Abstract
Viruses frequently carry out replication in specialized compartments within cells. The impact of these structures on virus replication is poorly understood. Recent research supports phase separation as a foundational principle for organization of cellular components with the potential to impact viral replication. In this review, phase separation is explored in the context of formation of viral replication centers, with an emphasis on the non-segmented negative strand RNA viruses. Consideration is given to the interplay between phase separation and the critical processes of viral transcription and genome replication, and the role of these regions in host-pathogen interactions is discussed. Finally, critical questions that must be addressed to fully understand how phase separation influences viral replication and the viral life cycle are presented, along with information on new approaches that could be used to make important breakthroughs in this intriguing field.
Keywords: phase separation, virus transcription and replication, NNSVs, inclusion bodies, viral life cycle, host-pathogen interactions
Viral inclusion body formation as a hallmark of viral infection
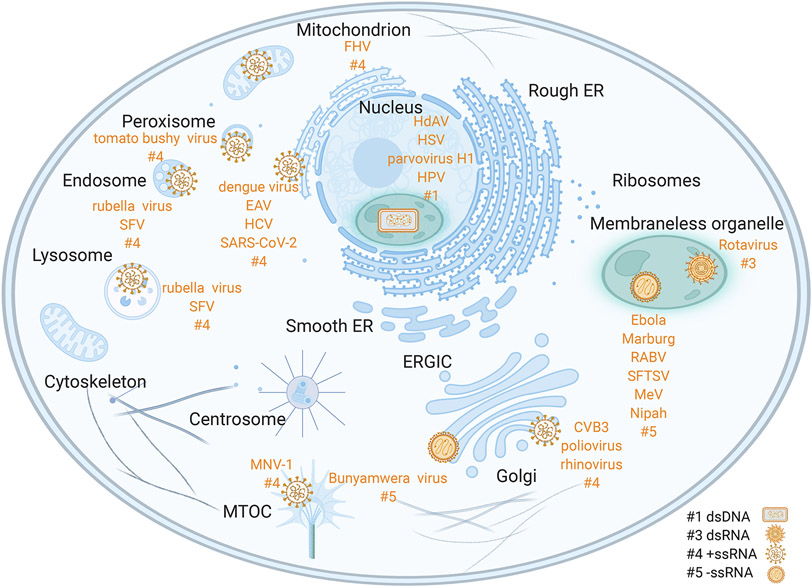
Viruses are defined by their absolute dependence on the host to facilitate viral replication and promote viral spread. In this context, viruses adopt different strategies to usurp and redirect host protein functions while protecting viral components from recognition by antiviral signaling molecules. During infection, many viruses induce the formation of discrete intracellular compartments. These viral inclusions have different intracellular locations depending on the virus. They can be associated with membranes derived from diverse organelles, such as the mitochondria (1), peroxisome (2), endosomes and lysosomes (3), endoplasmic reticulum (ER) (3-5), microtubule organizing center (MTOC) (6), and Golgi (7-10) (Fig. 1). Some positive-sense RNA virus infections lead to formation of double-membrane vesicles derived from diverse cellular membranes (11). For some other viruses, including dsDNA viruses, dsRNA viruses, and negative sense RNA viruses, viral inclusions are reported to be membraneless with some inside the nucleus (12-15) and the majority cytosolic (16-21). Disrupting virus-specific cellular assemblies offers a tantalizingly general strategy for antiviral treatments that in principle should minimize off-target toxicity. Despite this opportunity, many questions regarding the biophysical basis for viral compartmentalization remain.
Figure 1. Viral replication takes place in diverse membranous as well as membraneless organelles.
These viral replication compartments house essential steps of the viral life cycle and shield viral components from host defense systems. Sites of membrane-bound viral replication include mitochondria (Flock House virus, FHV (1)), peroxisomes (tomato bushy virus (2)), endosomes and lysosomes (rubella virus (3); Semliki Forest virus, SFV (3)), endoplasmic reticulum (ER) (dengue virus (4); equine arteritis virus, EAV (3), HCV (5), SARS-CoV-2 (5)), microtubule organizing center, MTOC (murine norovirus 1, MNV-1 (6)), Golgi (Bunyamwera virus (7); coxsackievirus B3, CVB3 (8), poliovirus (9), rhinovirus (10)). Sites of membraneless viral replication include cytosolic viral condensates (Ebola virus (10); Marburg virus (17); rabies virus, RABV (18); severe fever with thrombocytopenia syndrome virus, SFTSV (19); measles virus, MeV (20); Nipah virus (21)) and nuclear viral condensates (human adenovirus, HdAV (12); herpes simplex virus, HSV (13); parvovirus H1 (14); human papillomavirus, HPV (15)). The numbers at end of each group of viruses reflect the Baltimore classification (#1: dsDNA; #3: dsRNA; #4: +ssRNA; #5: -ssRNA). Created with Biorender.com.
The presence of viral inclusion bodies (IBs) was historically used to diagnose certain viral infections. As an example, the WI-38 cell line was originally developed in the 1960s to generate most human virus vaccines, including vaccines against adenoviruses, rubella, measles, mumps, poliovirus, and rabies virus. During its development, a critical consideration was that WI-38 cells were free of viral infection. With limited detection tools, indicators of viral infection included changing cellular morphology, detachment of cells from glass surfaces, and the development of “inclusion bodies” (22). Early electron microscopy studies further documented these observations of viral IB formation (23). Despite these observations, the actual function of IBs remained unclear.
IBs were initially thought of as “clumps of abnormal protein” or aggregated material targeted for degradation as a cytopathic effect (24-28). However, analyses over the subsequent decades revealed that these macromolecular structures establish physical platforms that are important for viral replication and viral assembly (27, 29). These viral IBs, also referred to as “virosomes”, “viral factories”, “viroplasms”, “liquid-like viral organelles”, and “membraneless replication compartments”, are often dynamic sites of concentrated host and viral proteins that are spherical in shape and required for viral RNA synthesis. Furthermore, the formation of viral IBs reorganizes the cellular architecture and cytoplasmic contents of a cell, including the cytoskeleton, a process which may disrupt immune response pathways and enable virus assembly and budding.
Recent advances in our understanding of these spherical moieties posit that viral IBs demonstrate many of the physical properties of liquid phases formed by phase separation (30-34). Viral IBs can fuse with other IBs to form even larger spherical bodies with differing surface tensions or densities, potentially leading to an exchange of molecules. More broadly, viral inclusions can be considered a type of biomolecular condensate. Biomolecular condensates are non-stochiometric assemblies that concentrate specific components with respect to the surrounding environment. While condensates can form via different mechanisms, the emerging de facto mode of assembly appears to be that of an intracellular phase transition (35). Although early work invoked liquid-liquid phase separation (LLPS) as the specific class of phase transition to explain many condensates, more recent analysis has revealed that condensates often behave as complex viscoelastic materials with intra-condensate architectures that preclude designation as a ‘simple’ liquid (36-38). As such, even for condensates with macroscopic liquid-like properties, we suggest it is more appropriate to refer to these simply as forming via intracellular phase transitions or though phase separation.
Over the last decade, there has been remarkable progress in understanding the molecular biophysics that contributes to phase separation in vitro (35, 39, 40). However, how the mechanisms that regulate phase separation impact viral replication or how phase separation impacts host and viral proteins within IBs are only starting to be appreciated. In most instances, key molecular mechanisms remain to be defined. Here we describe the formation, characteristics, and functions of viral IBs, with an emphasis on non-segmented negative strand RNA viruses (NNSVs). We also outline tools that are currently available to investigate viral IBs and their limitations. Finally, we offer our perspective on some of the current challenges in the understanding of viral IBs and the potential of targeting viral IBs for antiviral therapeutics.
Viral inclusion bodies as biomolecular condensates formed through phase separation
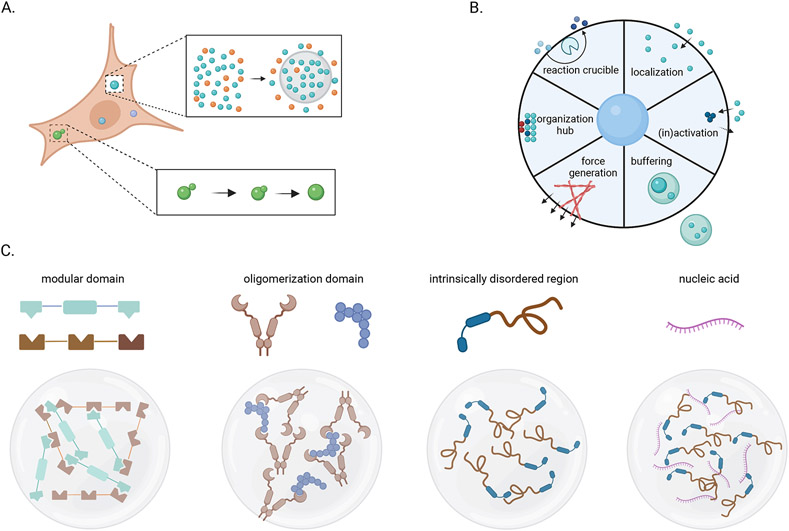
For many years, the mechanisms behind IB formation remained elusive, but recent studies have implicated phase separation as a likely driving force for this process. Work over the last decade has shown that phase separation is a ubiquitous phenomenon that appears to underlie the formation of many membraneless organelles in cells (39-46). In a simple two-component system (biomolecule and solvent) that undergoes phase separation, a spontaneous demixing event will occur when that biomolecule reaches a threshold concentration (also known as a saturation concentration), leading to a dense phase that co-exists with a corresponding dilute phase. In many cases, this dense phase is characterized by spherical liquid-like droplets, akin to droplets of vinegar in a sea of olive oil. The concentration of a molecule in the two phases can be orders of magnitude apart without the assistance of a physical barrier. The spherical liquid-like droplets can undergo fusion and fission, and in many cases, there exists a rapid exchange of individual molecules between the dense and dilute phases (Fig. 2A). As such, condensates formed by phase separation enable the formation of labile and environmentally-responsive intracellular structures that are poised to drive specific molecular processes. Condensates formed through phase separation can confer several functions, including controlled localization of molecules and promotion of biochemical reactions since partitioning within separated phases is different (46). Similarly, phase separation can provide an organizational hub or signaling platform and lead to the assembly of complexes (41, 46, 47). Condensates formed via phase separation can enable the activation/inactivation of components due to presence of other modulatory molecules (41). Depending on the mode of partitioning and interaction, condensates formed by phase separation also offer a means to buffer the concentration of specific components in the cytosol (48). Finally, the surface tension generated by a phase-separated assembly can exert mechanical force, bending membranes, displacing chromatin, or facilitating endo- or exo-cytosis (36) (Fig. 2B).
