Abstract
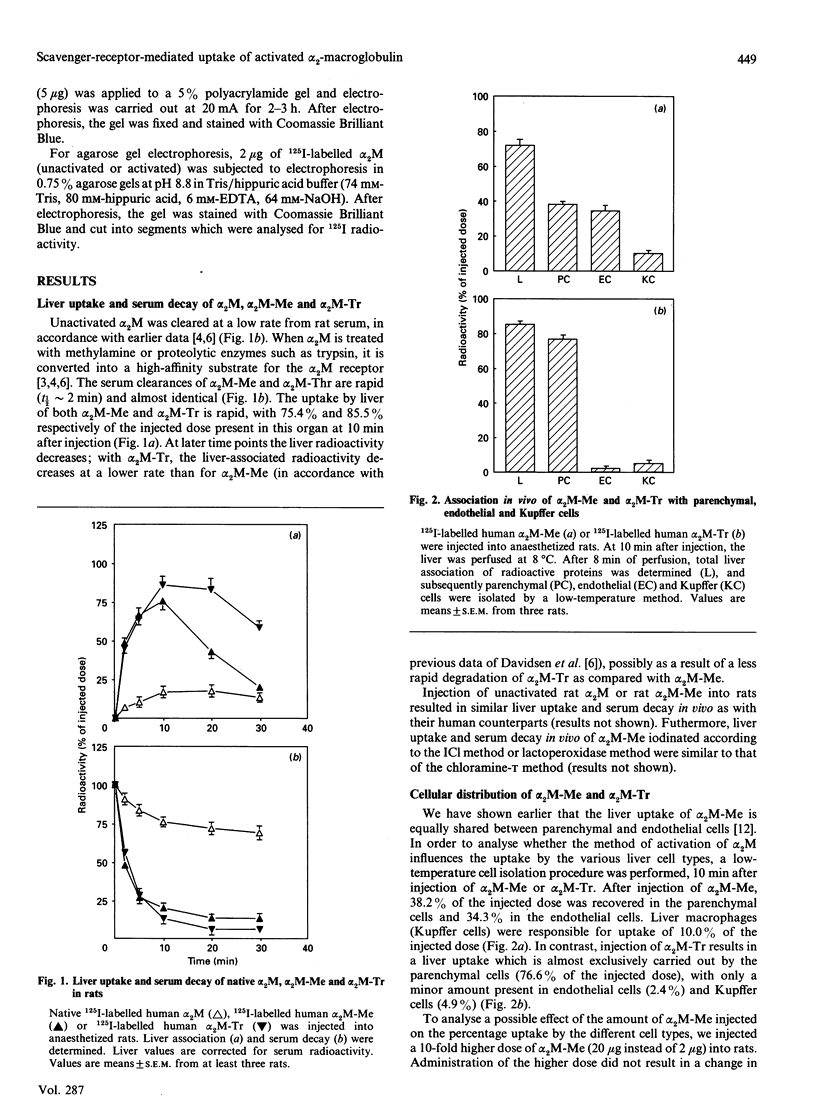
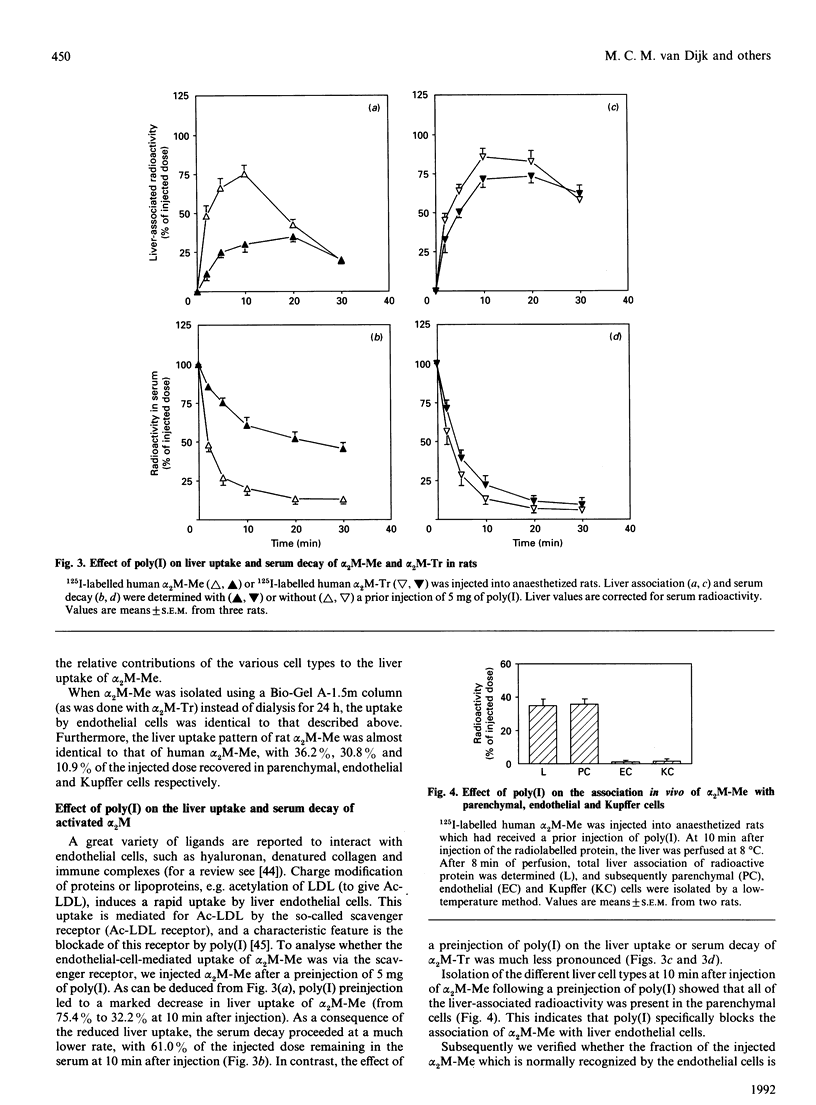
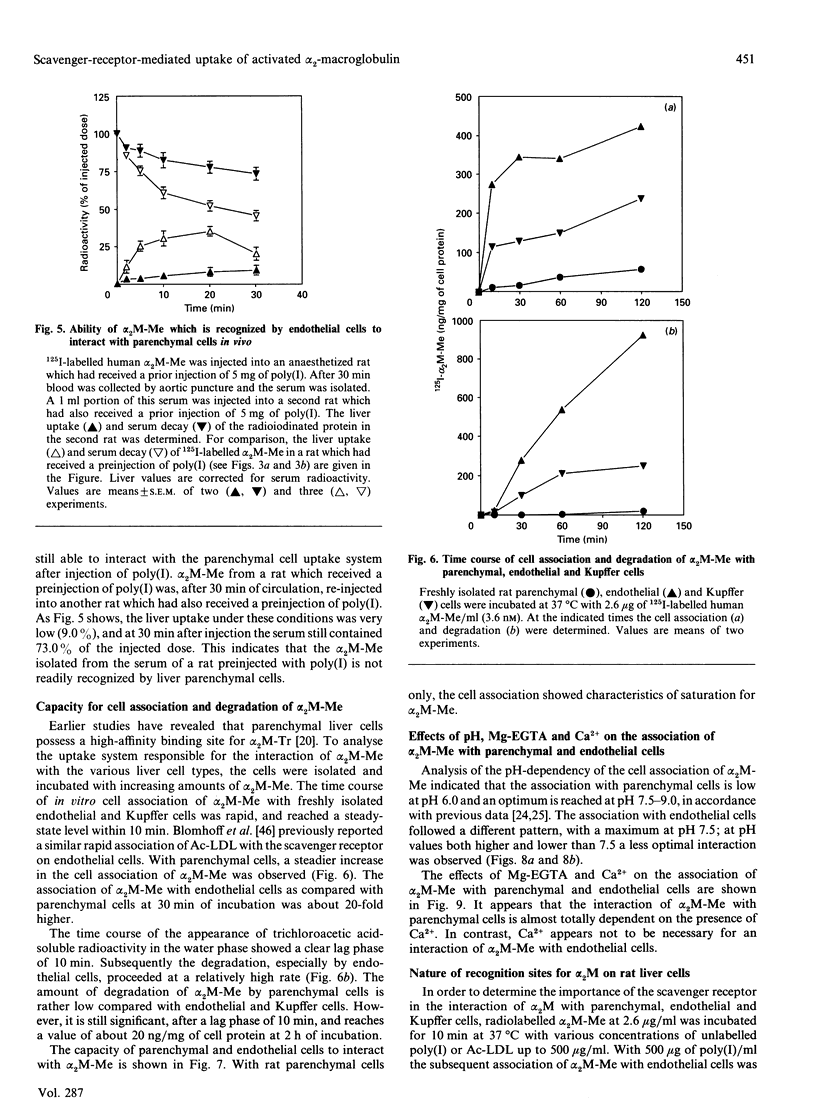
Alpha 2-Macroglobulin (alpha 2M) requires activation by small nucleophiles (e.g. methylamine; giving alpha 2M-Me) or proteolytic enzymes (e.g. trypsin; giving alpha 2M-Tr) in order to be rapidly removed from the circulation by the liver. Separation of rat liver cells into parenchymal, endothelial and Kupffer cells at 10 min after injection indicates that liver uptake of alpha 2M-Me is shared between parenchymal and endothelial cells, with relative contributions of 51.3% and 48.3% respectively of total liver-associated radioactivity. In contrast, alpha 2M-Tr is almost exclusively taken up by the parenchymal cells (90.1% of liver-associated radioactivity). A preinjection of 5 mg of poly(inosinic acid) decreased liver uptake of alpha 2M-Me to 39.9% of the control value, while it had no effect on liver uptake of alpha 2M-Tr. It appears that poly(inosinic acid) specifically reduces the uptake of alpha 2M-Me in vivo by endothelial cells, leaving uptake by parenchymal cells unaffected. In vitro studies with isolated liver cells indicate that the association of alpha 2M-Me with endothelial cells is 21-fold higher per mg of cell protein than with parenchymal cells. The capacity of endothelial cells to degrade alpha 2M-Me appears to be 46 times higher than that of parenchymal cells. Competition studies show that poly(inosinic acid) or acetylated low-density lipoprotein effectively competes with the association of alpha 2M-Me with endothelial and Kupffer cells, but association with parenchymal cells is unaffected. It is