Abstract
Objectives
To investigate the associations of physical activity (PA) and sedentary behaviour in early childhood with asthma and reduced lung function in later childhood within a large collaborative study.
Design
Pooling of longitudinal data from collaborating birth cohorts using meta-analysis of separate cohort-specific estimates and analysis of individual participant data of all cohorts combined.
Setting
Children aged 0–18 years from 26 European birth cohorts.
Participants
136 071 individual children from 26 cohorts, with information on PA and/or sedentary behaviour in early childhood and asthma assessment in later childhood.
Main outcome measure
Questionnaire-based current asthma and lung function measured by spirometry (forced expiratory volume in 1 s (FEV1), FEV1/forced vital capacity) at age 6–18 years.
Results
Questionnaire-based and accelerometry-based PA and sedentary behaviour at age 3–5 years was not associated with asthma at age 6–18 years (PA in hours/day adjusted OR 1.01, 95% CI 0.98 to 1.04; sedentary behaviour in hours/day adjusted OR 1.03, 95% CI 0.99 to 1.07). PA was not associated with lung function at any age. Analyses of sedentary behaviour and lung function showed inconsistent results.
Conclusions
Reduced PA and increased sedentary behaviour before 6 years of age were not associated with the presence of asthma later in childhood.
Keywords: Asthma, Exercise, Paediatric asthma
WHAT IS ALREADY KNOWN ON THIS TOPIC.
WHAT THIS STUDY ADDS
This study shows no indication that physical activity and sedentary behaviour in early childhood are associated with asthma or reduced lung function in later childhood.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study contributes to the growing knowledge of lifestyle and asthma. Physical activity seems not to play a role in asthma prevention.
Introduction
Asthma is the most common non-communicable chronic disease in childhood.1 Among lifestyle risk factors, obesity2 and physical inactivity3 have received particular attention. Results from cross-sectional studies suggest that adults and children with asthma might be less physically active compared with their peers.4,8 Since higher levels of physical activity (PA) have been found to be associated with better lung function and asthma control,3 the hypothesis emerged that PA could possibly protect against asthma development by influencing inflammatory processes important in the development of asthma. Fernandes et al9 showed that PA modulates pulmonary allergic inflammation by increasing anti-inflammatory cytokines and decreasing proinflammatory cells and mediators.
Several researchers have investigated possible longitudinal associations between PA and asthma onset and found contrasting results. In adult populations, some longitudinal studies found that low levels of PA at baseline were associated with higher asthma odds later in life.10,12 However, most studies did not find evidence for an association between PA and new-onset asthma in adults.13,16 In children, studies also show conflicting results. Cassim et al published a systematic review focusing on cohort studies in children with PA measurements preceding asthma and lung function outcomes.17 The results were highly inconsistent and showed insufficient evidence to suggest that PA influences the risk of new-onset asthma or improves lung function in children. In a recent study performed by our group, we did not find an association between PA at age 4–5 years and subsequent asthma at age 6–10 years in the KOALA Birth Cohort Study.18 However, in a small subgroup of children with both accelerometry and lung function data available, we observed an association between sedentary behaviour and subsequently lower lung function. In the literature, sedentary behaviour and physical inactivity have been regarded as two different entities.19 Sedentary behaviour is described as activities with an energy expenditure of 1.5 or less metabolic equivalent of a task in sitting, lying or reclining position, during wake time, which is not necessarily the same as physical inactivity (ie, not meeting the PA guidelines). Sherriff et al and Protudjer et al both found a positive association between screen time and new-onset asthma in childhood.20 21 Chen et al demonstrated a pathway from central obesity to childhood asthma, via physical fitness and sedentary behaviour.22
The relation between childhood PA and lung function has been described in a few studies before higher PA levels in childhood have been associated with higher lung function in adolescent boys23 and girls.24 These findings could be relevant for respiratory health across the life course since lung function has been positively associated with aerobic fitness, and higher fitness levels during childhood are associated with larger adult lung volumes.24
Limitations of earlier studies are the relatively small study sizes in childhood studies compared with adult studies and little information on sedentary behaviour and lung function. In this large collaborative study, we gathered information on PA, sedentary behaviour, asthma and lung function from birth to age 18 years from 26 cohorts in Europe. We aimed to investigate PA and sedentary behaviour in relation to asthma and lung function at different ages in childhood. Our hypothesis was that higher PA before the age of six protects against asthma development later in childhood and that sedentary behaviour increases asthma risk. We also hypothesised that a higher level of PA is positively associated with lung function in later childhood.
Methods
Design
Meta-analysis of cohort-specific association estimates from separate analyses of longitudinal data within the collaborating birth cohorts and individual participant data.
Study population
European cohorts identified from existing collaborations on childhood asthma or asthma-related outcomes (www.birthcohorts.net; www.birthcohortsenrieco.net; www.chicosproject.eu) were invited to participate if they had data on PA that preceded information on asthma. Criteria for exclusion of individual children were congenital birth defects and diseases (other than asthma) that could influence either PA or respiratory function (such as cystic fibrosis, intellectual disability, or rheumatic disorders).
37 potentially eligible cohorts were identified, 26 agreed to participate. Of the 11 studies not participating, 2 had no data on PA, 1 had no data on asthma and 5 studies only had cross-sectional data on PA and asthma. Three other cohorts did not reply or were not interested in participating. In total, we included 136 071 individual children from 26 birth cohorts across Europe.
Participating cohorts signed a data transfer agreement, and pseudonymised datasets were transferred to Maastricht University for analysis. Cohort-specific informed consent was signed by the parents or legal guardians in the original cohorts.
Patient and public involvement
No patients were involved in the design or implementation of this study.
Age groups
Cohorts were asked to provide their available exposure (PA, sedentary behaviour) and outcome data (asthma, lung function) for separate age groups: 0–2 years, 3–5 years, 6–8 years, 9–14 years and 15–18 years. If cohorts had multiple measurements for one age group, the age with the largest number of variables relevant to this study was selected.
PA and sedentary behaviour
Information on PA and sedentary behaviour was obtained by cohort-specific questionnaires in all cohorts and activity monitors (accelerometry, four cohorts). Parents were asked how much time on average their child spent on different physical activities, such as cycling, walking, playing outside, exercising and physical education lessons. In case both the child and its parents filled out a questionnaire, we selected the parent-reported data. The total amount of time being physically active was converted into hours per day. All cohorts had questionnaire-based information on PA for at least one age group totalling to 134 929 individual participants (available data per age group in table 1, detailed information for the individual cohorts in online supplemental appendix table A). 24 cohorts had information on sedentary behaviour for at least one age group in 117 473 participants. Sedentary behaviour was calculated as the amount of time (expressed as hours per day) the child on average spent on sedentary activities (eg, watching television, playing computer games, travelling by car and reading). To harmonise the data, the total amount of time spent on PA or sedentary behaviour was also categorised into (cohort-specific) tertiles.
Table 1. Data availability per cohort.
