesity among Pakistani primary school children. BMC Pediatr. 2011;11(1):1–10. 10.1186/1471-2431-11-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siddiqa M, Zubair A, Kamal A, Ijaz M, Abushal T. Prevalence and associated factors of stunting, wasting and underweight of children below five using quintile regression analysis (PDHS 2017–2018). Sci Rep. 2022;12(1):20326. 10.1038/s41598-022-24063-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mamabolo RL, Alberts M, Steyn NP, van de Waal HAD, Levitt NS. Prevalence and determinants of stunting and overweight in 3-year-old black South African children residing in the Central Region of Limpopo Province, South Africa. Public Health Nutr. 2005;8(5):501–8. 10.1079/PHN2005786 [DOI] [PubMed] [Google Scholar]
- 34.Fotso JC. Child health inequities in developing countries: differences across urban and rural areas. International Journal for Equity in Health. 2006;5(1):9. 10.1186/1475-9276-5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wieringa FT, Gauthier L, Greffeuille V, Som SV, Dijkhuizen MA, Laillou A, et al. Identification of Acute Malnutrition in Children in Cambodia Requires Both Mid Upper Arm Circumference and Weight-For-Height to Offset Gender Bias of Each Indicator. Nutrients. 2018;10(6):786. 10.3390/nu10060786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan S, Zaheer S, Safdar NF. Determinants of stunting, underweight and wasting among children < 5 years of age: evidence from 2012–2013 Pakistan demographic and health survey. BMC Public Health. 2019;19(1):1–15. 10.1186/s12889-019-6688-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das S, Rahman RM. Application of ordinal logistic regression analysis in determining risk factors of child malnutrition in Bangladesh. Nutr J. 2011;14(10):124. 10.1186/1475-2891-10-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajian-Tilaki K, Heidari B. Childhood Obesity, Overweight, Socio-Demographic and Life Style Determinants among Preschool Children in Babol, Northern Iran. Iran J Public Health. 2013;42(11):1283–91. [PMC free article] [PubMed] [Google Scholar]
- 39.Griffiths LJ, Smeeth L, Hawkins SS, Cole TJ, Dezateux C. Effects of infant feeding practice on weight gain from birth to 3 years. Arch Dis Child. 2009;94(8):577–82. 10.1136/adc.2008.137554 [DOI] [PubMed] [Google Scholar]
- 40.Ahmed SOM, Hamid HIA, Jothi Shanmugam A, Tia MMG, Alnassry SMA. Impact of exclusive breastfeeding on physical growth. Clin Nutr Open Sci. 2023;1(49):101–6. 10.1016/j.nutos.2023.04.008 [DOI] [Google Scholar]
- 41.Lee JW, Lee M, Lee J, Kim YJ, Ha E, Kim HS. The Protective Effect of Exclusive Breastfeeding on Overweight/Obesity in Children with High Birth Weight. J Korean Med Sci. 2019;34(10). 10.3346/jkms.2019.34.e85. Cited 2024 Jan 29. [DOI] [PMC free article] [PubMed]
- 42.Trevor N. Croft, Aileen M.J. Marshall, Courtney K. Allen. Guide to DHS Statistics. Rockville, Maryland, USA: ICF. Available from: https://dhsprogram.com/pubs/pdf/DHSG1/Guide_to_DHS_Statistics_DHS-7_v2.pdf. Cited 2022 Nov 22.