suggested that activation of alpha 2M by methylamine induces a charge distribution on the protein which triggers specific uptake by the scavenger receptor on endothelial cells. It is concluded that the uptake of alpha 2M-Me by the scavenger receptor might function as an additional system for the uptake of activated alpha 2M.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcom J. D., Tiller S. E., Dickerson K., Cravens J. L., Argraves W. S., Strickland D. K. The human alpha 2-macroglobulin receptor: identification of a 420-kD cell surface glycoprotein specific for the activated conformation of alpha 2-macroglobulin. J Cell Biol. 1990 Apr;110(4):1041–1048. doi: 10.1083/jcb.110.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijsterbosch M. K., Ziere G. J., Van Berkel T. J. Lactosylated low density lipoprotein: a potential carrier for the site-specific delivery of drugs to Kupffer cells. Mol Pharmacol. 1989 Sep;36(3):484–489. [PubMed] [Google Scholar]
- Björk I., Fish W. W. Evidence for similar conformational changes in alpha 2-macroglobulin on reaction with primary amines or proteolytic enzymes. Biochem J. 1982 Nov 1;207(2):347–356. doi: 10.1042/bj2070347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomhoff R., Drevon C. A., Eskild W., Helgerud P., Norum K. R., Berg T. Clearance of acetyl low density lipoprotein by rat liver endothelial cells. Implications for hepatic cholesterol metabolism. J Biol Chem. 1984 Jul 25;259(14):8898–8903. [PubMed] [Google Scholar]