Figure 2. Phase separation can lead to formation of membrane organelles.
A. Cell condensates formed through phase separation lack a membrane, and compartmentalization of biomolecules is driven by multivalent interactions that minimize the overall free energy such that chemically distinct cellular compartments form spontaneously (top right). Liquid-like condensates show many of the properties expected for liquids, including rapid intracondensate dynamics and the ability of two condensates to fuse rapidly into a single species (bottom right). B. Compartmentalization by phase separation enables diverse functions that can control subcellular biochemistry and information flow. C. Phase separation depends on multivalency that are encoded in biomolecules. These include modular binding domains connected in tandem, stoichiometric oligomerization domains that enable the formation of multivalent oligomers, intrinsically disordered regions with distributed sticker regions or residues, and long nucleic acid molecules that can function as platforms for higher-order assembly. Created with Biorender.com.
The intrinsic properties of the individual protein and nucleic acid components and their interactions define the properties of phase-separated compartments. One essential feature that drives phase separation is multivalency (47) (Fig. 2C). As the valency (number of binding sites of a molecule) increases, the threshold for phase separation decreases. Multivalency can come from structural domains in tandem, where each domain contributes to binding (47). The low affinity of individual interactions can enable liquid-like properties, although low-affinity interactions are not an essential feature for proteins that undergo phase separation (35). In addition, oligomerization domains from one of the binding partners can directly contribute to multivalency. Intrinsically disordered regions (IDR) are also often involved in phase separation, as they can in principle provide another source of multivalency; however, many (perhaps most) IDRs do not drive phase separation (49, 50). Finally, nucleic acid binding proteins may phase separate as a result of a linkage brought by nucleic acid binding and/or the presence of disordered regions (51). Long, multivalent nucleic acid molecules can act as a platform upon which multiple copies of nucleic acid binding proteins may bind, driving higher-order assembly via multiple distinct modes of interaction.
Phase separation can be regulated by changing valency through post-translational modifications, including phosphorylation (52) and ubiquitination (53), that directly perturb protein solution properties and remodel binding interfaces. This is also true for noncovalent modifications on the components from ligand binding (54). Temperature, ionic strength, osmolarity, pH, and crowding are additional environmental factors that modulate phase separation (40). Furthermore, biomolecular condensates can adopt a series of material properties and in many cases will mature over time, undergoing an apparent liquid-to-solid transition (55). These metastable states may proceed irreversibly to aggregate or form fibrils. Meanwhile, energy-dependent maintenance of droplet fluidity can be mediated by chaperones, disaggregases, and helicases, although mechanism that do not depend on ATP hydrolysis also exist (56-60).
Growing evidence supports the concept that intracellular phase transitions may account for formation of at least some viral IBs. The properties of many IBs that had been previously described (fusion, spherical nature, and lack of a membrane) are consistent with liquid-like droplets formed through phase separation. Many IBs readily deform when touching a physical barrier, disappear upon osmotic shock, and show rapid molecular re-arrangement when assessed by live cell imaging for kinetics and fluorescence recovery after photobleaching. As such, an emerging consensus argues that at least a subset of IBs form via spontaneous demixing transitions driven by phase separation.
Non-segmented negative strand viral inclusion bodies are phase separated viral replication factories
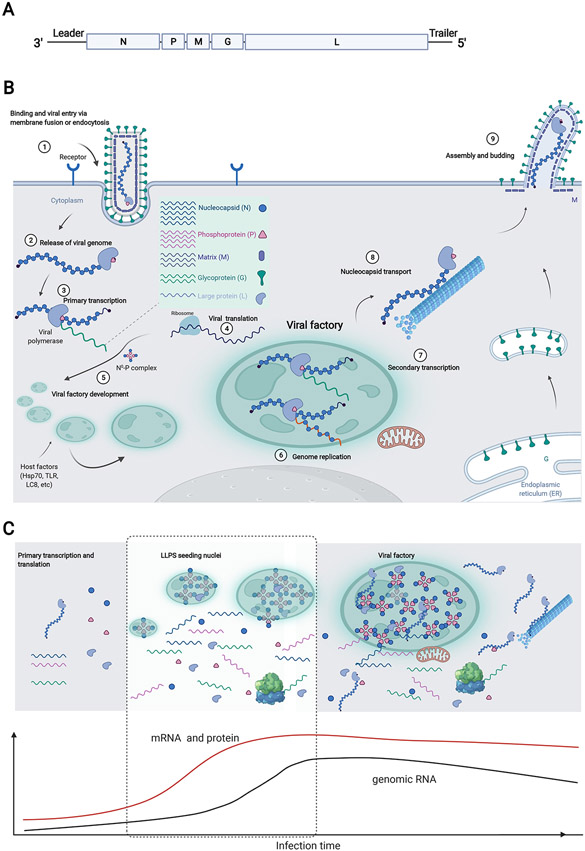
The non-segmented negative strand viruses (NNSVs), which encode multiple genes within the same RNA strand (Fig. 3A), have multiple examples of IBs with phase separation characteristics. Though there are differences between NNSV families and between individual viruses within each family, key elements of the life cycle are conserved between these viruses (illustrated for rabies virus (RABV) in Fig. 3B). Following viral entry, the nucleocapsid protein (N)-encapsidated viral genome must be transcribed to mRNA by the RNA-dependent RNA polymerase (L), along with the phosphoprotein (P) cofactor. The newly transcribed mRNA provides a template for translation of viral proteins, and transcript production is a gradient, with genes closest to the 3’-end of the genome produced at the highest level. The same viral proteins also promote antigenome and genome synthesis in a process that lags behind transcription (Fig. 3C). IBs enclose the viral replication machinery that drives transcription and replication in many viruses, highlighting their critical role in infection. For most viruses, the basic components of the viral replication complex comprise of the viral polymerase L, the P protein cofactor, and the N protein that encapsidates the viral genome. In the following sections we consider the specific details associated with several NNSVs.
Figure 3. Viral replication compartments in NNSVs.
A. The genomic structure of RABV as an example of an NNSV. The genome of RABV is a single-stranded, linear RNA of negative polarity with inverse-complementary 3' and 5' termini. Abbreviations: UTR, untranslated region; N, nucleocapsid protein; P, phosphoprotein; M, matrix protein; G, glycoprotein; L, large protein (with RNA-dependent RNA polymerase activity). B. Schematic of the viral replication cycle of RABV that involves the formation of membraneless viral factories after infection. Viral translational products from primary transcription form liquid-like membraneless viral factories, and host factors are recruited into these condensates. Replication takes place inside these viral factories. Encapsidated genomes are trafficked out of viral factories via cytoskeletal transport. A major driving force in viral IBs formation is the expression of N and P proteins, which are suggested as the basic scaffolds of IBs during infection. C. The phase separation of viral proteins drive the formation of viral factories that facilitate efficient transcription and replication. Primary transcription results in abundant translation of N and P, which undergoes phase separation and in turn assemble into viral factories. Full-length genome synthesis lags behind those of mRNA and viral proteins and coincides with replication factories establishment. Created with Biorender.com.
Rhabdoviruses:
Closer examination of two rhabdoviruses, RABV and vesicular stomatitis virus (VSV), has provided important insights into the roles of viral IBs and the first studies directly implicating phase separation in IB formation. Negri body formation in cells infected by RABV requires both N and P. These bodies grow during the early stages of viral infection into larger bodies that eventually transition to smaller structures at later stages of infection, with microtubule polymerization facilitating these transitions (61). In situ fluorescent hybridization showed that the genome, antigenome, and mRNA were located inside the Negri bodies. Active incorporation of BrUTP within the Negri body indicated that viral transcription and replication were occurring (61). The rapid re-arrangement of some components has been illustrated using FRAP experiments, which showed that proteins could freely diffuse within a Negri body yet diffusion rates differed from proteins in the surrounding cytosol, consistent with the formation of a distinct compartment. Negri bodies are small and spherical during early stages, fuse over time, and can be dissolved by hypotonic shock (62), all consistent with condensates formed through phase separation, although not strictly exclusionary of other mechanisms. FRAP experiments showed that the viral proteins could freely diffuse into the region, consistent with the lack of a membrane, and that diffusion rates within the inclusion differed from those in the surrounding, consistent with the conditions in the Negri body differing from that of the surrounding cytosol. Co-expression of the rabies virus N and P proteins resulted in cytoplasmic inclusions recapitulating Negri body properties. This minimal system revealed that an intrinsically disordered domain and the dimerization domain of P were essential for Negri bodies-like structures formation (62). In contrast to RABV, VSV primary viral transcription appears to take place in the cytoplasm prior to initiation of IB formation. IBs appear approximately four hours post-infection, and N, P, and the viral polymerase L are necessary for IB formation, as demonstrated with the use of a global protein synthesis inhibitor, puromycin, and protein-specific peptide-conjugated morpholino oligomers (63, 64). Similar to RABV, VSV IBs were shown to have liquid-like properties, including fusion, deformation and differences in diffusion rates inside the IB (64).