| 0–2 years | 3–5 years | 6–8 years | 9–14 years | 15–18 years | |||||||||||||||||||
| Name cohort | PA | Sed | Acc | PA | Sed | Acc | Asthma | LF | PA | Sed | Acc | Asthma | LF | PA | Sed | Acc | Asthma | LF | PA | Sed | Acc | Asthma | LF |
| ABCD | 0 | 0 | 0 | 2769 | 2836 | 0 | 0 | 0 | 0 | 0 | 0 | 2872 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ABIS | 0 | 0 | 0 | 7202 | 7127 | 0 | 6960 | 0 | 3925 | 3947 | 0 | 3845 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BAMSE | 0 | 0 | 0 | 0 | 0 | 0 | 3104 | 0 | 0 | 0 | 0 | 3039 | 1685 | 2712 | 2668 | 0 | 3040 | 0 | 2750 | 2860 | 0 | 3043 | 1962 |
| CHOP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 515 | 502 | 432 | 558 | 0 | 523 | 497 | 434 | 573 | 0 | 0 | 0 | 0 | 0 | 0 |
| COPSAC2000 | 0 | 0 | 0 | 0 | 143 | 236 | 272 | 0 | 0 | 31 | 40 | 272 | 259 | 0 | 0 | 0 | 272 | 0 | 0 | 0 | 0 | 272 | 0 |
| DNBC | 66 409 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 53 092 | 53 172 | 0 | 54 602 | 0 | 45 928 | 46 082 | 0 | 80 350 | 0 | 0 | 0 | 0 | 0 | 0 |
| EDEN | 753 | 734 | 0 | 608 | 629 | 0 | 100 | 876 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| G21 | 0 | 0 | 0 | 6975 | 5964 | 0 | 7125 | 0 | 5831 | 5831 | 0 | 5788 | 1605 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gen R | 0 | 0 | 0 | 3919 | 4027 | 0 | 0 | 0 | 3787 | 4390 | 0 | 4401 | 0 | 0 | 0 | 0 | 4484 | 4583 | 0 | 0 | 0 | 0 | 0 |
| GINIplus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3837 | 3848 | 0 | 3855 | 744 | 2695 | 3267 | 0 | 3300 | 626 | 2447 | 3047 | 0 | 3167 | 1822 |
| HUMIS | 0 | 694 | 0 | 686 | 685 | 0 | 681 | 0 | 292 | 369 | 0 | 371 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| INMA Asturias | 0 | 0 | 0 | 340 | 340 | 0 | 0 | 0 | 0 | 0 | 0 | 340 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| INMA Gipuzkoa | 0 | 0 | 0 | 351 | 351 | 0 | 0 | 284 | 0 | 0 | 0 | 351 | 331 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| INMA Menorca | 0 | 0 | 0 | 0 | 0 | 0 | 471 | 0 | 470 | 471 | 0 | 463 | 0 | 425 | 425 | 0 | 425 | 395 | 288 | 286 | 0 | 287 | 269 |
| INMA Sabadell | 0 | 0 | 0 | 534 | 534 | 0 | 0 | 415 | 0 | 0 | 0 | 534 | 433 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| INMA Valencia | 0 | 0 | 0 | 450 | 460 | 0 | 0 | 0 | 0 | 0 | 0 | 460 | 446 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| KOALA | 2089 | 2181 | 0 | 1787 | 1835 | 301 | 2009 | 0 | 1889 | 1944 | 367 | 1971 | 519 | 0 | 0 | 0 | 1810 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lifeways | 0 | 0 | 0 | 552 | 544 | 0 | 379 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 226 | 0 | 0 | 0 | 0 | 0 | 0 |
| LISA | 0 | 0 | 0 | 2409 | 2409 | 0 | 2346 | 0 | 2181 | 2185 | 0 | 2188 | 50 | 1429 | 1705 | 0 | 1756 | 111 | 1383 | 1661 | 0 | 1729 | 934 |
| LRC | 15 | 0 | 0 | 3671 | 3596 | 0 | 4937 | 0 | 4450 | 4381 | 0 | 4402 | 0 | 3244 | 3224 | 0 | 3635 | 499 | 0 | 0 | 0 | 659 | 0 |
| LucKi | 0 | 0 | 0 | 773 | 807 | 0 | 813 | 0 | 0 | 0 | 0 | 333 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PIAMA | 0 | 0 | 0 | 3439 | 3436 | 0 | 3506 | 0 | 3227 | 3229 | 0 | 3307 | 1055 | 2626 | 2629 | 0 | 2642 | 1292 | 2082 | 1995 | 0 | 1874 | 721 |
| SEATON | 0 | 0 | 0 | 0 | 0 | 0 | 199 | 0 | 0 | 0 | 0 | 0 | 0 | 212 | 0 | 0 | 206 | 147 | 0 | 0 | 0 | 156 | 126 |
| STEPS Study | 145 | 101 | 0 | 696 | 508 | 0 | 690 | 0 | 636 | 151 | 0 | 598 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SWS | 0 | 0 | 0 | 2542 | 2547 | 537 | 2547 | 0 | 0 | 0 | 0 | 1962 | 921 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| WHISTLER | 0 | 0 | 0 | 599 | 0 | 0 | 580 | 579 | 46 | 0 | 0 | 15 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total cohorts | 5 | 5 | 0 | 19 | 19 | 3 | 17 | 4 | 14 | 14 | 3 | 23 | 12 | 9 | 8 | 1 | 13 | 7 | 5 | 5 | 0 | 8 | 6 |
| Total participants | 69 411 | 3725 | 0 | 40 302 | 38 778 | 1074 | 37 495 | 2154 | 84 178 | 84 451 | 839 | 96 527 | 8068 | 59 794 | 60 497 | 434 | 102 719 | 7653 | 8950 | 9849 | 0 | 11 187 | 5834 |
Overview of data availability of participating cohorts. Numbers of observations for the different variables and age groups.
PA, physical activity; Sed, sedentary behaviour; Acc, accelerometry data; LF, lung function
PA as measured by accelerometry was available in four cohorts in 1905 children in total. Cohort-specific protocols with information on the type of activity monitor used, and intensity level cut-off values is presented in online supplemental appendix table A. Accelerometry data that were requested from the cohorts were mean activity counts per minute (cpm) per day, time spent in different intensity levels (sedentary, moderate to vigorous PA (MVPA)) and mean wear time per day. In general, children wore the activity monitor all day, also during school time.
Asthma and lung function
Asthma was measured using parent-completed ISAAC (International Study of Asthma and Allergies in Childhood) questionnaires in all cohorts (136 067 children).25 We requested different asthma definitions: parent-reported physician-diagnosed asthma, ISAAC-based current asthma18 and MeDALL (Mechanisms of the Development of Allergy) based current asthma.26 ISAAC-based current asthma was defined as presence of (1) physician-diagnosed asthma and (2) dyspnoea or wheeze in last 12 months, or (3) regular use of asthma medication in the last 12 months. MeDALL-based definition of current asthma was constructed requiring the presence of two out of three criteria (1) physician-diagnosed asthma, (2) wheeze in the last 12 months, (3) use of asthma medication in the last 12 months). Not all cohorts provided physician-diagnosed asthma, in that case it was replaced by asthma ever in order to complete the current asthma definitions. A detailed overview of information on asthma questions of each individual cohort is presented in online supplemental appendix table B. 25 cohorts provided asthma data at the age of 6–18 years (n=125 250 children), from which 24 cohorts provided physician-diagnosed asthma (n=95 122), 22 cohorts (n=117 143) had ISAAC-based current asthma definition and 21 cohorts had MeDALL-based current asthma definition (n=90 576). It has to be noted that these numbers do not add up because most cohorts provided more than one definition. The primary outcome was current asthma; a child was defined as a current asthma case if it had either physician-diagnosed asthma or met the ISAAC or MeDALL-based definition. Separate analyses were performed with ISAAC and MeDALL-based definition as outcome.
Lung function was measured by spirometry in seventeen cohorts, totalling to 19 314 individual participants. The spirometry was performed according to American Thoracic Society/European Respiratory Society guidelines.27 28 Measures of interest were forced expiratory volume in 1 s (FEV1) and FEV1/forced vital capacity (FVC) ratio. All lung function results were converted into sex-adjusted, age-adjusted and height-adjusted z-scores based on the Global Lung Initiative-2012 reference values.29
Statistical analysis
Data were analysed by using SPSS V.23.0 for Windows (SPSS). The main analysis consisted of PA and sedentary behaviour in hours/day at ages 0–2 years and 3–5 years with current asthma at age 6–18 years as outcome. Secondary analyses were performed with PA and sedentary behaviour, categorised in tertiles and by using accelerometry data, combined with current asthma at age 6–18 years as outcome. Age-specific analyses for PA and sedentary behaviour in each age group (ie, 0–2 years, 3–5 years, 6–8 years, 9–14 years) and asthma and lung function in the consecutive age group were performed in order to gain more insight into age dependent associations. In cohorts that had information available on wheeze and/or asthma at age 3–5 years, we were able to perform additional analyses excluding children with wheeze or asthma present at the time of exposure measurement, in order to reduce the risk of reverse causation or protopathic bias.
First, we performed cohort-specific regression analyses: logistic regression analysis was used for evaluating the associations of PA, sedentary behaviour and accelerometry with current asthma. Linear regression analysis was used for the associations of PA, sedentary behaviour and accelerometry with lung function z-scores. Cohort-specific association estimates were pooled using random effects meta-analysis in Review Manager (RevMan, V.5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Heterogeneity among studies was assessed using the χ2 test and Higgins I2 test.30 We excluded each separate cohort one by one to examine the influence of any particular cohort on the results. Second, we performed pooled analyses using individual participant data, using generalised linear and logistic mixed models with a random intercept for cohort. When there were too few cohorts to make a valid estimation of the variance, we used fixed effects models with cohort as a covariable. Usually, this occurred when only two or three cohorts had data availability for a specific age group.
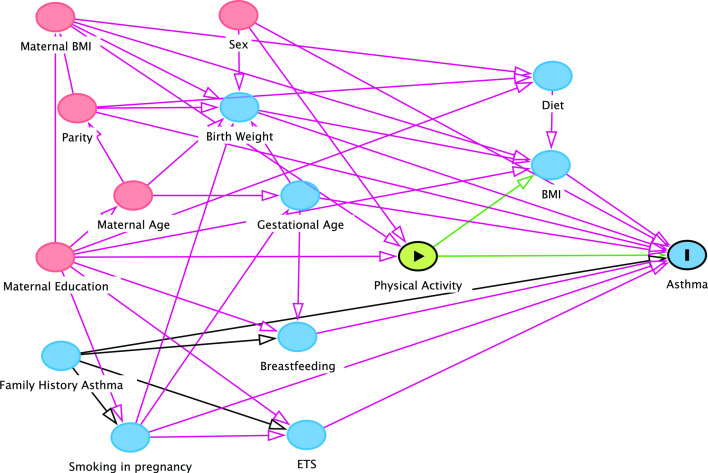
We considered the following potential confounders a priori: sex, gestational age at birth, birth weight, maternal smoking during pregnancy, environmental tobacco smoke, highest maternal education level, maternal age, maternal body mass index (BMI), breast feeding, parity, family history of asthma and family history of atopy. These variables were collected in cohort-specific parent-based questionnaires. Some cohorts measured height and weight of the child in order to calculate BMI, others used parent reported height and weight. A child’s BMI in each age group was converted into WHO z-scores adjusted for age and sex.31 We used a directed acyclic graph (DAG) approach in order to select the expected most important set of covariables for adjustment in multivariable models (figure 1). The graph was constructed using DAGitty V.2.3.32 We included the minimal sufficient adjustment set for estimating the total effect of PA on asthma as covariables in the multivariable analyses: maternal BMI, maternal education and sex. We assumed this confounder set to be the same for the other analyses (ie, sedentary behaviour and lung function). All analyses using accelerometry data were additionally adjusted for wear time. The child’s BMI was considered to have a potential interaction with PA and was, therefore, not adjusted for in the main analyses. Additional analyses were performed by testing for interaction between PA and BMI (z-scores as continuous variable) with and without adjustment for the other covariables. We reported such interactions if the interaction term was statistically significant (Wald test p<0.05).