- Blouin A., Bolender R. P., Weibel E. R. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977 Feb;72(2):441–455. doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTER W. O., SIMON A. B., ARMSTRONG W. D. Evans blue space in tissues of the rat. Am J Physiol. 1955 Nov;183(2):317–321. doi: 10.1152/ajplegacy.1955.183.2.317. [DOI] [PubMed] [Google Scholar]
- Casteleijn E., van Rooij H. C., van Berkel T. J., Koster J. F. Mechanism of glucagon stimulation of fructose-1,6-bisphosphatase in rat hepatocytes. Involvement of a low-Mr activator. FEBS Lett. 1986 Jun 9;201(2):193–197. doi: 10.1016/0014-5793(86)80607-x. [DOI] [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- Davidsen O., Christensen E. I., Gliemann J. The plasma clearance of human alpha 2-macroglobulin-trypsin complex in the rat is mainly accounted for by uptake into hepatocytes. Biochim Biophys Acta. 1985 Jul 30;846(1):85–92. doi: 10.1016/0167-4889(85)90113-2. [DOI] [PubMed] [Google Scholar]
- Debanne M. T., Bell R., Dolovich J. Characteristics of the macrophage uptake of proteinase-alpha-macroglobulin complexes. Biochim Biophys Acta. 1976 Apr 23;428(2):466–475. doi: 10.1016/0304-4165(76)90055-6. [DOI] [PubMed] [Google Scholar]
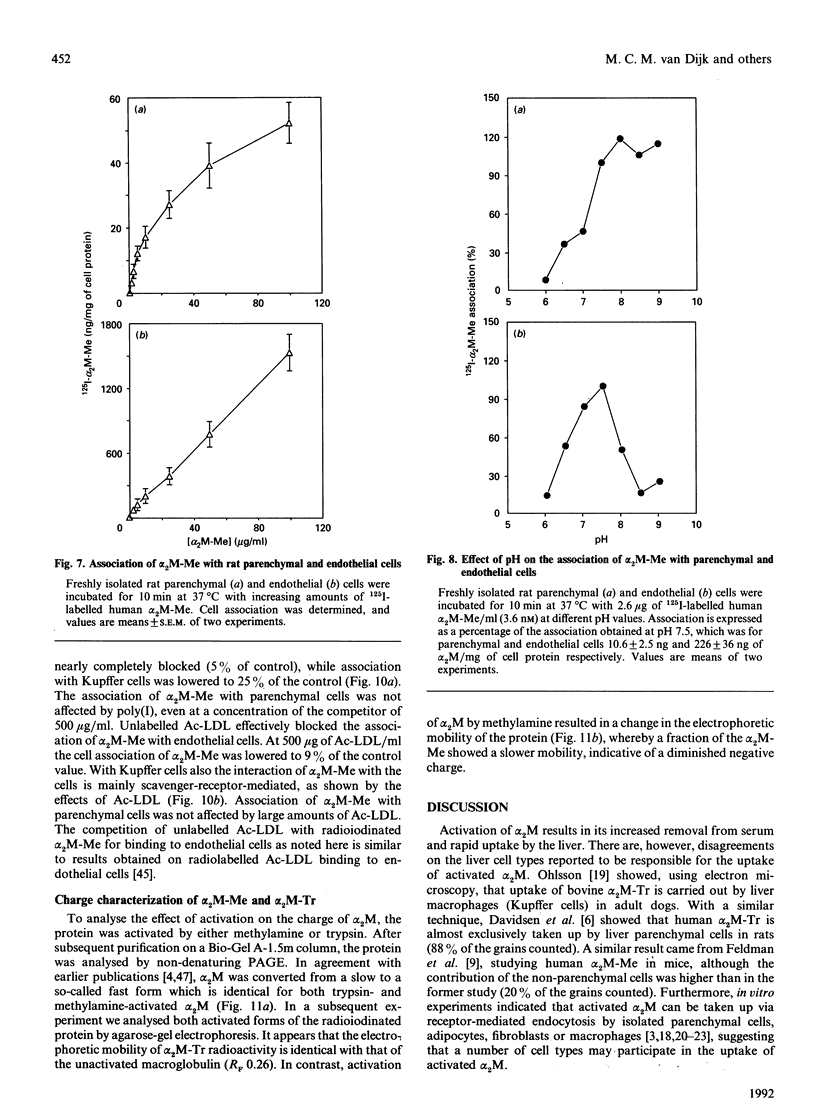
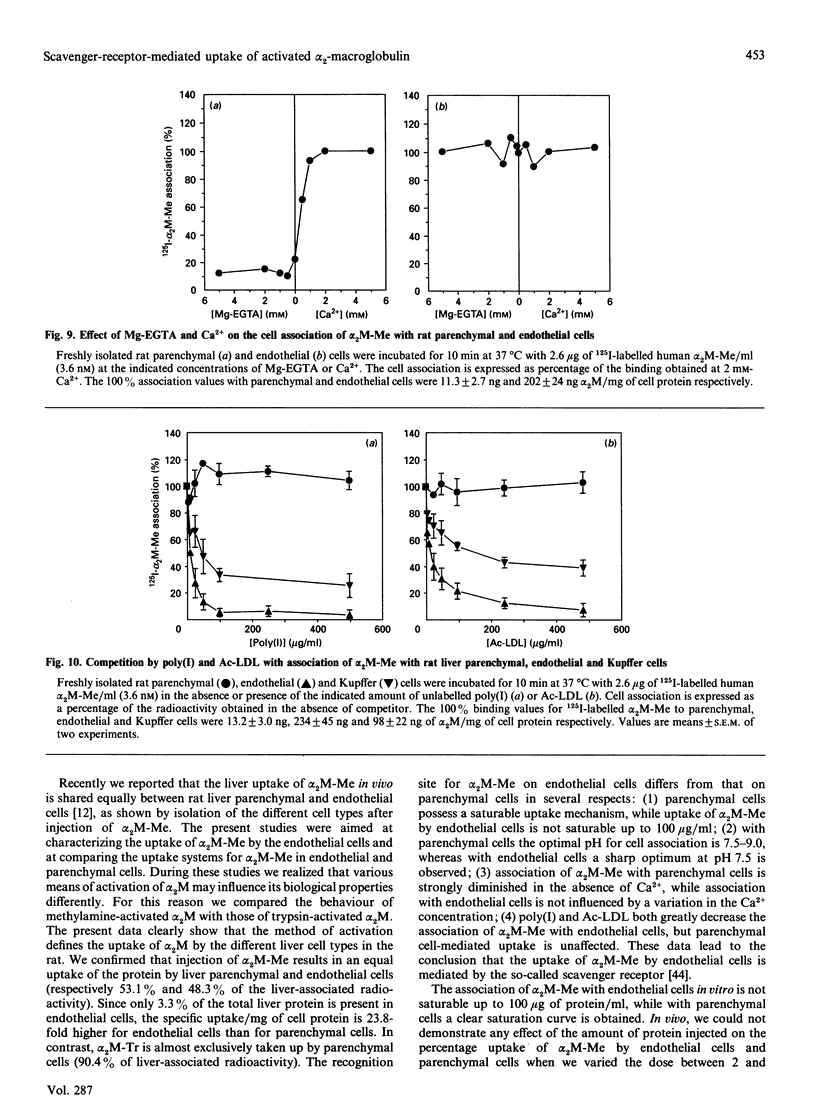
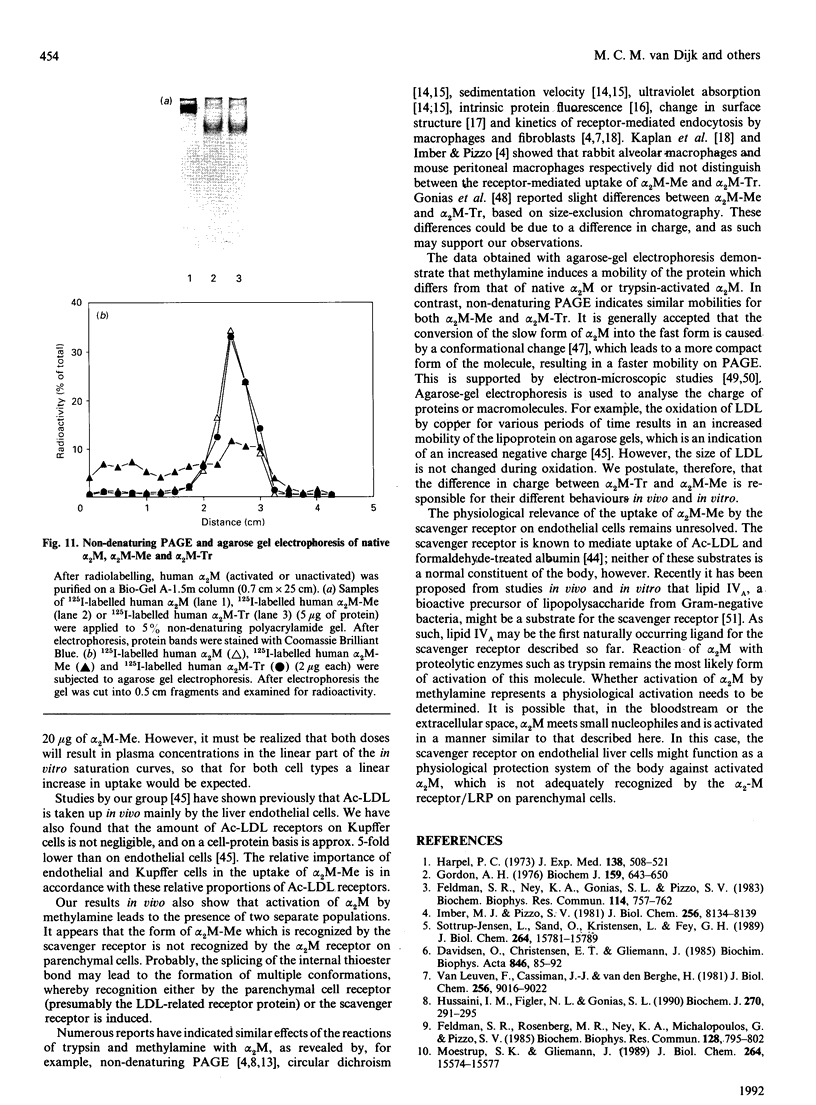
- Feldman S. R., Ney K. A., Gonias S. L., Pizzo S. V. In vitro binding and in vivo clearance of human alpha 2-macroglobulin after reaction with endoproteases from four different classes. Biochem Biophys Res Commun. 1983 Jul 29;114(2):757–762. doi: 10.1016/0006-291x(83)90845-8. [DOI] [PubMed] [Google Scholar]