Paramyxvoviruses:
Measles viruses, a member of the Paramyxoviridae family, replicates in cytoplasmic IBs that are linked to persistent infection that causes inclusion body encephalitis (65). The measles N and P proteins have been identified as the major components for IB formation, and these cytoplasmic viral factories have been shown to concentrate the viral RNA replication machinery but not to be membrane bound (66). Intriguingly, both dynein-mediated transport and phosphorylation of P facilitate growth of these organelles (20). Importantly, E. coli expressed, purified measles N and P protein can spontaneously phase separate under physiological salt conditions (67). Consistent with observations made for cellular IBs, the oligomerization domain and C-terminal X domain (XD) of P were crucial for phase separation. Intriguingly, a single point mutation in N (S491L) that is known to attenuate transcription also resulted in a loss of phase separation, hinting at a coupling between the two processes. Moreover, when RNA was added into this phase separation system, nucleocapsid assembly was accelerated (67). In contrast to what has been reported for measles and additional paramyxoviruses (68-70), a recent study of Nipah virus infected cells indicated that there were two separate types of IBs. Those that are perinuclear were highly dynamic but lacking the RNA species expected for a replication center, whereas those that were near the plasma membrane contained the viral matrix protein, and likely represent sites of assembly and budding (71).
Pneumoviruses:
IB formation and function has also been carefully examined for two pneumoviruses: human metapneumovirus (HMPV) and respiratory syncytial virus (RSV). For RSV, IBs contain all the viral proteins associated with the replication complex, including N, P, L, and M2-1, a transcription elongation factor. P and N are critical for IB formation, with the oligomeric state of N and its ability to bind RNA shown to be important (72). Both genomic RNA and viral mRNA are contained inside IBs. Intriguingly, super-resolution microscopy identified a sub-compartment, termed IB-associated granules (IBAGs), inside these IBs (73). There, newly synthesized mRNA accumulated with the viral transcription activator M2-1, while N, P, L and genomic RNA were excluded. Very recent work has also shown that RSV N and P expressed and purified from E. coli can phase separate when together, though neither was found to promote phase separation on their own (72). Formation of HMPV IB-like structures requires the viral N and P proteins (74). Work utilizing fluorescence in situ hybridization (FISH) demonstrated that HMPV IBs were the cytoplasmic sites of active transcription and replication (75). IB formation was consistent with an actin-dependent coalescence of multiple early replicative sites, in contrast to the importance of the microtubule network for RABV. Time course quantitative reverse transcription-PCR (qRT-PCR) analysis suggested that the coalescence of IBs is a strategy to efficiently replicate and transcribe the viral genome (75). Subsequent work also found that HMPV IBs display liquid-like properties; HMPV P was shown to phase separate on its own using purified proteins from E. coli (76). In addition to forming condensates in vitro, P also recruits the monomeric and oligomeric forms of N to phase separated condensates, suggesting that the proteins which promote this process can differ significantly between related systems (76).
Filoviruses:
In filovirus-infected cells, time course studies demonstrated that small IBs grow with time, undergoing fusion and fission events (16), consistent with phase separation. Interestingly, formation of larger IBs coincided with viral RNA replication. In contrast to the rhabdovirus and pneumovirus systems, the Ebola nucleoprotein (NP) alone has recently been shown to induce the formation of IB-like structures, the significance of which are not clear. Futhermore, other replication proteins localize inside IBs only when co-expressed with NP, suggesting the NP provides the driving force for IB formation (77). Ultrastructural studies show that Ebola IBs are not membrane-enclosed, although Ebola IBs are often found close to the ER, endosomal vesicles, and mitochondria (78, 79). It is therefore possible that filoviral IBs contact different compartments to favor different steps of viral RNA synthesis (transcription and translation, replication, condensation, assembly, and nucleocapsid transport).
Beyond NNSVs--bunyaviruses, rotaviruses and others:
While this review, and the majority of the work on phase separation in viral replication centers, has focused on NNSVs, several other RNA viruses have replication centers with phase separation properties. For the bunyaviruses, the nonstructural proteins (NSs) of severe fever with thrombocytopenia syndrome virus (SFTSV) induce formation of viral IBs that contain N and vRNA (19). Liquid droplets colocalize with the inclusions while inhibition of lipid metabolism reduces virus replication. During the replication cycle of rotavirus, electron-dense cytoplasmic inclusions are formed, where synthesis of dsRNA and its packaging into virion particles occur (80, 81). These viroplasms increase in size while their numbers decrease as they localize towards the perinuclear region and their maintenance depends on the microtubular network (82). For rotaviruses, nonstructural proteins NSP2 and NSP5 are sufficient to form membrane-free cytoplasmic inclusions. Interestingly, NSP2 phosphorylation-mediated liquid droplet interaction is critical for viroplasm formation (83). Both SFTSV and rotavirus viral IB studies suggest a previously undescribed link between lipid metabolism and virus replication. A number of other viral processes have recently been shown to be driven by phase separation, including SARS-CoV-2 N nucleocapsid assembly (84-90), retroviral nucleocapsid-driven genomic RNA positioning (91), herpes simplex virus-1 tegument formation (92), and Epstein-Barr virus EBNA2-regulated gene expression . Further discussion of these can be found elsewhere (31).
Linkage among phase separation, transcription, and replication in viral IBs
The multiple examples of viral replication sites that possess properties consistent with phase separated condensates implies that these spontaneously-forming cellular assemblies may offer a convenient sub-compartment for replication. As a result, there has been considerable interest in this important area. How are viral transcription and replication controlled, given that the same viral proteins promote both processes? Equally importantly, how does the process of phase separation contribute to viral transcription and replication?
For NNSVs, interaction between the P, N and L proteins is essential for transcription and replication, and the P and N proteins are also critical for IB formation and phase separation in most systems. The P protein varies in length (241 aa for RSV P, 340 aa for Ebola VP35, 507 aa for measles P), and interacts with both the monomeric, RNA-free N0 protein and the oligomeric RNA-bound N protein, with different domains of P involved in these key interactions (93-97). P also serves as the bridge between the L protein and the N-encapsidated genome (98). For the multifunctional roles of P, a central oligomerization domain is critical (93, 99-101) and enables the formation of an oligomeric and highly multivalent species which in turn can lower the concentration threshold needed for IB formation during early stage of viral infection. A recent model (32) proposes that early in infection, low concentrations of the viral proteins are present, with the majority of the newly synthesized N protein likely in its monomeric, RNA-free form. Proteins levels rise in the cytosol as mRNA production and protein translation increase, leading to a concentration of N and P sufficient to facilitate phase separation and IB formation (Fig. 3C). This model is consistent with the observation that purified N and P (62, 64, 67, 72), or purified P alone in the case of HMPV (76), are sufficient to drive phase separation in a concentration dependent manner. Accelerated transcription within the phase-separated region due to increased protein concentrations would then lead to further increases in viral proteins, and eventually to increasing genome replication (Fig. 3C).
Additional factors beyond the concentration of P and N are critical for transcription and genome replication, and similarly other factors likely strongly influence phase separation in IBs. The balance of N0 to N-RNA has been proposed to play a role in regulation of the switch between transcription and replication (102, 103), but evidence supporting this, or information on the differential effect of these two forms of N on phase separation, remains sparse. A recent study of purified proteins from HMPV did show that both N0 and N-RNA were recruited to P protein-formed droplets (76), but further work will be needed to understand how the changing ratio of these proteins over the course of infection affects both transcription/replication and phase separation. Relative nucleotide concentrations have been demonstrated to affect the balance between transcription and replication for RSV (104), and some IBs have been shown to localize near mitochondria (105), but the overall role of nucleotide levels in controlling transcription, replication, phase separation and the interplay between them remains to be explored.
RNA length, structure, and sequence are key additional determinants of condensate assembly, kinetics, and organization. Long multivalent RNA molecules offer a platform for protein-driven phase separation and can drive phase separation by mediating the formation of protein:RNA complexes that are themselves hypervalent (51). In addition to specific interactions with RNA binding proteins, long-range RNA-RNA interactions and the electrostatic contributions of negatively charged RNA molecules offer a complex biophysical landscape to tune condensate structure and function. The complex condensate architectures and temporal evolution of viral replication bodies may be driven by a combination of mRNAs corresponding to each of the viral genes along with N-encapsidated genomes and antigenomes, with the relative levels of these species changing over the course of infection. Intriguing work using purified measles N and P with added RNA demonstrated that RNP capsid formation is more efficient within phase separated condensates (67), suggesting that one additional role for phase separation may be to enhance genome/antigenome encapsidation rates. Recent work on RSV has indicated that the IBAG sub-compartments within IBs may be important in RNA localization, as these were shown to contain both viral mRNA and the viral protein M2-1, which is critical for transcription, but to exclude other viral proteins such as N, P or L (73). These results raise the possibility that, after transcription, viral mRNAs may be stored in specialized areas of IBs prior to export to the cytosol for translation, providing another area where formation of these phase-separated region may regulate the viral life cycle. The storage of mRNAs in condensates for later release mirrors observations made in germ granules, where dissolution of condensates is coincident with the release of mRNA molecules (106). RABV studies also hinted at the presence of subcompartments in IBs (107), but further work is needed to understand the ubiquitousness and functionality of sub-compartments within these viral replication factories.