Figure 1. Directed Acyclic Graph (DAG) of possible covariables on the association between physical activity and asthma. Minimal dataset was identified as sex, maternal education, and maternal BMI. BMI, body mass index. ETS, environmental tobacco smoke.
Results
Participant characteristics
The data available on exposure and outcome for each cohort are shown in table 1. At age 3–5 years, most cohorts had data available on PA and/or sedentary behaviour (ie, 19 cohorts). All cohorts except one (ie, EDEN) had data available for the age of 6 years and older. Characteristics of the study population are shown in online supplemental appendix table C.
PA and sedentary behaviour
Children were reported to be physically active for an average of 2.1 hours per day (all age groups combined), with children being the most active at age 6–8 years (mean 2.7 hours per day) and least active at age 9–14 years (mean 0.9 hours per day). Children engaged in sedentary behaviour for 2.7 hours per day on average over all age groups. At age 9–14 years sedentary behaviour peaked (mean 4.3 hours per day), whereas children aged 0–2 years were reported to spend the least amount of time engaging in sedentary behaviour (ie, screen time) (0.4 hours per day).
Accelerometry data showed an average mean count per minute per day of 400 cpm, varying from 323 at age 3–5 years to 606 at age 6–8 years. Large differences were observed between the different cohorts, depending on which type of accelerometer was used. Children spent on average 1.6 hours per day in MVPA, with children aged 9–14 years being the least active (1.4 hours in MVPA daily) compared with 3–5 year olds being the most active (1.8 hours in MVPA daily). The range of measured sedentary activity was very broad among the different cohorts (5.2–14.0 hours at age 3–5 years) due to differences in wear time: some cohorts included sleeping hours as wear time, while others limited the measuring time to waking hours.
Asthma
In total, 11.3% (n=14 112) of the children had current asthma at any age between 6 ando 18 years, ranging from 6.2% in G21 to 29.2% in LRC. When using parent-reported physician-diagnosed asthma only, 11.9% (n=11 349; range 3.8%–27.3%) of the children were defined as having asthma, compared with 7.4% (n=8633; range 1.7%–21.8%) according to the ISAAC-based current asthma definition and 7.9% (n=7155; range 2.5%–21.6%) according to the MeDALL-based current asthma definition.
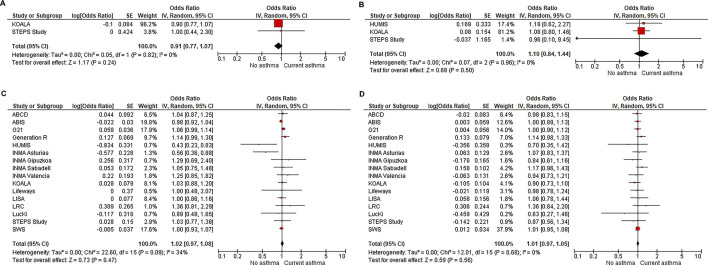
We found no association between PA at ages 0–2 years or 3–5 years and the presence of asthma at age 6–18 years. Meta-analysis of cohort-specific association estimates showed no association between PA in hours/day at age 3–5 years and asthma at age 6–18 years (adjusted OR 1.02, 95% CI 0.97 to 1.08) (figure 2). The pooled analysis of the individual participant data showed comparable results: adjusted OR 1.01, 95% CI 0.98 to 1.04 (table 2). When excluding wheeze and asthma at baseline (in a subgroup), no association was found either (adjusted OR 1.00, 95% CI 0.95 to 1.04) (online supplemental appendix table D). Also, analysing each asthma definition (ISAAC-based and MeDALL-based current asthma definition) separately did not reveal an association between PA and asthma (online supplemental appendix tables E,F), neither for PA measured by questionnaires nor for accelerometry. When PA was categorised in tertiles, only the youngest age group (0–2 years) showed a possible association between PA and lower asthma incidence: PA in the highest two tertiles at ages 0–2 years was associated with a lower asthma incidence at age 6–18 years compared with PA in the lowest tertile (adjusted OR highest tertile 0.80, 95% CI 0.68 to 0.95) (online supplemental appendix tables G,H). The result was driven by data of one cohort (DNBC) using the question: ‘Do you think he/she is more or less active than kids the same age?’. When this cohort was excluded, no association between PA in tertiles and subsequent asthma was found. The other two cohorts that had questionnaire-based information on PA at ages 0–2 years measured PA by the amount of time the child spent playing outside. No accelerometry data were available at this age.
Figure 2. Overview of meta-analyses of per-cohort longitudinal analyses on questionnaire derived physical activity and sedentary behaviour at ages 0–2 and 3–5 years and current asthma at age 6–18 years. (A) Exposure: physical activity in hours/day at ages 0–2 years—outcome: current asthma at age 6–18 years. (B) Exposure: sedentary behaviour in hours/day at ages 0–2 years—outcome: current asthma at age 6–18 years. (C) Exposure: physical activity in hours/day at age 3–5 years—outcome: current asthma at age 6–18 years. (D) Exposure: sedentary behaviour in hours/day at age 3–5 years—outcome: current asthma at age 6–18 years. Current asthma is defined as physician-diagnosed asthma, ISAAC-based current asthma definition or MeDALL-based current asthma definition. Per-cohort multivariable logistic regression using a random effects model with minimum data set as confounders (sex, maternal education level and maternal BMI for all cohorts except LRC which did not have information on maternal BMI, therefore, was corrected for sex and maternal education level). BMI, body mass index; ISAAC, International Study of Asthma and Allergies in Childhood.
Table 2. Longitudinal analyses on physical activity (PA), sedentary behaviour and current asthma between age 6 and 18 years.
| n (n asthma cases) | n cohorts | aOR (95% CI)* | ||
| Questionnaire based | ||||
| PA (hours/day) age 0–2 years | 2024 (282) | 2 | a | 0.91 (0.77 to 1.07) |
| Age 3–5 years | 21 927 (2204) | 16 | b | 1.01 (0.98 to 1.04) |
| Sedentary behaviour (hours/day) age 0–2 years | 2380 (329) | 3 | c | 1.05 (0.80 to 1.37) |
| Age 3–5 years | 21 643 (2180) | 15 | d | 1.03 (0.99 to 1.07) |
| Accelerometry | ||||
| Total activity (counts/min) age 3–5 years | 775 (131) | 2 | e | 1.00 (1.00 to 1.00) |
| Sedentary activity age 3–5 years | 775 (131) | 2 | e | 1.00 (0.86 to 1.16) |
| MVPA | ||||
| Age 3–5 years | 775 (131) | 2 | e | 0.99 (0.66 to 1.50) |
GeneralizedGeneralised logistic mixed models on questionnaire -based (PA) in hours per day, sedentary behaviour in hours per day, and accelerometry data at ages 0–2 years and 3–5 years; and current asthma at age 6–18 years.
Multivariable analyses corrected for sex, maternal education level, maternal BMI.
Included cohorts: (a) KOALA, STEPS Study, (b) ABCD, ABIS, G21, Generation R, HUMIS, INMA Asturias, INMA Gipuzkoa, INMA Sabadell, INMA Valencia, KOALA, Lifeways, LISA, LucKi, STEPS Study, SWS, Whistler, (c) HUMIS, KOALA, STEPS Study, (d) ABCD, ABIS, G21, Generation R, HUMIS, INMA Asturias, INMA Gipuzkoa, INMA Sabadell, INMA Valencia, KOALA, Lifeways, LISA, LucKi, STEPS Study, SWS. : KOALA, SWS.
(aORs) indicate the increase in odds of current asthma between age 6 and 18 years for each hour per day of parent reported PA or sedentary behaviour in the age periods between age 0 and 2 or 3 and 5 years; and time in sedentary activity or moderate to vigorous (MVPA) recorded by accelerometry between age 3 and 5 years. Current asthma is defined as physician -diagnosed asthma, ISAAC -based current asthma definition or MeDALL -based current asthma definition.
aORadjusted ORBMIbody mass indexISAACInternational Study of Asthma and Allergies in ChildhoodMeDALLMechanisms of the Development of AllergyMVPAmoderate to vigorous PA
Sedentary behaviour was not associated with the presence of asthma at subsequent follow-ups age 6–18 years, regardless of the PA method and asthma definition that was used. Accelerometry data for sedentary behaviour were also analysed for each cohort separately, due to large differences in wear time. In none of the separate analyses, nor the meta-analysis, an association between time spent in sedentary level and subsequent asthma was seen (online supplemental appendix figure A).