- 43.Lee AC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. 2013;1(1):e26–36. 10.1016/S2214-109X(13)70006-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho WK, Suh BK. Catch-up growth and catch-up fat in children born small for gestational age. Korean J Pediatr. 2016Jan;59(1):1–7. 10.3345/kjp.2016.59.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hokken-Koelega AC, De Ridder MA, Lemmen RJ, Den Hartog H, De Muinck Keizer-Schrama SM, Drop SL. Children born small for gestational age: do they catch up? Pediatr Res. 1995;38(2):267–71. 10.1203/00006450-199508000-00022 [DOI] [PubMed] [Google Scholar]
- 46.Karlberg J, Albertsson-Wikland K. Growth in Full- Term Small-for-Gestational-Age Infants: From Birth to Final Height. Pediatr Res. 1995;38(5):733–9. 10.1203/00006450-199511000-00017 [DOI] [PubMed] [Google Scholar]
- 47.Thompson CG, Kim RS, Aloe AM, Becker BJ. Extracting the Variance Inflation Factor and Other Multicollinearity Diagnostics from Typical Regression Results. Basic Appl Soc Psychol. 2017;39(2):81–90. 10.1080/01973533.2016.1277529 [DOI] [Google Scholar]
- 48.Holm S. A Simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70. [Google Scholar]
- 49.Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86(5):726–8. 10.2105/AJPH.86.5.726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anik AI, Rahman MM, Rahman MM, Tareque MI, Khan MN, Alam MM. Double burden of malnutrition at household level: a comparative study among Bangladesh, Nepal, Pakistan, and Myanmar. PLoS One. 2019;14(8):e0221274. 10.1371/journal.pone.0221274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jehn M, Brewis A. Paradoxical malnutrition in mother–child pairs: Untangling the phenomenon of over- and under-nutrition in underdeveloped economies. Econ Hum Biol. 2009;7(1):28–35. 10.1016/j.ehb.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 52.Oddo VM, Rah JH, Semba RD, Sun K, Akhter N, Sari M, et al. Predictors of maternal and child double burden of malnutrition in rural Indonesia and Bangladesh. Am J Clin Nutr. 2012;95(4):951–8. 10.3945/ajcn.111.026070 [DOI] [PubMed] [Google Scholar]
- 53.Fernald LC, Neufeld LM. Overweight with concurrent stunting in very young children from rural Mexico: prevalence and associated factors. Eur J Clin Nutr. 2007;61(5):623–32. 10.1038/sj.ejcn.1602558 [DOI] [PubMed] [Google Scholar]
- 54.Garrett JL, Ruel MT. Stunted Child-Overweight Mother Pairs: Prevalence and Association with Economic Development and Urbanization. Food Nutr Bull. 2005;26(2):209–21. 10.1177/156482650502600205 [DOI] [PubMed] [Google Scholar]
- 55.Doak CM, Adair LS, Bentley M, Monteiro C, Popkin BM. The dual burden household and the nutrition transition paradox. Int J Obes. 2005;29(1):129–36. 10.1038/sj.ijo.0802824 [DOI] [PubMed] [Google Scholar]
- 56.Doak CM, Adair LS, Monteiro C, Popkin BM. Overweight and Underweight Coexist within Households in Brazil, China and Russia. J Nutr. 2000;130(12):2965–71. 10.1093/jn/130.12.2965 [DOI] [PubMed] [Google Scholar]
- 57.Kosaka S, Umezaki M. A systematic review of the prevalence and predictors of the double burden of malnutrition within households. Br J Nutr. 2017;117(8):1118–27. 10.1017/S0007114517000812 [DOI] [PubMed] [Google Scholar]
- 58.Davis JN, Oaks BM, Engle-Stone R. The double burden of malnutrition: a systematic review of operational definitions. Curr Dev Nutr. 2020;4(9):nzaa127. 10.1093/cdn/nzaa127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biswas T, Townsend N, Magalhaes RJS, Hasan M, Mamun A. Patterns and determinants of the double burden of malnutrition at the household level in South and Southeast Asia. Eur J Clin Nutr. 2021;75(2):385–91. 10.1038/s41430-020-00726-z [DOI] [PubMed] [Google Scholar]
- 60.Popkin BM, Corvalan C, Grummer-Strawn LM. Dynamics of the double burden of malnutrition and the changing nutrition reality. The Lancet. 2020;395(10217):65–74. 10.1016/S0140-6736(19)32497-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wells JC, Sawaya AL, Wibaek R, Mwangome M, Poullas MS, Yajnik CS, et al. The double burden of malnutrition: aetiological pathways and consequences for health. Lancet. 2020;395(10217):75–88. 10.1016/S0140-6736(19)32472-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shrimpton R, Rokx C. The Double Burden of Malnutrition]. World Bank; 2012. 1. (Health, Nutrition and Population Discussion Papers). Available from: http://elibrary.worldbank.org/doi/abs/10.1596/27417 Cited 2022 Dec 7.
- 63.World Health Organization. World Health Organization. Double burden of malnutrition. Available from: https://www.who.int/nutrition/double-burden-malnutrition/en/. Cited 2022 Dec 7.
- 64.Jaacks LM, Kavle J, Perry A, Nyaku A. Programming maternal and child overweight and obesity in the context of undernutrition: current evidence and key considerations for low- and middle-income countries. Public Health Nutr. 2017;20(7):1286–96. 10.1017/S1368980016003323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horta BL, de Loret Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015;104(S467):30–7. 10.1111/apa.13133 [DOI] [PubMed] [Google Scholar]
- 66.Ottawa charter for health promotion. Health Promot Int. 1986;1(4):405.
- 67.United Nations. Transforming our world: the 2030 agenda for sustainable development. New York, NY: United Nations; 2015. Available from: https://sdgs.un.org/sites/default/files/publications/21252030%20Agenda%20for%20Sustainable%20Development%20web.pdf. Cited 2024 Jan 29.