The complex interactions regulating phase separation and viral transcription and replication likely alter significantly during the course of the infection, due to changes in both the constituents within the IB, and the resulting physical properties of the condensate itself. In addition to changes in the concentrations of viral proteins and RNAs, host components resident in IBs may modify over time. A number of host cell proteins have been identified in IBs, though little information is available about the timing of this localization during infection or their concentrations. Chaperone proteins play key roles in the maintenance of proper folding for viral proteins such and N and L. For example, hsp90 is present in IBs and is critical for L protein function for both measles and Nipah virus (108), and for maintenance of RSV L protein levels (109) and mumps L maturation (110). The actin-binding protein cofilin has been implicated in HPIV3 IB formation and viral replication (69). Addition of a casein kinase 2 inhibitor or mutation of two potential phosphorylation sites on MeV P protein resulted in alteration of IB size, implicating protein phosphorylation and host cell kinases in regulation of these structures (20). Interestingly, this study of MeV IBs also demonstrated using FRAP analysis that larger IBs showed distinct biophysical properties from smaller ones, with the larger ones displaying greatly reduced exchange with the cytosol and slower internal diffusion rates (20). Studies of RSV (111) and IAV (112) IBs in response to hypotonic shock also suggest a reduction in liquid-like behavior as condensates grow in size over time. As mentioned, viral condensates can mature from liquid-like to gel-like states in a process termed maturation (51), a process that can be driven by changes in component concentrations, as well as the acquisition of local structure within condensates. Despite these observations, the effects of maturation and the associated changes in condensate material properties on viral processes remain unclear.
Interactions with cellular components outside the IB can also be critical for their formation and dynamics. The initial formation of HMPV IBs requires the actin cytoskeleton (75), while the microtubule network is critical for RABV Negri body dynamics (61) and movement of ejected RNPs to form new Negri bodies or be assembled into viral particles (62). These dynamic processes may include intercellular movement of replication compartments (113), as IB-like structures have been observed in actin-containing connections between cells (114).
Formation of replication structures that utilize phase separation for initial membraneless organelles is not mutually exclusive with later developments making use of membrane structures. Negri bodies from RABV infected cells are initially not membrane bound, but then associate with a double membrane derived from the rough ER as infection progresses (18). Bunyamwera virus infection results in fragmented Golgi stacks (115). Oropouche viral factories attract ESCRT components to modify Golgi cisternae (116). Junin virus N protein induces discrete structures associated with autophagy membranes (116). PIV3 virus IBs are surrounded by a fragment ER membrane and are enriched with PI4P that facilitate replication (117).
Viral IBs provide a new compartment for virus-host interactions
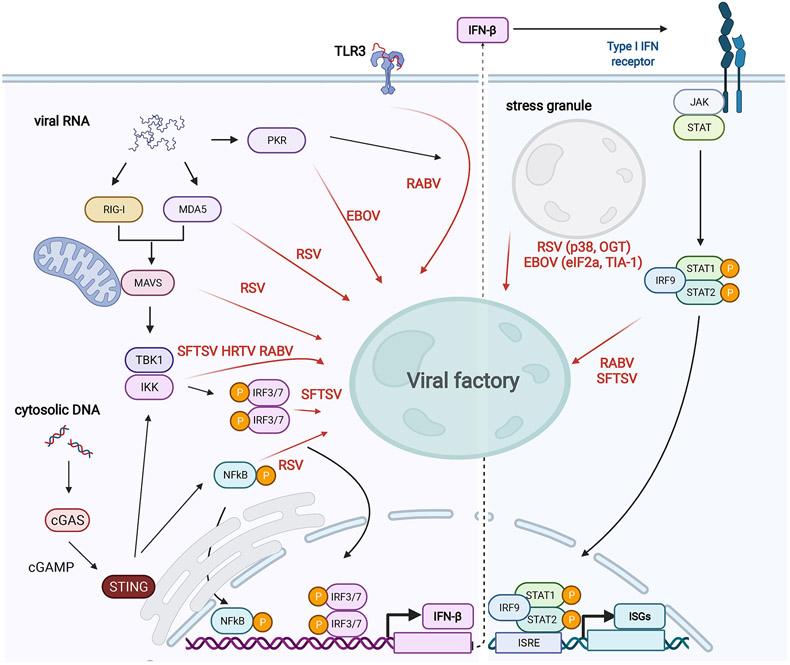
The formation of IBs through phase separation also appears to play a critical role in the battle between the virus and the host, contributing both by shielding key signals from the host cell innate immune system, and by sequestering components of the innate immune system to block their ability to initiate a response. For the RNA viruses, TLR3, RIG-I and MDA5 are key players in the recognition of dsRNA as a pathogen-associated molecular pattern (PAMP). Activation of these pattern recognition receptors (PRRs) leads to signaling through mitochondrial antiviral-signaling protein (MAVS) and other innate immune response proteins and initiation of interferon production (118, 119) (Figure 4). Interestingly, TLR3 was shown to re-localize to Negri bodies during RABV infection, and the absence of TLR3 blocked Negri body formation (120), suggesting that in some cases the virus can hijack these pathways to help promote replication body formation. Very little is known about how these host cell factors influence the biophysical properties of inclusion bodies, but in some cases, direct interactions with viral proteins that are key for phase separation have been demonstrated.
Figure 4. Viral IBs in innate immune evasion.
Viral factories counteract the host IFN response Viral factories can sequester key components (MDA5, MAVS, IKK, IRF3/7, NFκB, PKR, STAT1/2, and essential stress granule components) from innate immune signaling pathways that detect pathogen-associated molecular patterns including cytosolic nucleic acids. Abbreviations: PKR, protein kinase R; TLR3, Toll-like receptor 3; RIG-I, retinoic acid-inducible gene I; MDA5, melanoma differentiation-associated protein 5; TBK1, TANK binding kinase 1; IRF3/7, interferon regulatory factor 3/7; cGAS, cyclic GMP–AMP synthase; STING, stimulator of interferon genes; IKK, IκB kinase; OGT, O-Linked N-Acetylglucosamine (GlcNAc) Transferase; HRTV, Heartland virus. Created with Biorender.com.
IBs, as with other condensates, concentrate some components within them while excluding others. The dsRNA generated during RNA virus replication is a potent activator of the innate immune system, but localization to IBs may reduce this response. Early studies with parainfluenza virus 5 demonstrated that the virus remained primarily quiescent during the initial period of infection, with the genome present in inclusion bodies and only limited replication occurring. Significant increases in viral proteins and RNA synthesis was then seen only after the host cell anti-viral state had been blocked (68, 121). Encapsidation by the N protein likely also alters the innate immune response, and a recent study using microRNA to deplete Ebola or influenza virus NP protein found both a decrease in viral replication and an increase in the host antiviral response when NP levels were reduced (122). Intriguingly, modifications of viral RNA within the inclusions may also be important, as recruitment of RNA modification enzymes to inclusions has recently been shown to occur during RSV (123) and HMPV (124) infection. The resulting m6A methylation of viral RNA has been shown to be important for reduction of the host antiviral response for a number of RNA viral families (125).
The key viral proteins involved in IB formation and phase separation have been shown to interact with elements of the innate immune system in multiple systems, often resulting in recruitment of these host proteins to the inclusions. RSV infection results in translocation of MDA5 and MAVS to IBs, where both closely co-localize with the RSV N protein (105). RABV P has been shown to bind STAT 1 and STAT2, preventing translocation to the nucleus (126, 127). RABV P also blocks phosphorylation of interferon regulatory factor 3 (IRF-3; (128)). The bunyavirus severe fever with thrombocytopenia syndrome virus (SFTSV) nonstructural proteins interact with IRF7, again resulting in inclusion body sequestration (129). Finally, the Ebola virus (130) and Marburg virus VP35 proteins inhibit the function of the dsRNA-responsive kinase PKR, though recent work with Ebola VP35 indicates this is a cell-type specific function (131).
In a number of cases, key host proteins have been shown to be sequestered to IBs, though the mechanisms of recruitment have not yet been identified. NFκB has been shown to be rerouted to IBs in RSV infected cells, inhibiting innate immune signaling (111), and the signaling proteins p38 and OGT involved in stress response similarly retarget to RSV inclusions (132). Ebola virus inclusions have been shown to contain stress granule proteins, which form granules within the inclusion but not canonical stress granules (133), and the Ebola VP35 protein was suggested to play a key role in preventing stress granule formation. Infection with SFTSV (134)–results in relocalization of the key signaling molecules TBK1 and IKKε to viral inclusion bodies (134). Future studies will be needed to dissect the interplay between the biophysical properties of the inclusions and the recruitment of these host proteins.
Tools for studying viral IB formation and major questions to be addressed
Our understanding of the significance of phase separation in the context of virus replication is still in the early stages. Thus, a multitude of questions remain to be addressed on the formation and function of viral IBs. Very basic information about the interactions regulating the formation, maturation, and function of IBs has been determined and discussed above. Interactions formed by viral-viral components as well as viral-host components are likely to play a role in the process. Importantly, the temporal impact of induced host components and their impact on virus replication in the context of phase separation are not well defined at present. Below, we describe some opportunities as areas of potential impact in the future.