Age-specific analyses of PA and sedentary behaviour and asthma in the consecutive age group showed no associations at any age in the multivariable analyses (online supplemental appendix tables I–L), except again for PA in tertiles at ages 0–2 years and asthma at age 6–8.
The interaction term child’s BMI×PA was tested in both univariable and multivariable models but was not statistically significant at any age (online supplemental appendix tables M,N).
Lung function
No associations between questionnaire-based PA and lung function in the age-specific analyses were observed at any age (online supplemental appendix tables O,P). Children who spent more time in MVPA at age 3–5 years (as measured by accelerometry) had a higher FEV1 at age 6–8 years (B 0.27 SD, 95% CI 0.07 to 0.46). This means that every 1 hour per day more engaging in MVPA level at age 3–5 years results in a 0.27 SD (reported as z-score) higher FEV1 at age 6–8 years. This association disappeared when we excluded the children with wheeze or asthma at baseline (online supplemental appendix tables Q,P).
For questionnaire-based sedentary behaviour, children who engaged more time in sedentary behaviour at age 6–8 years had a slightly higher FEV1 at age 9–14 years (B 0.03 SD, 95% CI 0.00 to 0.06 for every additional hour of sedentary behaviour per day). Children aged 9–14 years old who spent more time in sedentary behaviour had slightly higher FEV1/FVC at age 15–18 years (B 0.04 SD, 95% CI 0.00 to 0.07). Children who displayed more time in sedentary behaviour (as measured by accelerometry) at age 3–5 years had a lower FEV1 at age 6–8 years (B –0.13 SD, 95% CI –0.20 to –0.06). This association persisted after excluding children with wheeze or asthma at baseline. At all other ages no association between PA or sedentary behaviour and lung function was observed.
Discussion
Overall, in this large collaborative study, we found no evidence that PA or sedentary behaviour during early childhood was associated with the presence of asthma in later childhood. Both PA measured by questionnaire and by accelerometry showed no association. This is in line with more recent studies that have shown that PA is not associated with subsequent asthma in childhood.17 18 Cassim et al performed a bidirectional longitudinal analysis on PA and childhood asthma and found no association in any direction.33 Recently, Russell et al described the association between PA and asthma incidence over 10 years in a multicentre study and found no benefit from vigorous PA in reducing the risk of asthma development in adults.34 Garcia-Aymerich et al performed hypothetical interventions on BMI and PA in 76 470 asthma-free women and found no effect of PA intervention on new-onset asthma.35
Unfortunately, we were not able to collect reliable information on PA in the youngest age group (under 2 years) to draw conclusions for this age. Habitual PA in infants and toddlers differs from PA at older ages, and no validated questionnaires on PA at this young age exist. Earlier studies on this subject have stressed the importance of using accelerometry for measuring PA in infants and toddlers.36 37 However, a recent systematic review and meta-analysis on accelerometry in infants and toddlers showed that accelerometry measurements in infants still are inconclusive due to a lack of existing validated cut-points at this age. In toddlers (ie, in general 1–3 years) validated cut-points are available for some accelerometer devices (eg, Actigraph) but consistency and reliability remains problematic.38
We found no clear associations between PA and lung function at any age (0–18 years). The analyses of sedentary behaviour and lung function measured a few years later showed a few associations: questionnaire-based sedentary behaviour at age 6–8 years was associated with a marginally higher FEV1 at age 9–14 years, whereas accelerometry measured sedentary behaviour at age 3–5 years was associated with a slightly lower FEV1 at age 6–8. FEV1/FVC was lower at age 15–18 years when children had spent more time in sedentary behaviour at age 9–14 years. All other analyses on sedentary behaviour and lung function showed no associations.
In the literature, we only found one study that focused on the longitudinal association between sedentary behaviour and lung function in childhood: da Silva et al39 found that adolescents who spent less time in sedentary behaviour at ages 11–18 years had higher FVC at age 18 years. Earlier studies on PA and lung function are inconsistent: cross-sectionally, no association between PA and lung function in adolescents was found.40 In contrast to studies in adults, where a weak positive association between higher PA level and FEV1 was found.41 Longitudinally, in adolescents and young adults, aerobic fitness was positively associated with FEV1 and FVC but not with FEV1/FVC.42 It is possible that our findings are the result of chance finding because of multiple testing. The clinical relevance of these small differences is not known either.
Obesity was a priori considered to have a possible interaction with PA in relation to asthma. However, models including BMI×PA as interaction term did not show any modifying effect of BMI on the association between PA and asthma. Bédard et al investigated the role of PA in the obesity-asthma link in adult women and found an independent association between obesity and asthma but no independent causal effect of PA on asthma.16
The most important strength of this study is that it is a large collaboration of 26 European birth cohorts, which all delivered individual-level information on PA, sedentary behaviour and asthma from 0 to 18 years. By including children from different geographical areas residual confounding was indirectly taken into account. By virtue of the longitudinal design, with information on several age groups, we were able to reduce the risk of reverse causality. We evaluated protopathic bias by excluding children with asthma and wheeze in the 12 months preceding the exposure date. In this asthma-and-wheeze free population, there was no association between PA levels or sedentary behaviour and new-onset asthma at ages 6–8 years. Unfortunately, we were not able to perform repeated measures analysis as most cohorts had only one or two measurements of PA, all at different ages.
An important limitation of this study is the heterogeneity in data collection between the different cohorts. Especially the data on PA and sedentary behaviour differed across the cohorts. For example, some cohorts had more detailed questionnaires on PA than others, some included questions on school activities while others only included activities outside of school hours. To harmonise the data, we performed additional analyses after conversion into tertiles. These showed comparable results. Accelerometry data also showed large differences across the cohorts due to different methodologies, especially for the time spent in sedentary activity level. However, the separate cohort-specific analyses displayed comparable results and meta-analysis showed little statistical heterogeneity. Asthma outcome data were less heterogeneous: all cohorts used ISAAC core questionnaires and/or MeDALL-based asthma definition and separate analyses on these different asthma outcomes showed comparable results. Recruitment bias could also be an issue: most birth cohorts consist of relatively highly educated parents, which is a selection of the real population. Low socioeconomic status is a known risk factor for severe asthma and is possibly under-represented in this study.43 44
This study focuses on the association between PA and asthma development later in childhood. It is important to notice that this study did not focus on asthma severity, which can still be related to PA and sedentary behaviour, for example, due to symptoms of breathlessness.
In conclusion, we found no indication of a relation between PA and sedentary behaviour in early childhood and asthma in later childhood. There is very sparse information about the PA levels in the youngest age group (under 2 years) and subsequent asthma so no conclusion can be drawn for this age. The results of the effects of PA and sedentary behaviour on lung function were inconsistent.
We thank the parents and children who participated in this study for their efforts. We thank Bjorn Winkens for his help in the data analyses.
Overview of the included cohorts:
supplementary material
The funders had no role in the design and conduct of the cohort study, nor in the present work should it be statistical analysis, manuscript preparation and decision to submit it for publication. GlaxoSmithKline had no role in study design, in the collection, analysis and interpretation of data, in the writing of the report and in the decision to submit the report for publication.