- 68.Singh Thakur J, Nangia R, Singh S. Progress and challenges in achieving noncommunicable diseases targets for the sustainable development goals. FASEB Bioadv. 2021;3(8):563–8. 10.1096/fba.2020-00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nishtar S, Faruqui AMA, Mattu MA, Mohamud KB, Ahmed A. The National Action Plan for the Prevention and Control of Non-communicable Diseases and Health Promotion in Pakistan-Cardiovascular diseases. J Pak Med Assoc. 2004;54(12 Suppl 3):S14–25. [PubMed] [Google Scholar]
- 70.The World Bank, South Asia Human Development, Health, Nutrition, and Population. NCDs Policy Brief: Pakistan. Washington, DC: The World Bank; 2011. Available from: https://documents1.worldbank.org/curated/pt/552281505992200763/pdf/119408-BRI-P114171-PUBLIC-NCD-PK.pdf. Cited 2024 Jan 29.
- 71.Jafar TH, Haaland BA, Rahman A, Razzak JA, Bilger M, Naghavi M, et al. Non-communicable diseases and injuries in Pakistan: strategic priorities. Lancet. 2013;381(9885):2281–90. 10.1016/S0140-6736(13)60646-7 [DOI] [PubMed] [Google Scholar]
- 72.Roshan R, Hamid S, Mashhadi SF. Non-communicable diseases in pakistan; a health system perspective: non-communicable diseases in Pakistan. Pak Armed Forces Med J. 2018;68(2):394–9. [Google Scholar]
- 73.Khan SD, Jafar TH, Siddiqi K, Ahmad T, Khan AA, Samad Z. Data on non-communicable diseases: A missed opportunity in Pakistan. J Glob Health. 2023;13:03045. 10.7189/jogh.13.03045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Asim M, Nawaz Y. Child Malnutrition in Pakistan: Evidence from Literature. Children. 2018;5(5):60. 10.3390/children5050060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.District Government Tharparkar. Analysis of Nutritional Data Community-Based Management of Acute Malnutrition Project. Sindh, Pakistan: District Government Tharparkar; 2014. [Google Scholar]
- 76.Achakzai P, Khan R. Nutritional status and associated factors among children less than five years of age in tehsil Zarghoon town, district Quetta, Baluchistan. J Ayub Med Coll Abbottabad. 2016;28(1):146–51. [PubMed] [Google Scholar]
- 77.Khan GN, Turab A, Khan MI, Rizvi A, Shaheen F, Ullah A, et al. Prevalence and associated factors of malnutrition among children under-five years in Sindh, Pakistan: a cross-sectional study. BMC Nutrition. 2016;2(1):69. 10.1186/s40795-016-0112-4 [DOI] [Google Scholar]
- 78.Ali W, Ayub A, Hussain H. Prevalence and associated risk factors of under nutrition among children aged 6 to 59 months in internally displaced persons of Jalozai Camp, District Nowshera, Khyber PakhtunkhwA. J Ayub Med Coll Abbottabad. 2015;27(3):556–9. [PubMed] [Google Scholar]
- 79.Gul R, Kibria Z. Prevalence and predeterminants of malnutrition in children under 3 years of age in the two rural communities of PeshawaR. Khyber Medical University Journal. 2013;5(4):190–4.
- 80.Trang NHHD, Hong TK, van der Ploeg HP, Hardy LL, Kelly PJ, Dibley MJ. Longitudinal Sedentary Behavior Changes in Adolescents in Ho Chi Minh City. Am J Prev Med. 2013;44(3):223–30. 10.1016/j.amepre.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 81.Biadgilign S, Mgutshini T, Gebremichael B, Haile D, Berhanu L, Chitekwe S, et al. Correlates of sedentary time among children and adolescents in Ethiopia: a cross-sectional Study. Pediatr Exerc Sci. 2022;1(aop):1–8. 10.1123/pes.2021-0077 [DOI] [PubMed] [Google Scholar]
- 82.Carrillo-Larco RM, Miranda JJ, Bernabé-Ortiz A. Wealth index and risk of childhood overweight and obesity: evidence from four prospective cohorts in Peru and Vietnam. Int J Public Health. 2016;61(4):475–85. 10.1007/s00038-015-0767-7 [DOI] [PubMed] [Google Scholar]
- 83.Neupane SKCP, Doku DT. Overweight and obesity among women: analysis of demographic and health survey data from 32 Sub-Saharan African Countries. BMC Public Health. 2016;16(1):30. 10.1186/s12889-016-2698-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available with DHS program. The data can be requested at: https://dhsprogram.com/data/available-datasets.cfm