In terms of viral-viral interactions, work in multiple systems has demonstrated a key role for the viral P and N proteins (62, 64, 67, 72) in driving phase separation of purified proteins or IB formation in cells. Key regions in the P and N proteins have been identified (64, 72, 107) . However, the influence of the different forms of N (the RNA-free N0 monomer or the N-RNA oligomer) remains to be determined, as does the impact of RNA and nucleotide levels on the biophysical properties of these inclusions. Also poorly understood is the potential role of post-translational modifications. Studies from other systems have demonstrated key roles for protein phosphorylation (135) and ubiquitination (53) on phase separation. A recent study on measles inclusion found a role for P phosphorylation and the host kinase CK2 in modulation of inclusion body size (20), but the mechanism for this, or the role of phosphorylation in regulation of other viral replication regions remains to be defined. In vitro phase separation biochemical studies using purified protein are powerful tools to dissect how protein components regulate phase separation and condensate assembly/disassembly. Biochemical tools that regulate oligomerization, such as SpyTag (136) with different oligomerization features, and optogenetic tools, such as Corelet with photoinduced phase separating oligomers (137), are especially useful to perturb phase separation systems for testable hypothesis generation. Synthetic cell engineering is another exciting field for designer membraneless organelles to artificially control cellular behaviors (138, 139).
Viral-host interactions have been defined in the context of virus entry, replication, and egress, yet only a few studies have evaluated the impact of viral-host interactions on phase separation and replication. A small number of host proteins have been shown to be recruited to IBs, but major questions remain about the proteome within IBs. Importantly, how host protein recruitment or exclusion changes over the course of infection and how host proteins modulate phase separation remains unexplored. In addition, for RNA viruses, whether such interactions result in differential impact on viral replication and transcription remains to be characterized. It is suspected that many cellular proteins are recruited due to their similar physiochemical properties and interactions with the viral scaffold proteins. Such interactions might be dynamic and transient, requiring more live cell imaging and time-lapse studies, as well as innovative tools, to capture transient interactions. It is also critical to distinguish recruitment that is unavoidable vs. recruitment that is functionally important - just because a protein enters an IB does not necessarily mean its recruitment is meaningful for viral replication.
Research on the composition of IBs in cells will be an exciting field, although challenging since the liquid properties will make IB purification difficult. In particular, accurate reconstitution of IBs in vitro will be challenged by the inherent sensitivity of phase behavior to crowding and solution conditions (pH, osmolytes, ions, small molecules etc.). Fluorescence-based tools such as fluorescent timers (140), which encode temporal information to characterize evolution and heterogeneity of IBs, may be useful for assessing kinetics in vivo. In addition, cells with IBs are amenable to analysis by fluorescence microscopy and flow cytometry of whole cells or isolated IBs if those IBs can be purified without dissolution.
Given the transient nature of interactions within condensates, proximity labelling offers a burgeoning set of tools for characterizing intracondensate-interactomes (141). APEX2 is an engineered peroxidase that can be genetically targeted to cellular regions of interest (142, 143). Fluorescent protein tagging in tandem with APEX2, which has also been described, will provide live cell imaging to validate that tagging does not influence functional outcome. In addition, the localization of the APEX2 construct can be examined at higher resolution by electron microscopy (EM) because APEX2 also functions as a genetically encoded EM tag. Correlative light and electron microscopy (CLEM) (144) can be complemented with cryoelectron tomography and sub-tomogram averaging (145), elucidating how viral and host factors come together to confer replication organelles form and function.
Research on measles virus (20), RSV (111) and IAV (112) IBs all indicate a change in IBs and a reduction in condensate dynamics over the course of infection in a process that has been termed maturation (51). However, the key interactions leading to this change, the effect of this on virus replication and transcription, and the ubiquitousness of this finding in other viruses are all unknown. One intriguing hypothesis is that maturation of IBs to a less dynamic state could provide a storage site for viral genomes, potentially contributing to the observed long-term persistence for some viruses (146). Recent work has shown that phosphorylation of the P protein may regulate persistence in parainfluenza virus 5 (147), but how that phosphorylation affects phase separation and IBs is not yet known.
While IBs are known to be dynamic structures which can change over time, the functional implications of changes in IB composition, material state, and subcellular location are unknown for most viruses. The identification of two populations of IBs in Nipah-infected cells (71) with different properties and potentially different functions suggests it will be critical to monitor whether there are different varieties of IBs that co-exist within infected cells. A role for both the actin (75) and microtubule (62) cytoskeleton in IB dynamics has been reported, but many details about the interactions are unknown. In addition, while IB-like structures have been reported in cellular connections (114), whether movement of replication centers is a bona fide mechanism for cell-to-cell spread (113) remains to be determined.
Finally, the therapeutic value of phase separation properties of IBs remains to be fully explored. If in fact phase separation is important for virus replication, then modulation of phase separation could be a route towards potent and general antiviral therapeutics. To this end, a recent study revealed that a drug that disrupts the organization of RSV IBs leading to condensate hardening was shown to effectively block viral replication in RSV-infected mice (148), demonstrating the proof of principle that affecting these regions can dramatically alter viral infection. However, off-target effects would need to be carefully monitored, as the maturation and hardening of phase-separated assemblies has been implicated in progression of a number of neurodegenerative diseases (149, 150). If, alternatively, phase separation is unimportant for viral replication, it still offers a convenient macroscopic readout to assess nanoscopic phenomenon in vitro and in vivo (85). If this is true, the value of studying phase separation in viral replication stems from biophysical insights that phase separated assemblies offer, notably as convenient markers for drug screens, measuring the impact of mutations, or assessing for co-recruitment of host factors. In much the same way that IBs were originally used to identify viral infection in WI-38 cells, drugs that impede phase separation in vitro may offer a convenient means to identify compounds that could disrupt essential molecular interactions in vivo. Taken together, the value of using phase separation as a framework in biology is not to demonstrate specific physical states, but to provide predictive insight into molecular mechanism.
References
- 1.Kopek BG, Settles EW, Friesen PD, Ahlquist P. 2010. Nodavirus-Induced Membrane Rearrangement in Replication Complex Assembly Requires Replicase Protein A, RNA Templates, and Polymerase Activity. Journal of Virology 84: 12492–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCartney AW, Greenwood JS, Fabian MR, White KA, Mullen RT. 2005. Localization of the tomato bushy stunt virus replication protein p33 reveals a peroxisome-to-endoplasmic reticulum sorting pathway. The Plant cell 17: 3513–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller S, Krijnse-Locker J. 2008. Modification of intracellular membrane structures for virus replication. Nature Reviews Microbiology 6: 363–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paul D, Bartenschlager R. 2015. Flaviviridae Replication Organelles: Oh, What a Tangled Web We Weave. Annual Review of Virology 2: 289–310 [DOI] [PubMed] [Google Scholar]
- 5.Chukkapalli V, Randall G. 2014. Hepatitis C virus replication compartment formation: mechanism and drug target. Gastroenterology 146: 1164–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyde JL, Gillespie LK, Mackenzie JM. 2012. Mouse norovirus 1 utilizes the cytoskeleton network to establish localization of the replication complex proximal to the microtubule organizing center. Journal of Virology 86: 4110–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontana J, López-Montero N, Elliott RM, Fernández JJ, Risco C. 2008. The unique architecture of Bunyamwera virus factories around the Golgi complex. Cell Microbiol 10: 2012–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garmaroudi FS, Marchant D, Hendry R, Luo H, Yang D, et al. 2015. Coxsackievirus B3 replication and pathogenesis. Future Microbiol 10: 629–53 [DOI] [PubMed] [Google Scholar]
- 9.Sandoval IV, Carrasco L. 1997. Poliovirus infection and expression of the poliovirus protein 2B provoke the disassembly of the Golgi complex, the organelle target for the antipoliovirus drug Ro-090179. Journal of Virology 71: 4679–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roulin PS, Lötzerich M, Torta F, Tanner LB, van Kuppeveld FJ, et al. 2014. Rhinovirus uses a phosphatidylinositol 4-phosphate/cholesterol counter-current for the formation of replication compartments at the ER-Golgi interface. Cell Host Microbe 16: 677–90 [DOI] [PubMed] [Google Scholar]