Footnotes
Funding: The authors received no specific funding for this article. Funding information per cohort: ABCD: The ABCD study has been supported by grants from The Netherlands Organisation for Health Research and Development (ZonMW) and The Netherlands Heart Foundation. ABIS: Special thanks to the participating families in the ABIS study, and all staff at Obstetric departments and Well-Baby Clinics. ABIS has been supported by Swedish Research Council (K2005-72X-11242-11A and K2008-69X-20826-01-4) and the Swedish Child Diabetes Foundation (Barndiabetesfonden), JDRF Wallenberg Foundation (K 98-99D-12813-01A), Medical Research Council of Southeast Sweden (FORSS), and the Swedish Council for Working Life and Social Research (FAS2004-1775) and Östgöta Brandstodsbolag. BAMSE: This BAMSE birth cohort was supported by grants from the Swedish Research Council, the Swedish Research Council for Health, Working Life and Welfare, Formas, the Swedish Heart-Lung Foundation, the Swedish Asthma and Allergy Research Foundation, Region Stockholm (ALF project, and for cohort and database maintenance), and the European Research Council (TRIBAL, grant agreement 757919). CHOP: The CHOP study reported herein have been carried out with partial financial support from the Commission of the European Community, specific RTD Programme 'Quality of Life and Management of Living Resources', within the European Union's Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition under grant agreement no. 289346, partial financial support from Polish Ministry of Science and Higher Education (2571/7.PR/2012/2), the EU H2020 project PHC-2014-DynaHealth under grant no. 633595 and the European Research Council Advanced Grant META-GROWTH (ERC-2012-AdG-no.322605). COPSAC2000: All funding received by COPSAC is listed on www.copsac.com. The Lundbeck Foundation (Grant no R16-A1694); The Ministry of Health (Grant no 903516); Danish Council for Strategic Research (Grant no 0603-00280B) and The Capital Region Research Foundation have provided core support to the COPSAC research center. DNBC: The Danish National Birth Cohort was established with a significant grant from the Danish National Research Foundation. Additional support was obtained from the Danish Regional Committees, the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, the Health Foundation and other minor grants. The DNBC Biobank has been supported by the Novo Nordisk Foundation and the Lundbeck Foundation. EDEN: EU FP7 Framework MedAll project, National Institute for Research in Public Health (IRESP TGIR Cohorte Santé 2008 Program); National Agency for Research (ANR non-thematic programme); French Speaking Association for the Study of Diabetes and Metabolism (Alfediam); Mutuelle Générale de l’Éducation Nationale; Nestlé; French National Institute for Health Education (INPES); Paris‐Sud University; French National Istitute for Population Health Surveillance (InVS); French Agency for Environment Security (AFFSET); French Ministry of Health Perinatal Program; Inserm Nutrition Research Program; Institut Fédératif de Recherche and Cohort Program; French Ministry of Research; EURIP and FIRE doctoral school–Programme Bettencourt; Fondation pour la Recherche Médicale (FRM). G21: Generation XXI was supported by the European Regional Development Fund (ERDF) through the Operational Programme Competitiveness and Internationalisation and national funding from the Foundation for Science and Technology (FCT), Portuguese Ministry of Science, Technology and Higher Education under the project 'HIneC: When do health inequalities start? Understanding the impact of childhood social adversity on health trajectories from birth to early adolescence' (POCI-01-0145-FEDER-029567; Reference PTDC/SAU-PUB/29567/2017). It is also supported by the Unidade de Investigação em Epidemiologia–Instituto de Saúde Pública da Universidade do Porto (EPIUnit) (UIDB/04750/2020), Administração Regional de Saúde Norte (Regional Department of Ministry of Health) and Fundação Calouste Gulbenkian; PhD Grant SFRH/BD/108742/2015 (to SS) co-funded by FCT and the Human Capital Operational Programme (POCH/FSE Program); ACS is founded by a FCT Investigator contracts IF/01060/2015. Generation R: The Generation R Study is made possible by financial support from the Erasmus Medical Centre, Rotterdam, the Erasmus University Rotterdam and The Netherlands Organization for Health Research and Development. The project received funding for projects from the European Union's Horizon 2020 research and innovation programme (LIFECYCLE, grant agreement No 733206, 2016; EUCAN-Connect grant agreement No 824989; ATHLETE, grant agreement No 874583). LD received funding from the European Union's Horizon 2020 cofunded programme ERA-Net on Biomarkers for Nutrition and Health (ERA HDHL) (ALPHABET project (no 696295; 2017), ZonMW The Netherlands (no 529051014; 2017)). GINIplus: The GINIplus study was mainly supported for the first 3 years of the Federal Ministry for Education, Science, Research and Technology (interventional arm) and Helmholtz Zentrum Munich (former GSF) (observational arm). The 4 years, 6 years, 10 years and 15 years follow-up examinations of the GINIplus study were covered from the respective budgets of the five study centres (Helmholtz Zentrum Munich (former GSF), Research Institute at Marien-Hospital Wesel, LMU Munich, TU Munich and from 6 years onwards also from IUF - Leibniz Research-Institute for Environmental Medicine at the University of Düsseldorf) and a grant from the Federal Ministry for Environment (IUF Düsseldorf, FKZ 20462296). Further, the 15-year follow-up examination of the GINIplus study was supported by the Commission of the European Communities, the 7th Framework Program: MeDALL project, and as well by the companies Mead Johnson and Nestlé. The authors thank all the families for their participation in the GINIplus study. Furthermore, we thank all members of the GINIplus Study Group for their excellent work. The GINIplus Study group consists of the following: Institute of Epidemiology, Helmholtz Zentrum München, German Research Center for Environmental Health, Neuherberg (Heinrich J, Brüske I, Schulz H, Flexeder C, Zeller C, Standl M, Schnappinger M, Ferland M, Thiering E, Tiesler C); Department of Pediatrics, Marien-Hospital, Wesel (Berdel D, von Berg A); Ludwig-Maximilians-University of Munich, Dr von Hauner Children’s Hospital (Koletzko S); Child and Adolescent Medicine, University Hospital rechts der Isar of the Technical University Munich (Bauer CP, Hoffmann U); IUF- Environmental Health Research Institute, Düsseldorf (Schikowski T, Link E, Klümper C, Krämer U, Sugiri D). HUMIS: HUMIS is supported by the Research Council of Norway (NevroNor, grant number 226402). INMA Asturias: This study was funded by grants from, FIS-FEDER: PI04/2018, PI09/02311, PI13/02429, PI18/00909; Obra Social Cajastur/Fundación Liberbank, and Universidad de Oviedo. We thank Fundación NOE Alimerka. INMA Gipuzkoa: This study was funded by grants from Instituto de Salud Carlos III (FIS-PI06/0867, FIS-PI09/00090, FIS-PI13/02187 include FEDER funds), CIBERESP, Department of Health of the Basque Government (2005111093, 2009111069, 2013111089 and 2015111065), and the Provincial Government of Gipuzkoa (DFG06/002, DFG08/001 and DFG15/221) and annual agreements with the municipalities of the study area (Zumarraga, Urretxu, Legazpi, Azkoitia y Azpeitia y Beasain). INMA Menorca: This study was funded by grants from Instituto de Salud Carlos III (Red INMA G03/176; CB06/02/0041; 97/0588; 00/0021-2; PI061756; PS0901958; PI14/00677 incl. FEDER funds), CIBERESP, Beca de la IV convocatoria de Ayudas a la Investigación en Enfermedades Neurodegenerativas de La Caixa, and EC Contract No. QLK4-CT-2000-00263. INMA Sabadell: This study was funded by grants from Instituto de Salud Carlos III (Red INMA G03/176; CB06/02/0041; PI041436; PI081151 incl. FEDER funds; CPII/00018), CIBERESP, Generalitat de Catalunya-CIRIT 1999SGR 00241, Generalitat de Catalunya-AGAUR 2009 SGR 501, Fundació La marató de TV3 (090430), EU Commission (261357). ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya. INMA Valencia: This study was funded by grants from UE (FP7-ENV-2011 cod 282957 and HEALTH.2010.2.4.5-1), Spain: ISCIII (Red INMA G03/176, CB06/02/0041; FIS-FEDER: PI03/1615, PI04/1509, PI04/1112, PI04/1931, PI05/1079, PI05/1052, PI06/1213, PI07/0314, PI09/02647, PI11/01007, PI11/02591, PI11/02038, PI13/1944, PI13/2032, PI14/00891, PI14/01687, PI16/1288, PI17/00663, and 19/1338; Miguel Servet-FEDER CP11/00178, CP15/00025 and CPII16/00051), Generalitat Valenciana: FISABIO (UGP 15-230, UGP-15-244, UGP-15-249, and AICO 2020/285), and Alicia Koplowitz Foundation 2017. KOALA: The KOALA cohort study was cofinanced by Friesland Foods (now FrieslandCampina), Netherlands Asthma Foundation (grant numbers 3.2.07.022 and 3.2.03.48) and Netherlands Heart Foundation (grant number 2014 T037), the Netherlands Organization for Health Research and Development (ZonMw Prevention Program number 1.210-00-090). The funding sources had no role in the study design and the collection, analysis and interpretation of data and the writing of the article and the decision to submit it for publication. Lifeways: The Lifeways study has been funded by the Health Research Board, Ireland, and the Irish Department of Health and Children’s Health Promotion Policy Unit. LISA: The LISA study was mainly supported by grants from the Federal Ministry for Education, Science, Research and Technology and in addition from Helmholtz Zentrum Munich (former GSF), Helmholtz Centre for Environmental Research—UFZ, Leipzig, Research Institute at Marien-Hospital Wesel, Pediatric Practice, Bad Honnef for