- Feldman S. R., Rosenberg M. R., Ney K. A., Michalopoulos G., Pizzo S. V. Binding of alpha 2-macroglobulin to hepatocytes: mechanism of in vivo clearance. Biochem Biophys Res Commun. 1985 Apr 30;128(2):795–802. doi: 10.1016/0006-291x(85)90117-2. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Davidsen O. Characterization of receptors for alpha 2-macroglobulin-trypsin complex in rat hepatocytes. Biochim Biophys Acta. 1986 Jan 23;885(1):49–57. doi: 10.1016/0167-4889(86)90037-6. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Davidsen O., Moestrup S. K. Characterization, size estimation and solubilization of alpha-macroglobulin complex receptors in liver membranes. Biochim Biophys Acta. 1989 Apr 28;980(3):326–332. doi: 10.1016/0005-2736(89)90320-9. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Larsen T. R., Sottrup-Jensen L. Cell association and degradation of alpha 2-macroglobulin-trypsin complexes in hepatocytes and adipocytes. Biochim Biophys Acta. 1983 Mar 31;756(2):230–237. doi: 10.1016/0304-4165(83)90096-x. [DOI] [PubMed] [Google Scholar]
- Gonias S. L., Figler N. L. Electron microscopy studies of alpha 2-macroglobulin conformational intermediates obtained by derivatization with cis-dichlorodiammineplatinum (II). J Biol Chem. 1989 Jun 5;264(16):9565–9570. [PubMed] [Google Scholar]
- Gonias S. L., Reynolds J. A., Pizzo S. V. Physical properties of human alpha 2-macroglobulin following reaction with methylamine and trypsin. Biochim Biophys Acta. 1982 Aug 10;705(3):306–314. doi: 10.1016/0167-4838(82)90252-7. [DOI] [PubMed] [Google Scholar]
- Gonias S. L., Roche P. A., Pizzo S. V. Purification and characterization of human alpha 2-macroglobulin conformational variants by non-ideal high performance size-exclusion chromatography. Biochem J. 1986 Apr 15;235(2):559–567. doi: 10.1042/bj2350559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. H. The alpha macroglobulins of rat serum. Biochem J. 1976 Dec 1;159(3):643–650. doi: 10.1042/bj1590643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton R. Y., Golenbock D. T., Penman M., Krieger M., Raetz C. R. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991 Jul 25;352(6333):342–344. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- Harpel P. C. Studies on human plasma alpha 2-macroglobulin-enzyme interactions. Evidence for proteolytic modification of the subunit chain structure. J Exp Med. 1973 Sep 1;138(3):508–521. doi: 10.1084/jem.138.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussaini I. M., Figler N. L., Gonias S. L. The structure of alpha 2-macroglobulin-methylamine after papain digestion as determined by electron microscopy. Biochem J. 1990 Sep 1;270(2):291–295. doi: 10.1042/bj2700291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V. Clearance and binding of two electrophoretic "fast" forms of human alpha 2-macroglobulin. J Biol Chem. 1981 Aug 10;256(15):8134–8139. [PubMed] [Google Scholar]
- Kaplan J. Evidence for reutilization of surface receptors for alpha-macroglobulin.protease complexes in rabbit alveolar macrophages. Cell. 1980 Jan;19(1):197–205. doi: 10.1016/0092-8674(80)90401-8. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Ray F. A., Keogh E. A. Recognition of nucleophile-treated alpha 2-macroglobulin by the alveolar macrophage alpha-macroglobulin . protease complex receptor. J Biol Chem. 1981 Aug 10;256(15):7705–7707. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lonberg-Holm K., Reed D. L., Roberts R. C., Hebert R. R., Hillman M. C., Kutney R. M. Three high molecular weight protease inhibitors of rat plasma. Isolation, characterization, and acute phase changes. J Biol Chem. 1987 Jan 5;262(1):438–445. [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Moestrup S. K., Gliemann J. Analysis of ligand recognition by the purified alpha 2-macroglobulin receptor (low density lipoprotein receptor-related protein). Evidence that high affinity of alpha 2-macroglobulin-proteinase complex is achieved by binding to adjacent receptors. J Biol Chem. 1991 Jul 25;266(21):14011–14017. [PubMed] [Google Scholar]
- Moestrup S. K., Gliemann J. Purification of the rat hepatic alpha 2-macroglobulin receptor as an approximately 440-kDa single chain protein. J Biol Chem. 1989 Sep 15;264(26):15574–15577. [PubMed] [Google Scholar]
- Moestrup S. K., Kaltoft K., Sottrup-Jensen L., Gliemann J. The human alpha 2-macroglobulin receptor contains high affinity calcium binding sites important for receptor conformation and ligand recognition. J Biol Chem. 1990 Jul 25;265(21):12623–12628. [PubMed] [Google Scholar]
- Nagelkerke J. F., Barto K. P., van Berkel T. J. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J Biol Chem. 1983 Oct 25;258(20):12221–12227. [PubMed] [Google Scholar]
- Nelles L. P., Hall P. K., Roberts R. C. Human alpha-2-macroglobulin. Studies on the electrophoretic heterogeneity. Biochim Biophys Acta. 1980 May 29;623(1):46–56. doi: 10.1016/0005-2795(80)90006-9. [DOI] [PubMed] [Google Scholar]
- Ohlsson K. Elimination of 125-I-trypsin alpha-macroglobulin complexes from blood by reticuloendothelial cells in dogs. Acta Physiol Scand. 1971 Feb;81(2):269–272. doi: 10.1111/j.1748-1716.1971.tb04900.x. [DOI] [PubMed] [Google Scholar]