- 11.Wolff G, Melia CE, Snijder EJ, Bárcena M. 2020. Double-Membrane Vesicles as Platforms for Viral Replication. Trends in Microbiology 28: 1022–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hidalgo P, Pimentel A, Mojica-Santamaría D, von Stromberg K, Hofmann-Sieber H, et al. 2021. Evidence That the Adenovirus Single-Stranded DNA Binding Protein Mediates the Assembly of Biomolecular Condensates to Form Viral Replication Compartments. Viruses 13: 1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang L, Godinez WJ, Kim I-H, Tektonidis M, Lanerolle Pd, et al. 2011. Herpesviral replication compartments move and coalesce at nuclear speckles to enhance export of viral late mRNA. Proceedings of the National Academy of Sciences 108: E136–E44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cziepluch C, Lampel S, Grewenig A, Grund C, Lichter P, Rommelaere J. 2000. H-1 Parvovirus-Associated Replication Bodies: a Distinct Virus-Induced Nuclear Structure. Journal of Virology 74: 4807–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swindle CS, Zou N, Van Tine BA, Shaw GM, Engler JA, Chow LT. 1999. Human papillomavirus DNA replication compartments in a transient DNA replication system. Journal of Virology 73: 1001–09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoenen T, Shabman RS, Groseth A, Herwig A, Weber M, et al. 2012. Inclusion bodies are a site of ebolavirus replication. Journal of Virology 86: 11779–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolnik O, Stevermann L, Kolesnikova L, Becker S. 2015. Marburg virus inclusions: A virus-induced microcompartment and interface to multivesicular bodies and the late endosomal compartment. Eur J Cell Biol 94: 323–31 [DOI] [PubMed] [Google Scholar]
- 18.Nikolic J, Le Bars R, Lama Z, Scrima N, Lagaudrière-Gesbert C, et al. 2017. Negri bodies are viral factories with properties of liquid organelles. Nature Communications 8: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Qi X, Liang M, Li C, Cardona CJ, et al. 2014. Roles of viroplasm-like structures formed by nonstructural protein NSs in infection with severe fever with thrombocytopenia syndrome virus. FASEB J 28: 2504–16 [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Su JM, Samuel CE, Ma D. 2019. Measles virus forms inclusion bodies with properties of liquid organelles. Journal of Virology 93: e00948–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ringel M, Heiner A, Behner L, Halwe S, Sauerhering L, et al. 2019. Nipah virus induces two inclusion body populations: Identification of novel inclusions at the plasma membrane. PLOS Pathogens 15: e1007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wadman M. 2018. The vaccine race : science, politics, and the human costs of defeating disease. New York, NY, Penguin Books, 2018 [Google Scholar]
- 23.Pavelka M, Roth J. 2010. Viral Inclusions. In Functional Ultrastructure: Atlas of Tissue Biology and Pathology, pp. 22–23. Vienna: Springer Vienna [Google Scholar]
- 24.Liu L. 2014. Fields Virology, 6th Edition. Clinical Infectious Diseases 59: 613–13 [Google Scholar]
- 25.Reissig M, Howes DW, Melnick JL. 1956. Sequence of morphological changes in epithelial cell cultures infected with poliovirus. Journal of Experimental Medicine 104: 289–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robbins FC, Enders JF, Weller TH. 1950. Cytopathogenic effect of poliomyelitis viruses in vitro on, human embryonic tissues. Proc Soc Exp Biol Med 75: 370–4 [DOI] [PubMed] [Google Scholar]
- 27.Netherton C, Moffat K, Brooks E, Wileman T. 2007. A guide to viral inclusions, membrane rearrangements, factories, and viroplasm produced during virus replication. Adv Virus Res 70: 101–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinkerton H. 1950. The morphology of viral inclusions and their practical importance in the diagnosis of human disease. American Journal of Clinical Pathology 20: 201–7 [DOI] [PubMed] [Google Scholar]
- 29.Wileman T. 2007. Aggresomes and pericentriolar sites of virus assembly: Cellular defense or viral design? Annual Review of Microbiology 61: 149–67 [DOI] [PubMed] [Google Scholar]
- 30.Dolnik O, Gerresheim GK, Biedenkopf N. 2021. New perspectives on the biogenesis of viral inclusion bodies in negative-sense RNA virus infections. Cells 10: 1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Etibor TA, Yamauchi Y, Amorim MJ. 2021. Liquid biomolecular condensates and viral lifecycles: Review and perspectives. Viruses 13: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez N, Camporeale G, Salgueiro M, Borkosky SS, Visentin A, et al. 2021. Deconstructing virus condensation. PLoS Pathog 17: e1009926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nevers Q, Albertini AA, Lagaudriere-Gesbert C, Gaudin Y. 2020. Negri bodies and other virus membrane-less replication compartments. Biochim Biophys Acta Mol Cell Res 1867: 118831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su JM, Wilson MZ, Samuel CE, Ma D. 2021. Formation and function of liquid-like viral factories in negative-sense single-stranded RNA virus infections. Viruses 13: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi J-M, Holehouse AS, Pappu RV. 2020. Physical principles underlying the complex biology of intracellular phase transitions. Annual Review of Biophysics 49: 107–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergeron-Sandoval L-P, Kumar S, Heris HK, Chang CLA, Cornell CE, et al. 2021. Endocytic proteins with prion-like domains form viscoelastic condensates that enable membrane remodeling. Proceedings of the National Academy of Sciences 118: e2113789118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanders DW, Kedersha N, Lee DSW, Strom AR, Drake V, et al. 2020. Competing protein-RNA interaction networks control multiphase intracellular organization. Cell 181: 306–24.e28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jawerth L, Fischer-Friedrich E, Saha S, Wang J, Franzmann T, et al. 2020. Protein condensates as aging Maxwell fluids. Science 370: 1317–23 [DOI] [PubMed] [Google Scholar]
- 39.Hyman AA, Weber CA, Jülicher F. 2014. Liquid-liquid phase separation in biology. Annual Review of Cell and Developmental Biology 30: 39–58 [DOI] [PubMed] [Google Scholar]
- 40.Dignon GL, Best RB, Mittal J. 2020. Biomolecular Phase Separation: From molecular driving forces to macroscopic properties. Annu Rev Phys Chem 71: 53–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyon AS, Peeples WB, Rosen MK. 2021. A framework for understanding the functions of biomolecular condensates across scales. Nature Reviews Molecular Cell Biology 22: 215–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chong PA, Forman-Kay JD. 2016. Liquid-liquid phase separation in cellular signaling systems. Curr Opin Struct Biol 41: 180–86 [DOI] [PubMed] [Google Scholar]
- 43.Alberti S. 2017. Phase separation in biology. Curr Biol 27: R1097–R102 [DOI] [PubMed] [Google Scholar]
- 44.Alberti S, Dormann D. 2019. Liquid-liquid phase separation in disease. Annu Rev Genet 53:171–194 [DOI] [PubMed] [Google Scholar]
- 45.Andre AAM, Spruijt E. 2020. Liquid-liquid phase separation in crowded environments. Int J Mol Sci 21:5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banani SF, Lee HO, Hyman AA, Rosen MK. 2017. Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 18: 285–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li P, Banjade S, Cheng HC, Kim S, Chen B, et al. 2012. Phase transitions in the assembly of multivalent signalling proteins. Nature 483: 336–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oltsch F KA, Julicher F, Hyman AA, Zechner C. 2019. Phase separation provides a mechanism to reduce noise in cells. bioRxiv 524231. [DOI] [PubMed] [Google Scholar]
- 49.Martin EW, Holehouse AS, Peran I, Farag M, Incicco JJ, et al. 2020. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science 367: 694–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin EW, Holehouse AS. 2020. Intrinsically disordered protein regions and phase separation: sequence determinants of assembly or lack thereof. Emerg Top Life Sci 4: 307–29 [DOI] [PubMed] [Google Scholar]
- 51.Lin Y, Protter DS, Rosen MK, Parker R. 2015. Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol Cell 60: 208–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Na Z, Luo Y, Cui DS, Khitun A, Smelyansky S, et al. 2021. Phosphorylation of a human microprotein promotes dissociation of biomolecular condensates. J Am Chem Soc 143: 12675–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dao TP, Kolaitis RM, Kim HJ, O'Donovan K, Martyniak B, et al. 2018. Ubiquitin modulates liquid-liquid phase separation of UBQLN2 via disruption of multivalent interactions. Mol Cell 69: 965–78 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han D, Longhini AP, Zhang X, Hoang V, Wilson MZ, Kosik KS. 2022. Dynamic assembly of the mRNA m6A methyltransferase complex is regulated by METTL3 phase separation. PLOS Biology 20: e3001535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, et al. 2015. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162: 1066–77 [DOI] [PubMed] [Google Scholar]
- 56.Wheeler JR, Matheny T, Jain S, Abrisch R, Parker R. 2016. Distinct stages in stress granule assembly and disassembly. eLife 5: e18413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo Y, Na Z, Slavoff SA. 2018. P-Bodies: Composition, properties, and functions. Biochemistry 57: 2424–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu H, Lu S, Gasior K, Singh D, Vazquez-Sanchez S, et al. 2021. HSP70 chaperones RNA-free TDP-43 into anisotropic intranuclear liquid spherical shells. Science 371: eabb4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel A, Malinovska L, Saha S, Wang J, Alberti S, et al. 2017. ATP as a biological hydrotrope. Science 356: 753–56 [DOI] [PubMed] [Google Scholar]
- 60.Boczek EE, Fürsch J, Niedermeier ML, Jawerth L, Jahnel M, et al. 2021. HspB8 prevents aberrant phase transitions of FUS by chaperoning its folded RNA-binding domain. eLife 10: e69377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lahaye X, Vidy A, Pomier C, Obiang L, Harper F, et al. 2009. Functional characterization of Negri bodies (NBs) in rabies virus-infected cells: Evidence that NBs are sites of viral transcription and replication. J Virol 83: 7948–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nikolic J, Le Bars R, Lama Z, Scrima N, Lagaudriere-Gesbert C, et al. 2017. Negri bodies are viral factories with properties of liquid organelles. Nat Commun 8: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heinrich BS, Cureton DK, Rahmeh AA, Whelan SP. 2010. Protein expression redirects vesicular stomatitis virus RNA synthesis to cytoplasmic inclusions. PLoS Pathog 6: e1000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heinrich BS, Maliga Z, Stein DA, Hyman AA, Whelan SPJ. 2018. Phase Transitions Drive the Formation of Vesicular Stomatitis Virus Replication Compartments. mBio 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kurtzke JF. 1956. Inclusion body encephalitis. A Nonfatal Case 6: 371–71 [DOI] [PubMed] [Google Scholar]