the first 2 years. The 4 years, 6 years, 10 years and 15 years follow-up examinations of the LISA study were covered from the respective budgets of the involved partners (Helmholtz Zentrum Munich (former GSF), Helmholtz Centre for Environmental Research—UFZ, Leipzig, Research Institute at Marien-Hospital Wesel, Pediatric Practice, Bad Honnef, IUF—Leibniz-Research Institute for Environmental Medicine at the University of Düsseldorf) and in addition by a grant from the Federal Ministry for Environment (IUF Düsseldorf, FKZ 20462296). Further, the 15-year follow-up examination of the LISA study was supported by the Commission of the European Communities, the 7th Framework Program: MeDALL project. The authors thank all the families for their participation in the LISA study. Furthermore, we thank all members of the LISA Study Group for their excellent work. The LISA Study group consists of the following: Helmholtz Zentrum München, German Research Center for Environmental Health, Institute of Epidemiology, Munich (Heinrich J, Schnappinger M, Brüske I, Ferland M, Schulz H, Zeller C, Standl M, Thiering E, Tiesler C, Flexeder C); Department of Pediatrics, Municipal Hospital 'St. Georg', Leipzig (Borte M, Diez U, Dorn C, Braun E); Marien Hospital Wesel, Department of Pediatrics, Wesel (von Berg A, Berdel D, Stiers G, Maas B); Pediatric Practice, Bad Honnef (Schaaf B); Helmholtz Centre of Environmental Research—UFZ, Department of Environmental Immunology/Core Facility Studies, Leipzig (Lehmann I, Bauer M, Röder S, Schilde M, Nowak M, Herberth G, Müller J); Technical University Munich, Department of Pediatrics, Munich (Hoffmann U, Paschke M, Marra S); Clinical Research Group Molecular Dermatology, Department of Dermatology and Allergy, Technische Universität München (TUM), Munich (Ollert M, J. Grosch). LRC: All phases of this study were supported by the Swiss National Science Foundation (grants: SNF 320030_182628, 32003B_162820, PDFMP3 137033, 32003B_162820, 32003B_144068, PZ00P3_147987) and Asthma UK 07/048. LUCKI: This study was supported by Maastricht University and the Public Health Service South Limburg. PIAMA: The Prevention and Incidence of Asthma and Mite Allergy Study has been funded by grants from the Netherlands Organization for Health Research and Development; the Netherlands Organization for Scientific Research; the Lung Foundation of the Netherlands; the Netherlands Ministry of Planning, Housing and the Environment; the Netherlands Ministry of Health, Welfare and Sport; and the National Institute for Public Health and the Environment. SEATON: Medical Research Council, Grant number: 80219, MR/K001035/1; Asthma UK, Grant numbers: 00/011, 02/017. STEPS Study: The Academy of Finland (grant no. 123571 and 121659); the Juho Vainio Foundation; the Foundation for Pediatric Research; the Finnish Medical Foundation. SWS: The SWS was supported by grants from the Medical Research Council (MC_UU_12011/4), Dunhill Medical Trust, British Heart Foundation, Food Standards Agency (contract no N05071), British Lung Foundation. National Institute for Health Research Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton National Health Service Foundation Trust, the European Union’s Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition (grant 289346) and European Union’s Horizon 2020 research and innovation programme under grant agreement No 733206 (LifeCycle). WHISTLER: The authors (from the WHISTLER birth cohort) received no specific funding for this article. The WHISTLER birth cohort was supported with a grant from the Netherlands Organization for Health Research and Development (grant no. 2001-1-1322) and by an unrestricted grant from GlaxoSmithKline Netherlands.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: All parents gave written informed consent. Ethical approval was obtained from the local authorised institutional review boards of the individual cohorts: ABCD: The Central Committee on Research Involving Human Subjects in the Netherlands, the medical ethics review committees of the participating hospitals and the Registration of the Municipality of Amsterdam approved the protocol of the ABCD study and written informed consent of all the participants was obtained. ABIS: The ABIS project was approved by the Research Ethics Committees of the Faculty of Health Science at the University of Linköping, Linköping, Sweden and the Medical Faculty at the University of Lund, Lund, Sweden (Dnr 99227, Dnr 99321). BAMSE: The BAMSE study and subsequent follow-ups were approved by the Regional Ethical Review Board, Karolinska Institutet, Stockholm, Sweden, and all parents provided informed consent for data collection and analysis. CHOP:Belgium: Comitè d’Ethique Medicale de Centre Hospitalier Chretien Liege; No. OM87Germany: Bayerische Landesärztekammer Ethik-Kommission, No. 02070Italy: Azienda Ospedaliera San Paolo Comitato Etico, No 14/2002Poland: Instytut Pomnik-Centrum Zdrowia Dziecka Komitet Etyczny, No 243/KE/2001Spain: Comité ético de investigación clínica del Hospital Universitario de Tarragona Joan XXIII, Comité ético de investigación clínica del Hospital Universitario Sant Joan de ReusCOPSAC2000: The study was conducted in accordance with the guiding principles of the Declaration of Helsinki and was approved by the Local Ethics Committee (COPSAC2000: KF 01-289/96, COPSAC2000 18 University College Hospital, Galway, Ireland; St. Vincent’s University Hospital, Dublin, Ireland; Irish College of General Practitioners; National University of Ireland, Galway, Ireland; University College Dublin, Ireland. LISA: For the LISA study, the ethical approval was given by the Bavarian Board of Physicians (12067), the Board of Physicians of Saxony (EK-BR02/13-1) and the Board of Physicians of North Rhine-Westphalia (2012446). LRC: The Leicestershire Health Authority Research Ethics Committee approved this study. LUCKI: The study was approved by the medical ethics committee of Maastricht University and Academic Hospital of Maastricht, Netherlands (MEC 09-4-058). PIAMA: Start project—Rotterdam, MEC (Medisch Ethische Commisie Erasmus Universiteit Rotterdam/Academische Ziekenhuizen Rotterdam) 132.636/1994/39, 13 June 1994 and 137.326/1994/130, 16 February 1995—Groningen, MEC (Medisch Ethische Commisie Academisch ziekenhuis Groningen) 94/08/92, 26 August 1994—Utrecht/Bilthoven, MEC-TNO (Medisch Ethische Commisie—Toegepast Natuurwetenschappelijk Onderzoek) 95/50, 28 February 1996 Age 4 years Utrecht, CCMO (Centrale Commissie Mensgebonden Onderzoek) P000777C, 25 September 2000 Age 8 years Utrecht, CCMO (Centrale Commissie Mensgebonden Onderzoek) P04.0071C, 5 August 2004 (Utrecht, METC-protocol number 04—101/K, 27 July 2004; Rotterdam, P04.0071C/MEC 2004-152, 1 July 2004; Groningen, P04.0071C/ M 4.019912, 28 June 2004) Age 12 years Utrecht, METC (Medisch Ethische ToetsingsCommissie) protocol number 07-337/K, 20 May 2008 Age 16 years Utrecht, METC (Medisch Ethische ToetsingsCommissie) protocol number 12-019/K, 25 May 2012; Amendement 1, 12 July 2012; Amendement 2, 20 September 2012; Groningen, METC (Medisch Ethische ToetsingsCommissie) protocol number 12-019/K; Amendement, 16 August 2012 Age 18 years Utrecht (Medisch Ethische Toetsingscommissie), onderzoeksvoorstel 15/170, PIAMA studie Preventie en Incidentie van Astma en Mijt Allergie, opvragen van huisarts JGZ en PRN gegevens SEATON: North of Scotland Research Ethics Committee (13/NS/0108). STEPS Study: The STEPS Study was approved by the Ethics Committee of the Hospital District of Southwest Finland (27 February 2007). SWS: The SWS received ethics approval for all waves of the cohort study from Southampton and South-West Hampshire Local Research Ethics Committee. WHISTLER: The WHISTLER birth cohort study was approved by the paediatric Medical Ethical Committee of the University Medical Center Utrecht. Parents gave informed consent for participating in the study. Participants gave informed consent to participate in the study before taking part.
Data availability free text: The cohort-specific datasets are available to interested researchers on reasonable request, provided the release is consistent with the obtained consent of the study participants of the cohort. This will not be possible for all cohorts involved. Ethical approval might be necessary to be obtained for the release and a data transfer agreement must be accepted.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Marianne Eijkemans, Email: m.eijkemans@alumni.maastrichtuniversity.nl.
Monique Mommers, Email: monique.mommers@maastrichtuniversity.nl.
Margreet W Harskamp-van Ginkel, Email: w.m.harskampvanginkel@amsterdamumc.nl.
Tanja G M Vrijkotte, Email: t.vrijkotte@amsterdamumc.nl.
Johnny Ludvigsson, Email: Johnny.Ludvigsson@liu.se.
Åshild Faresjö, Email: ashild.olsen.faresjo@liu.se.
Anna Bergström, Email: Anna.Bergstrom@ki.se.
Sandra Ekström, Email: Sandra.ekstrom@ki.se.
Veit Grote, Email: Veit.Grote@med.uni-muenchen.de.
Berthold Koletzko, Email: Berthold.Koletzko@med.uni-muenchen.de.
Klaus Bønnelykke, Email: kb@copsac.com.
Anders Ulrik Eliasen, Email: anders.eliasen@dbac.dk.
Peter Bager, Email: pbg@ssi.dk.
Mads Melbye, Email: mmelbye@stanford.edu.
Isabella Annesi-Maesano, Email: isabella.annesi-maesano@inserm.fr.
Nour Baïz, Email: nour.baiz@iplesp.upmc.fr.
Henrique Barros, Email: hbarros@med.up.pt.
Ana Cristina Santos, Email: acsantos@med.up.pt.
Liesbeth Duijts, Email: l.duijts@erasmusmc.nl.
Sara M Mensink-Bout, Email: s.mensink-bout@erasmusmc.nl.
Claudia Flexeder, Email: claudia.flexeder@helmholtz-muenchen.de.
Sibylle Koletzko, Email: Sibylle.Koletzko@med.uni-muenchen.de.
Tamara Schikowski, Email: Tamara.Schikowski@IUF-Duesseldorf.de.
Merete Åse Eggesbø, Email: Merete.Eggesbo@fhi.no.