- Redgrave T. G., Roberts D. C., West C. E. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975 May 12;65(1-2):42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Smedsrød B., Pertoft H., Gustafson S., Laurent T. C. Scavenger functions of the liver endothelial cell. Biochem J. 1990 Mar 1;266(2):313–327. doi: 10.1042/bj2660313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Petersen T. E., Magnusson S. A thiol-ester in alpha 2-macroglobulin cleaved during proteinase complex formation. FEBS Lett. 1980 Dec 1;121(2):275–279. doi: 10.1016/0014-5793(80)80361-9. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Sand O., Kristensen L., Fey G. H. The alpha-macroglobulin bait region. Sequence diversity and localization of cleavage sites for proteinases in five mammalian alpha-macroglobulins. J Biol Chem. 1989 Sep 25;264(27):15781–15789. [PubMed] [Google Scholar]
- Straight D. L., McKee P. A. Effect of protease binding by alpha 2-macroglobulin on intrinsic fluorescence. Biochemistry. 1982 Sep 14;21(19):4550–4556. doi: 10.1021/bi00262a006. [DOI] [PubMed] [Google Scholar]
- Strickland D. K., Bhattacharya P., Olson S. T. Kinetics of the conformational alterations associated with nucleophilic modification of alpha 2-macroglobulin. Biochemistry. 1984 Jul 3;23(14):3115–3124. doi: 10.1021/bi00309a002. [DOI] [PubMed] [Google Scholar]
- Tapon-Bretaudiére J., Bros A., Couture-Tosi E., Delain E. Electron microscopy of the conformational changes of alpha 2-macroglobulin from human plasma. EMBO J. 1985 Jan;4(1):85–89. doi: 10.1002/j.1460-2075.1985.tb02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Berkel T. J., De Rijke Y. B., Kruijt J. K. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats. Recognition by various scavenger receptors on Kupffer and endothelial liver cells. J Biol Chem. 1991 Feb 5;266(4):2282–2289. [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van Den Berghe H. Demonstration of an alpha2-macroglobulin receptor in human fibroblasts, absent in tumor-derived cell lines. J Biol Chem. 1979 Jun 25;254(12):5155–5160. [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van den Berghe H. Functional modifications of alpha 2-macroglobulin by primary amines. I. Characterization of alpha 2 M after derivatization by methylamine and by factor XIII. J Biol Chem. 1981 Sep 10;256(17):9016–9022. [PubMed] [Google Scholar]
- Van Tol A., Van 't Hooft F. M., Van Gent T. Discrepancies in the catabolic pathways of rat and human low density lipoproteins as revealed by partial hepatectomy in the rat. Atherosclerosis. 1978 Apr;29(4):449–457. doi: 10.1016/0021-9150(78)90173-9. [DOI] [PubMed] [Google Scholar]
- van Berkel T. J., Dekker C. J., Kruijt J. K., van Eijk H. G. The interaction in vivo of transferrin and asialotransferrin with liver cells. Biochem J. 1987 May 1;243(3):715–722. doi: 10.1042/bj2430715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk M. C., Ziere G. J., Boers W., Linthorst C., Bijsterbosch M. K., van Berkel T. J. Recognition of chylomicron remnants and beta-migrating very-low-density lipoproteins by the remnant receptor of parenchymal liver cells is distinct from the liver alpha 2-macroglobulin-recognition site. Biochem J. 1991 Nov 1;279(Pt 3):863–870. doi: 10.1042/bj2790863. [DOI] [PMC free article] [PubMed] [Google Scholar]