- 66.Guseva S, Milles S, Jensen MR, Schoehn G, Ruigrok RW, Blackledge M. 2020. Structure, dynamics and phase separation of measles virus RNA replication machinery. Curr Opin Virol 41: 59–67 [DOI] [PubMed] [Google Scholar]
- 67.Guseva S, Milles S, Jensen MR, Salvi N, Kleman JP, et al. 2020. Measles virus nucleo- and phosphoproteins form liquid-like phase-separated compartments that promote nucleocapsid assembly. Sci Adv 6: eaaz7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carlos TS, Young DF, Schneider M, Simas JP, Randall RE. 2009. Parainfluenza virus 5 genomes are located in viral cytoplasmic bodies whilst the virus dismantles the interferon-induced antiviral state of cells. J Gen Virol 90: 2147–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Y, Zhang C, Lu N, Deng X, Zang G, et al. 2019. Involvement of actin-regulating factor cofilin in the inclusion body formation and RNA synthesis of human parainfluenza virus type 3 via interaction with the nucleoprotein. Front Microbiol 10: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang S, Jiang Y, Cheng Q, Zhong Y, Qin Y, Chen M. 2017. Inclusion body fusion of human parainfluenza virus type 3 regulated by acetylated alpha-tubulin enhances viral replication. J Virol 91: e01802–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ringel M, Heiner A, Behner L, Halwe S, Sauerhering L, et al. 2019. Nipah virus induces two inclusion body populations: Identification of novel inclusions at the plasma membrane. PLoS Pathog 15: e1007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galloux M, Risso-Ballester J, Richard CA, Fix J, Rameix-Welti MA, Eleouet JF. 2020. Minimal elements required for the formation of respiratory syncytial virus cytoplasmic inclusion bodies in vivo and in vitro. mBio 11: e01202–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rincheval V, Lelek M, Gault E, Bouillier C, Sitterlin D, et al. 2017. Functional organization of cytoplasmic inclusion bodies in cells infected by respiratory syncytial virus. Nat Commun 8: 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Derdowski A, Peters TR, Glover N, Qian R, Utley TJ, et al. 2008. Human metapneumovirus nucleoprotein and phosphoprotein interact and provide the minimal requirements for inclusion body formation. J Gen Virol 89: 2698–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cifuentes-Munoz N, Branttie J, Slaughter KB, Dutch RE. 2017. Human metapneumovirus induces formation of inclusion bodies for efficient genome replication and transcription. J Virol 91: e01282–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boggs KB, Cifuentes-Munoz N, Edmonds K, El Najjar F, Ossandon C, et al. 2021. Human metapneumovirus P protein independently drives phase separation and recruits N protein to liquid-like inclusion bodies. bioRxiv 021.09.24.461765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miyake T, Farley CM, Neubauer BE, Beddow TP, Hoenen T, et al. 2020. Ebola virus inclusion body formation and RNA synthesis are controlled by a novel domain of nucleoprotein interacting with VP35. Journal of Virology 94: e02100–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoenen T, Shabman RS, Groseth A, Herwig A, Weber M, et al. 2012. Inclusion bodies are a site of ebolavirus replication. Journal of Virology 86: 11779–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmidt ML, Hoenen T. 2017. Characterization of the catalytic center of the Ebola virus L polymerase. PLOS Neglected Tropical Diseases 11: e0005996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Papa G, Borodavka A, Desselberger U. 2021. Viroplasms: Assembly and functions of rotavirus replication factories. Viruses 13: 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Criglar JM, Crawford SE, Estes MK. 2021. Plasmid-based reverse genetics for probing phosphorylation-dependent viroplasm formation in rotaviruses. Virus Res 291: 198193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eichwald C, Arnoldi F, Laimbacher AS, Schraner EM, Fraefel C, et al. 2012. Rotavirus viroplasm fusion and perinuclear localization are dynamic processes requiring stabilized microtubules. PloS ONE 7: e47947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Criglar JM, Crawford SE, Zhao B, Smith HG, Stossi F, et al. 2020. A genetically engineered rotavirus NSP2 phosphorylation mutant impaired in viroplasm formation and replication shows an early interaction between vNSP2 and cellular lipid droplets. Journal of Virology 94: e00972–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carlson CR, Asfaha JB, Ghent CM, Howard CJ, Hartooni N, et al. 2020. Phosphoregulation of Phase Separation by the SARS-CoV-2 N protein suggests a biophysical basis for its dual functions. Mol Cell 80: 1092–103 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cubuk J, Alston JJ, Incicco JJ, Singh S, Stuchell-Brereton MD, et al. 2021. The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA. Nat Commun 12: 1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iserman C, Roden CA, Boerneke MA, Sealfon RSG, McLaughlin GA, et al. 2020. Genomic RNA elements drive phase separation of the SARS-CoV-2 nucleocapsid. Mol Cell 80: 1078–91 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jack A, Ferro LS, Trnka MJ, Wehri E, Nadgir A, et al. 2021. SARS-CoV-2 nucleocapsid protein forms condensates with viral genomic RNA. PLoS Biol 19: e3001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu S, Ye Q, Singh D, Cao Y, Diedrich JK, et al. 2021. The SARS-CoV-2 nucleocapsid phosphoprotein forms mutually exclusive condensates with RNA and the membrane-associated M protein. Nat Commun 12: 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perdikari TM, Murthy AC, Ryan VH, Watters S, Naik MT, Fawzi NL. 2020. SARS-CoV-2 nucleocapsid protein phase-separates with RNA and with human hnRNPs. EMBO J 39: e106478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu C, Qavi AJ, Hachim A, Kavian N, Cole AR, et al. 2021. Characterization of SARS-CoV-2 nucleocapsid protein reveals multiple functional consequences of the C-terminal domain. iScience 24: 102681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Monette A, Niu M, Chen L, Rao S, Gorelick RJ, Mouland AJ. 2020. Pan-retroviral nucleocapsid-mediated phase separation regulates genomic RNA positioning and trafficking. Cell Rep 31: 107520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Metrick CM, Koenigsberg AL, Heldwein EE. 2020. Conserved outer tegument component UL11 from herpes simplex virus 1 is an intrinsically disordered, RNA-binding protein. mBio 11: e00810–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Castagné N, Barbier A, Bernard J, Rezaei H, Huet JC, et al. 2004. Biochemical characterization of the respiratory syncytial virus P-P and P-N protein complexes and localization of the P protein oligomerization domain. J Gen Virol 85: 1643–53 [DOI] [PubMed] [Google Scholar]
- 94.Su Z, Wu C, Shi L, Luthra P, Pintilie GD, et al. 2018. Electron cryo-microscopy structure of Ebola virus nucleoprotein reveals a mechanism for nucleocapsid-like assembly. Cell 172: 966–78.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leung Daisy W, Borek D, Luthra P, Binning Jennifer M, Anantpadma M, et al. 2015. An intrinsically disordered peptide from Ebola virus VP35 controls viral RNA synthesis by modulating nucleoprotein-RNA interactions. Cell Reports 11: 376–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bankamp B, Horikami SM, Thompson PD, Huber M, Billeter M, Moyer SA. 1996. Domains of the measles virus N protein required for binding to P protein and self-assembly. Virology 216: 272–7 [DOI] [PubMed] [Google Scholar]
- 97.Tawar RG, Duquerroy S, Vonrhein C, Varela PF, Damier-Piolle L, et al. 2009. Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. Science 326: 1279–83 [DOI] [PubMed] [Google Scholar]
- 98.Pyle JD, Whelan SPJ, Bloyet LM. 2021. Structure and function of negative-strand RNA virus polymerase complexes. Enzymes 50: 21–78 [DOI] [PubMed] [Google Scholar]
- 99.Luthra P, Jordan DS, Leung DW, Amarasinghe GK, Basler CF. 2015. Ebola virus VP35 interaction with dynein LC8 regulates viral RNA synthesis. J Virol 89: 5148–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chanthamontri CK, Jordan DS, Wang W, Wu C, Lin Y, et al. 2019. The Ebola viral protein 35 N-terminus is a parallel tetramer. Biochemistry 58: 657–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bloyet LM, Schramm A, Lazert C, Raynal B, Hologne M, et al. 2019. Regulation of measles virus gene expression by P protein coiled-coil properties. Sci Adv 5: eaaw3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nilsson-Payant BE, Blanco-Melo D, Uhl S, Escudero-Pérez B, Olschewski S, et al. 2021. Reduced nucleoprotein availability impairs negative-sense RNA virus replication and promotes host recognition. Journal of Virology 95: e02274–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fearns R, Peeples ME, Collins PL. 1997. Increased expression of the N Protein of respiratory syncytial virus stimulates minigenome replication but does not alter the balance between the synthesis of mRNA and antigenome. Virology 236: 188–201 [DOI] [PubMed] [Google Scholar]