Virissa Lenters, Email: V.C.Lenters@umcutrecht.nl.
Guillermo Fernández-Tardón, Email: gfernanta@gmail.com.
Mikel Subiza-Perez, Email: m-subiza@euskadi.eus.
Judith Garcia-Aymerich, Email: judith.garcia@isglobal.org.
Mónica López-Vicente, Email: m.lopez-vicente@erasmusmc.nl.
Jordi Sunyer, Email: jordi.sunyer@isglobal.org.
Maties Torrent, Email: maties.torrent@ssib.es.
Ferran Ballester, Email: ballester_fer@gva.es.
Cecily Kelleher, Email: cecily.kelleher@ucd.ie.
John Mehegan, Email: john.mehegan@ucd.ie.
Andrea von Berg, Email: avb.rodehorst@gmx.de.
Gunda Herberth, Email: gunda.herberth@ufz.de.
Marie Standl, Email: marie.standl@helmholtz-muenchen.de.
Claudia E Kuehni, Email: claudia.kuehni@ispm.unibe.ch.
Eva S L Pedersen, Email: eva.pedersen@ispm.unibe.ch.
Maria Jansen, Email: maria.jansen@ggdzl.nl.
Ulrike Gehring, Email: U.Gehring@uu.nl.
Jolanda M A Boer, Email: jolanda.boer@rivm.nl.
Graham Devereux, Email: graham.devereux@lstmed.ac.uk.
Steve Turner, Email: s.w.turner@abdn.ac.uk.
Ville Peltola, Email: vilpel@utu.fi.
Hanna Lagström, Email: hanna.lagstrom@utu.fi.
Hazel M Inskip, Email: hmi@mrc.soton.ac.uk.
Katharine C Pike, Email: katharine.pike@uhbw.nhs.uk.
Geertje W Dalmeijer, Email: G.W.Dalmeijer@umcutrecht.nl.
Cornelis K van der Ent, Email: K.vanderEnt@umcutrecht.nl.
Carel Thijs, Email: c.thijs@maastrichtuniversity.nl.
Data availability statement
Due to data protection reasons, the datasets generated during the current study cannot be made publicly available.
References
- 1.WHO Asthma. https://www.who.int/news-room/q-a-detail/asthma n.d. Available.
- 2.Noal RB, Menezes AMB, Macedo SEC, et al. Childhood body mass index and risk of asthma in adolescence: a systematic review. Obes Rev. 2011;12:93–104. doi: 10.1111/j.1467-789X.2010.00741.x. [DOI] [PubMed] [Google Scholar]
- 3.Cordova-Rivera L, Gibson PG, Gardiner PA, et al. A systematic review of associations of physical activity and sedentary time with asthma outcomes. J Allergy Clin Immunol Pract. 2018;6:1968–81. doi: 10.1016/j.jaip.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Strom MA, Silverberg JI. Associations of physical activity and sedentary behavior with Atopic disease in United States children. J Pediatr. 2016;174:247–53. doi: 10.1016/j.jpeds.2016.03.063. [DOI] [PubMed] [Google Scholar]
- 5.Corbo GM, Forastiere F, De Sario M, et al. Wheeze and asthma in children: associations with body mass index, sports, television viewing, and diet. Epidemiology. 2008;19:747–55. doi: 10.1097/EDE.0b013e3181776213. [DOI] [PubMed] [Google Scholar]
- 6.van ’t Hul AJ, Frouws S, van den Akker E, et al. Decreased physical activity in adults with bronchial asthma. Respir Med. 2016;114:72–7. doi: 10.1016/j.rmed.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Lam K-M, Yang Y-H, Wang L-C, et al. Physical activity in school-aged children with asthma in an urban city of Taiwan. Pediatr Neonatol. 2016;57:333–7. doi: 10.1016/j.pedneo.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Xu M, Lodge CJ, Lowe AJ, et al. Are adults with asthma less physically active? A systematic review and meta-analysis. J Asthma. 2021;58:1426–43. doi: 10.1080/02770903.2020.1810273. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes P, de Mendonça Oliveira L, Brüggemann TR, et al. Physical exercise induces Immunoregulation of TREG, M2, and pDCs in a lung allergic inflammation model. Front Immunol. 2019;10:854. doi: 10.3389/fimmu.2019.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huovinen E, Kaprio J, Koskenvuo M. Factors associated to Lifestyle and risk of adult onset asthma. Respir Med. 2003;97:273–80. doi: 10.1053/rmed.2003.1419. [DOI] [PubMed] [Google Scholar]
- 11.Lucke J, Waters B, Hockey R, et al. Trends in women’s risk factors and chronic conditions: findings from the Australian longitudinal study on women’s health. Womens Health (Lond) 2007;3:423–32. doi: 10.2217/17455057.3.4.423. [DOI] [PubMed] [Google Scholar]
- 12.Russell MA, Janson C, Real FG, et al. Physical activity and asthma: a longitudinal and multi-country study. J Asthma. 2017;54:938–45. doi: 10.1080/02770903.2017.1281293. [DOI] [PubMed] [Google Scholar]
- 13.Benet M, Varraso R, Kauffmann F, et al. The effects of regular physical activity on adult-onset asthma incidence in women. Respir Med. 2011;105:1104–7. doi: 10.1016/j.rmed.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Beckett WS, Jacobs DR, Yu X, et al. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med. 2001;164:2045–50. doi: 10.1164/ajrccm.164.11.2004235. [DOI] [PubMed] [Google Scholar]
- 15.Brumpton BM, Langhammer A, Ferreira MAR, et al. Physical activity and incident asthma in adults: the HUNT study, Norway. BMJ Open. 2016;6:e013856. doi: 10.1136/bmjopen-2016-013856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bédard A, Serra I, Dumas O, et al. Time-dependent associations between body composition, physical activity, and current asthma in women: a marginal structural modeling analysis. Am J Epidemiol. 2017;186:21–8. doi: 10.1093/aje/kwx038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassim R, Dharmage SC, Koplin JJ, et al. Does physical activity strengthen lungs and protect against asthma in childhood? a systematic review. Pediatr Allergy Immunol. 2019;30:739–51. doi: 10.1111/pai.13105. [DOI] [PubMed] [Google Scholar]
- 18.Eijkemans M, Mommers M, Remmers T, et al. Physical activity and asthma development in childhood: prospective birth cohort study. Pediatr Pulmonol. 2020;55:76–82. doi: 10.1002/ppul.24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Ploeg HP, Hillsdon M. Is sedentary behaviour just physical inactivity by another name. Int J Behav Nutr Phys Act. 2017;14:142. doi: 10.1186/s12966-017-0601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherriff A, Maitra A, Ness AR, et al. Association of duration of television viewing in early childhood with the subsequent development of asthma. Thorax. 2009;64:321–5. doi: 10.1136/thx.2008.104406. [DOI] [PubMed] [Google Scholar]
- 21.Protudjer J, Kozyrskyj AL, McGavock JM, et al. High screen time is associated with asthma in overweight Manitoba youth. J Asthma. 2012;49:935–41. doi: 10.3109/02770903.2012.724753. [DOI] [PubMed] [Google Scholar]
- 22.Chen YC, Tu YK, Huang KC, et al. Pathway from central obesity to childhood asthma. physical fitness and sedentary time are leading factors. Am J Respir Crit Care Med. 2014;189:1194–203. doi: 10.1164/rccm.201401-0097OC. [DOI] [PubMed] [Google Scholar]
- 23.da Silva BGC, Wehrmeister FC, Quanjer PH, et al. Physical activity in early adolescence and pulmonary function gain from 15 to 18 years of age in a birth cohort in Brazil. J Phys Act Health. 2016;13:1164–73. doi: 10.1123/jpah.2016-0056. [DOI] [PubMed] [Google Scholar]
- 24.Roda C, Mahmoud O, Peralta GP, et al. Physical-activity Trajectories during childhood and lung function at 15 years: findings from the ALSPAC cohort. Int J Epidemiol. 2020;49:131–41. doi: 10.1093/ije/dyz128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beasley R. Worldwide variation in prevalence of symptoms of asthma, allergic Rhinoconjunctivitis, and Atopic Eczema: ISAAC. Lancet. 1998;351:1225–32. doi: 10.1016/S0140-6736(97)07302-9. [DOI] [PubMed] [Google Scholar]
- 26.Gehring U, Wijga AH, Hoek G, et al. Exposure to air pollution and development of asthma and Rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med. 2015;3:933–42. doi: 10.1016/S2213-2600(15)00426-9. [DOI] [PubMed] [Google Scholar]
- 27.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–61. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 28.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of Spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 29.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for Spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–43. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 31.de Onis M, WHO MULTICENTRE GROWTH REFERENCE STUDY GROUP WHO child growth standards based on length/height, weight and age. Acta Paediatr. 2006;95:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 32.Dagitty. http://www.dagitty.net/dags.html n.d. Available.