- 104.Cressey TN, Noton SL, Nagendra K, Braun MR, Fearns R. 2018. Mechanism for de novo initiation at two sites in the respiratory syncytial virus promoter. Nucleic Acids Res 46: 6785–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lifland AW, Jung J, Alonas E, Zurla C, Crowe JE Jr., Santangelo PJ. 2012. Human respiratory syncytial virus nucleoprotein and inclusion bodies antagonize the innate immune response mediated by MDA5 and MAVS. J Virol 86: 8245–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sankaranarayanan M, Emenecker RJ, Wilby EL, Jahnel M, Trussina I, et al. 2021. Adaptable P body physical states differentially regulate bicoid mRNA storage during early Drosophila development. Dev Cell 56: 2886–901 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nikolic J, Civas A, Lama Z, Lagaudriere-Gesbert C, Blondel D. 2016. Rabies virus infection induces the formation of stress granules closely connected to the viral factories. PLoS Pathog 12: e1005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bloyet LM, Welsch J, Enchery F, Mathieu C, de Breyne S, et al. 2016. HSP90 chaperoning in addition to phosphoprotein required for folding but not for supporting enzymatic activities of measles and Nipah virus L polymerases. J Virol 90: 6642–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Munday DC, Wu W, Smith N, Fix J, Noton SL, et al. 2015. Interactome analysis of the human respiratory syncytial virus RNA polymerase complex identifies protein chaperones as important cofactors that promote L-protein stability and RNA synthesis. J Virol 89: 917–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Katoh H, Kubota T, Nakatsu Y, Tahara M, Kidokoro M, Takeda M. 2017. Heat shock protein 90 ensures efficient mumps virus replication by assisting with viral polymerase complex formation. J Virol 91: e02220–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jobe F, Simpson J, Hawes P, Guzman E, Bailey D. 2020. Respiratory syncytial virus sequesters NF-kappaB subunit p65 to cytoplasmic inclusion bodies to inhibit innate immune signaling. J Virol 94: e01380–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alenquer M, Vale-Costa S, Etibor TA, Ferreira F, Sousa AL, Amorim MJ. 2019. Influenza A virus ribonucleoproteins form liquid organelles at endoplasmic reticulum exit sites. Nat Commun 10: 1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cifuentes-Munoz N, Dutch RE, Cattaneo R. 2018. Direct cell-to-cell transmission of respiratory viruses: The fast lanes. PLoS Pathog 14: e1007015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.El Najjar F, Cifuentes-Muñoz N, Chen J, Zhu H, Buchholz UJ, et al. 2016. Human metapneumovirus Induces Reorganization of the Actin Cytoskeleton for Direct Cell-to-Cell Spread. PLoS Pathog 12: e1005922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Novoa RR, Calderita G, Cabezas P, Elliott RM, Risco C. 2005. Key Golgi factors for structural and functional maturation of bunyamwera virus. Journal of Virology 79: 10852–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barbosa NS, Mendonça LR, Dias MVS, Pontelli MC, da Silva EZM, et al. 2018. ESCRT machinery components are required for Orthobunyavirus particle production in Golgi compartments. PLoS Pathog 14: e1007047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Z, Guo D, Qin Y, Chen M. 2019. PI4KB on inclusion bodies formed by ER membrane remodeling facilitates replication of human parainfluenza virus type 3. Cell Reports 29: 2229–42.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kawai T, Akira S. 2006. Innate immune recognition of viral infection. Nature Immunology 7: 131–37 [DOI] [PubMed] [Google Scholar]
- 119.Chatterjee S, Basler CF, Amarasinghe GK, Leung DW. 2016. Molecular mechanisms of innate immune inhibition by non-segmented negative-sense RNA viruses. Journal of Molecular Biology 428: 3467–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ménager P, Roux P, Mégret F, Bourgeois JP, Le Sourd AM, et al. 2009. Toll-like receptor 3 (TLR3) plays a major role in the formation of rabies virus Negri Bodies. PLoS Pathog 5: e1000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fearns R, Young DF, Randall RE. 1994. Evidence that the paramyxovirus simian virus 5 can establish quiescent infections by remaining inactive in cytoplasmic inclusion bodies. J Gen Virol 75 ( Pt 12): 3525–39 [DOI] [PubMed] [Google Scholar]
- 122.Nilsson-Payant BE, Blanco-Melo D, Uhl S, Escudero-Perez B, Olschewski S, et al. 2021. Reduced Nucleoprotein Availability Impairs Negative-Sense RNA Virus Replication and Promotes Host Recognition. J Virol 95: e02274–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xue M, Zhao BS, Zhang Z, Lu M, Harder O, et al. 2019. Viral N(6)-methyladenosine upregulates replication and pathogenesis of human respiratory syncytial virus. Nat Commun 10: 4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lu M, Zhang Z, Xue M, Zhao BS, Harder O, et al. 2020. N(6)-methyladenosine modification enables viral RNA to escape recognition by RNA sensor RIG-I. Nat Microbiol 5: 584–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lu M, Xue M, Wang HT, Kairis EL, Ahmad S, et al. 2021. Nonsegmented negative-sense RNA viruses utilize N (6)-Methyladenosine (m(6)A) as a common strategy to evade host innate immunity. J Virol 95: e02274–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brzozka K, Finke S, Conzelmann KK. 2006. Inhibition of interferon signaling by rabies virus phosphoprotein P: activation-dependent binding of STAT1 and STAT2. J Virol 80: 2675–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vidy A, Chelbi-Alix M, Blondel D. 2005. Rabies virus P protein interacts with STAT1 and inhibits interferon signal transduction pathways. J Virol 79: 14411–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brzozka K, Finke S, Conzelmann KK. 2005. Identification of the rabies virus alpha/beta interferon antagonist: phosphoprotein P interferes with phosphorylation of interferon regulatory factor 3. J Virol 79: 7673–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hong Y, Bai M, Qi X, Li C, Liang M, et al. 2019. Suppression of the IFN-alpha and -beta Induction through Sequestering IRF7 into Viral Inclusion Bodies by Nonstructural Protein NSs in Severe Fever with Thrombocytopenia Syndrome Bunyavirus Infection. J Immunol 202: 841–56 [DOI] [PubMed] [Google Scholar]
- 130.Feng Z, Cerveny M, Yan Z, He B. 2007. The VP35 protein of Ebola virus inhibits the antiviral effect mediated by double-stranded RNA-dependent protein kinase PKR. J Virol 81: 182–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hume A, Muhlberger E. 2018. Marburg virus viral protein 35 inhibits protein kinase R activation in a cell type-specific manner. J Infect Dis 218: S403–S08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fricke J, Koo LY, Brown CR, Collins PL. 2013. p38 and OGT sequestration into viral inclusion bodies in cells infected with human respiratory syncytial virus suppresses MK2 activities and stress granule assembly. J Virol 87: 1333–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nelson EV, Schmidt KM, Deflube LR, Doganay S, Banadyga L, et al. 2016. Ebola virus does not induce stress granule formation during infection and sequesters stress granule proteins within viral inclusions. J Virol 90: 7268–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ning YJ, Wang M, Deng M, Shen S, Liu W, et al. 2014. Viral suppression of innate immunity via spatial isolation of TBK1/IKKepsilon from mitochondrial antiviral platform. J Mol Cell Biol 6: 324–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Na Z, Luo Y, Cui DS, Khitun A, Smelyansky S, et al. 2021. Phosphorylation of a human microprotein promotes dissociation of biomolecular condensates. Journal of the American Chemical Society 143: 12675–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Khairil Anuar INA, Banerjee A, Keeble AH, Carella A, Nikov GI, Howarth M. 2019. Spy&Go purification of SpyTag-proteins using pseudo-SpyCatcher to access an oligomerization toolbox. Nature Communications 10: 1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bracha D, Walls MT, Wei M-T, Zhu L, Kurian M, et al. 2018. Mapping local and global liquid phase behavior in living cells using photo-oligomerizable seeds. Cell 175: 1467–80.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Garabedian MV, Wang W, Dabdoub JB, Tong M, Caldwell RM, et al. 2021. Designer membraneless organelles sequester native factors for control of cell behavior. Nature Chemical Biology 17: 998–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hastings RL, Boeynaems S. 2021. Designer condensates: A toolkit for the biomolecular architect. Journal of Molecular Biology 433: 166837. [DOI] [PubMed] [Google Scholar]
- 140.Subach FV, Subach OM, Gundorov IS, Morozova KS, Piatkevich KD, et al. 2009. Monomeric fluorescent timers that change color from blue to red report on cellular trafficking. Nature Chemical Biology 5: 118–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Youn JY, Dyakov BJA, Zhang J, Knight JDR, Vernon RM, et al. 2019. Properties of stress granule and P-body proteomes. Mol Cell 76: 286–94 [DOI] [PubMed] [Google Scholar]
- 142.Hung V, Udeshi ND, Lam SS, Loh KH, Cox KJ, et al. 2016. Spatially resolved proteomic mapping in living cells with the engineered peroxidase APEX2. Nature Protocols 11: 456–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Martell JD, Deerinck TJ, Lam SS, Ellisman MH, Ting AY. 2017. Electron microscopy using the genetically encoded APEX2 tag in cultured mammalian cells. Nature Protocols 12: 1792–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de Boer P, Hoogenboom JP, Giepmans BNG. 2015. Correlated light and electron microscopy: ultrastructure lights up! Nature Methods 12: 503–13 [DOI] [PubMed] [Google Scholar]
- 145.Wan W, Briggs JAG. 2016. Chapter Thirteen - Cryo-Electron Tomography and Subtomogram Averaging. In Methods in Enzymology, ed. Crowther RA pp. 329–67: Academic Press; [DOI] [PubMed] [Google Scholar]
- 146.Randall RE, Griffin DE. 2017. Within host RNA virus persistence: mechanisms and consequences. Curr Opin Virol 23: 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Young DF, Wignall-Fleming EB, Busse DC, Pickin MJ, Hankinson J, et al. 2019. The switch between acute and persistent paramyxovirus infection caused by single amino acid substitutions in the RNA polymerase P subunit. PLoS Pathog 15: e1007561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Risso-Ballester J, Galloux M, Cao J, Le Goffic R, Hontonnou F, et al. 2021. A condensate-hardening drug blocks RSV replication in vivo. Nature 595: 596–99 [DOI] [PubMed] [Google Scholar]
- 149.Carey JL, Guo L. 2022. Liquid-liquid phase separation of TDP-43 and FUS in physiology and pathology ofnNeurodegenerative diseases. Front Mol Biosci 9: 826719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boyko S, Surewicz WK. 2022. Tau liquid-liquid phase separation in neurodegenerative diseases. Trends Cell Biol S0962-8924(22)00026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]