- 33.Cassim R, Milanzi E, Koplin JJ, et al. Physical activity and asthma: cause or consequence? a Bidirectional longitudinal analysis. J Epidemiol Community Health. 2018;72:770–5. doi: 10.1136/jech-2017-210287. [DOI] [PubMed] [Google Scholar]
- 34.Russell MA, Dharmage S, Fuertes E, et al. The effect of physical activity on asthma incidence over 10 years: population-based study. ERJ Open Res. 2021;7:00970-2020. doi: 10.1183/23120541.00970-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Aymerich J, Varraso R, Danaei G, et al. Incidence of adult-onset asthma after hypothetical interventions on body mass index and physical activity: an application of the parametric G-formula. Am J Epidemiol. 2014;179:20–6. doi: 10.1093/aje/kwt229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardon G, Van Cauwenberghe E, De Bourdeaudhuij I. What do we know about physical activity in infants and toddlers: a review of the literature and future research directions. Science & Sports . 2011;26:127–30. doi: 10.1016/j.scispo.2011.01.005. [DOI] [Google Scholar]
- 37.Prioreschi A, Micklesfield LK. A Scoping review examining physical activity measurement and levels in the first 2 years of life. Child Care Health Dev. 2016;42:775–83. doi: 10.1111/cch.12382. [DOI] [PubMed] [Google Scholar]
- 38.Bruijns BA, Truelove S, Johnson AM, et al. Infants' and toddlers' physical activity and sedentary time as measured by accelerometry: a systematic review and meta-analysis. Int J Behav Nutr Phys Act. 2020;17:14. doi: 10.1186/s12966-020-0912-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.da Silva BGC, Menezes AMB, Wehrmeister FC, et al. Screen-based sedentary behavior during adolescence and pulmonary function in a birth cohort. Int J Behav Nutr Phys Act. 2017;14:82. doi: 10.1186/s12966-017-0536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith MP, von Berg A, Berdel D, et al. Physical activity is not associated with spirometric indices in lung-healthy German youth. Eur Respir J. 2016;48:428–40. doi: 10.1183/13993003.01408-2015. [DOI] [PubMed] [Google Scholar]
- 41.Luzak A, Karrasch S, Thorand B, et al. Association of physical activity with lung function in lung-healthy German adults: results from the KORA Ff4 study. BMC Pulm Med. 2017;17:215. doi: 10.1186/s12890-017-0562-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hancox RJ, Rasmussen F. Does physical fitness enhance lung function in children and young adults. Eur Respir J. 2018;51:1701374. doi: 10.1183/13993003.01374-2017. [DOI] [PubMed] [Google Scholar]
- 43.Mielck A, Reitmeir P, Wjst M. Severity of childhood asthma by socioeconomic status. Int J Epidemiol. 1996;25:388–93. doi: 10.1093/ije/25.2.388. [DOI] [PubMed] [Google Scholar]
- 44.Bacon SL, Bouchard A, Loucks EB, et al. Individual-level socioeconomic status is associated with worse asthma morbidity in patients with asthma. Respir Res. 2009;10:125. doi: 10.1186/1465-9921-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Eijsden M, Vrijkotte TGM, Gemke RJBJ, et al. Cohort profile: the Amsterdam born children and their development (ABCD) study. Int J Epidemiol. 2011;40:1176–86. doi: 10.1093/ije/dyq128. [DOI] [PubMed] [Google Scholar]
- 46.Nygren M, Carstensen J, Koch F, et al. Experience of a serious life event increases the risk for childhood type 1 diabetes: the ABIS population-based prospective cohort study. Diabetologia. 2015;58:1188–97. doi: 10.1007/s00125-015-3555-2. [DOI] [PubMed] [Google Scholar]
- 47.Wickman M, Kull I, Pershagen G, et al. The BAMSE project: presentation of a prospective longitudinal birth cohort study. Pediatr Allergy Immunol. 2002;13:11–3. doi: 10.1034/j.1399-3038.13.s.15.10.x. [DOI] [PubMed] [Google Scholar]
- 48.Weber M, Grote V, Closa-Monasterolo R, et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr. 2014;99:1041–51. doi: 10.3945/ajcn.113.064071. [DOI] [PubMed] [Google Scholar]
- 49.Bisgaard H. The Copenhagen prospective study on asthma in childhood (COPSAC): design, rationale, and baseline data from a longitudinal birth cohort study. Ann Allergy Asthma Immunol. 2004;93:381–9. doi: 10.1016/S1081-1206(10)61398-1. [DOI] [PubMed] [Google Scholar]
- 50.Olsen J, Melbye M, Olsen SF, et al. The Danish national birth cohort--its background, structure and aim. Scand J Public Health. 2001;29:300–7. doi: 10.1177/14034948010290040201. [DOI] [PubMed] [Google Scholar]
- 51.Heude B, Forhan A, Slama R, et al. Cohort profile: the EDEN mother-child cohort on the Prenatal and early postnatal determinants of child health and development. Int J Epidemiol. 2016;45:353–63. doi: 10.1093/ije/dyv151. [DOI] [PubMed] [Google Scholar]
- 52.Larsen PS, Kamper-Jørgensen M, Adamson A, et al. Pregnancy and birth cohort resources in Europe: a large opportunity for Aetiological child health research. Paediatr Perinat Epidemiol. 2013;27:393–414. doi: 10.1111/ppe.12060. [DOI] [PubMed] [Google Scholar]
- 53.Kooijman MN, Kruithof CJ, van Duijn CM, et al. The generation R study: design and cohort update 2017. Eur J Epidemiol. 2016;31:1243–64. doi: 10.1007/s10654-016-0224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berg A v, Krämer U, Link E, et al. Impact of early feeding on childhood Eczema: development after nutritional intervention compared with the natural course - the Giniplus study up to the age of 6 years. Clin Exp Allergy. 2010;40:627–36. doi: 10.1111/j.1365-2222.2009.03444.x. [DOI] [PubMed] [Google Scholar]
- 55.Lenters V, Iszatt N, Forns J, et al. Early-life exposure to persistent organic Pollutants (OCPs, PBDEs, PCBs, PFASs) and attention-deficit/hyperactivity disorder: A multi-Pollutant analysis of a Norwegian birth cohort. Environ Int. 2019;125:33–42. doi: 10.1016/j.envint.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 56.Guxens M, Ballester F, Espada M, et al. Cohort profile: the INMA--Infancia Y Medio Ambiente--(Environment and childhood) Int J Epidemiol . 2012;41:930–40. doi: 10.1093/ije/dyr054. [DOI] [PubMed] [Google Scholar]
- 57.Kummeling I, Thijs C, Penders J, et al. Etiology of Atopy in infancy: the KOALA birth cohort study. Pediatr Allergy Immunol. 2005;16:679–84. doi: 10.1111/j.1399-3038.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 58.O’Mahony D, Fallon UB, Hannon F, et al. The Lifeways cross-generation study: design, recruitment and data management considerations. Ir Med J. 2007;100:suppl. [PubMed] [Google Scholar]
- 59.Heinrich J, Brüske I, Cramer C, et al. Giniplus and Lisaplus - design and selected results of two German birth cohorts about natural course of Atopic diseases and their determinants. Allergol Select . 2017;1:85–95. doi: 10.5414/ALX01455E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuehni CE, Brooke AM, Strippoli M-PF, et al. Cohort profile: the Leicester respiratory cohorts. Int J Epidemiol. 2007;36:977–85. doi: 10.1093/ije/dym090. [DOI] [PubMed] [Google Scholar]
- 61.de Korte-de Boer D, Mommers M, Creemers HMH, et al. Lucki birth cohort study: rationale and design. BMC Public Health. 2015;15:934. doi: 10.1186/s12889-015-2255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wijga AH, Kerkhof M, Gehring U, et al. Cohort profile: the prevention and incidence of asthma and mite allergy (PIAMA) birth cohort. Int J Epidemiol. 2014;43:527–35. doi: 10.1093/ije/dys231. [DOI] [PubMed] [Google Scholar]
- 63.Martindale S, McNeill G, Devereux G, et al. Antioxidant intake in pregnancy in relation to Wheeze and Eczema in the first two years of life. Am J Respir Crit Care Med. 2005;171:121–8. doi: 10.1164/rccm.200402-220OC. [DOI] [PubMed] [Google Scholar]
- 64.Allan KM, Prabhu N, Craig LCA, et al. Maternal vitamin D and E intakes during pregnancy are associated with asthma in children. Eur Respir J. 2015;45:1027–36. doi: 10.1183/09031936.00102214. [DOI] [PubMed] [Google Scholar]
- 65.Lagström H, Rautava P, Kaljonen A, et al. Cohort profile: steps to the healthy development and well-being of children (the STEPS study) Int J Epidemiol. 2013;42:1273–84. doi: 10.1093/ije/dys150. [DOI] [PubMed] [Google Scholar]
- 66.Inskip HM, Godfrey KM, Robinson SM, et al. Cohort profile: the Southampton women’s survey. Int J Epidemiol. 2006;35:42–8. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katier N, Uiterwaal CSPM, de Jong BM, et al. The wheezing illnesses study Leidsche Rijn (WHISTLER): rationale and design. Eur J Epidemiol. 2004;19:895–903. doi: 10.1023/b:ejep.0000040530.98310.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to data protection reasons, the datasets generated during the current study cannot be made publicly available.