Abstract
Objective
This study aimed to identify the physicochemical and phenotypic characteristics of circulating Extracellular Vesicles (EVs) in the plasma of patients with SLE, with or without Lupus Nephritis (LN), and their potential utility as disease biomarkers.
Methods
Plasma-circulating EVs were concentrated using differential centrifugation from adult female patients (n=38) who met the ‘American College of Rheumatology/European Alliance of Associations for Rheumatology 2019’ criteria for SLE diagnosis with (LN) or without LN (nLN), confirmed by renal biopsy. Controls (n=18) were healthy volunteers matched by gender and similar age. The structure, size and Energy Dispersion Spectrum (EDS) of EVs were observed by electron microscopy. The surface charge and size distribution were evaluated using dynamic light scattering. The counts and phenotype of EVs from patients (SLE-EVs) and controls (Ctrl-EVs) were obtained using flow cytometry. Non-parametric statistical tests and exploratory analysis of multiple variables were performed. The discriminatory power of some variables as potential biomarkers of the disease was also evaluated.
Results
Circulating EVs were heterogeneous in morphology and size, but SLE-EVs reached larger diameters than Ctrl-EVs (p<0.0001). Small SLE-EVs and large SLE-EVs were increased compared with Ctrl-EV (p<0.0001 and p<0.05, respectively). Likewise, patients with SLE (LN or nLN) had higher concentrations of large EVs compared with controls (p<0.001 and p<0.0001, respectively). SLE-EVs showed a different EDS (p<0.001) and were less electronegative (p<0.0001) than Ctrl-EVs. EV-CD45+, EV-CD14+ and EV-IgM+ were more frequent in patients with SLE compared with controls (p<0.001, p<0.05 and p<0.001, respectively). The concentrations of large EVs and EV-IgM+ allowed better discrimination of patients from controls.
Conclusions
Plasma-circulating EVs from patients with SLE with and without nephritis are increased in peripheral blood and have different physicochemical properties than controls. Characteristics of EVs such as larger size and the presence of IgM on the surface could help discriminate patients from controls.
Keywords: Lupus Erythematosus, Systemic; Lupus Nephritis; Autoimmunity
WHAT IS ALREADY KNOWN ON THIS TOPIC
SLE is considered one of the most challenging diseases in medicine. While some studies have examined the number and phenotypic characteristics of Extracellular Vesicles (EVs) in SLE, others have focused on understanding their cargo and how they contribute to the development of the disease. However, there is still a need for further exploration into the physiochemical properties of these EVs to gain a more comprehensive understanding of their role in SLE.
WHAT THIS STUDY ADDS
We demonstrate that SLE-EVs counts were increased in the plasma of patients with SLE, and they differ in their physicochemical characteristics compared with Ctrl-EVs.
We found that large EV-CD45+ and EV-CD14+ were increased in patients with SLE, and they are forming IgM immunocomplexes.
We identified that the concentrations of large EVs and EV-IgM+ allowed better discrimination of patients with SLE from controls.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our study proposes EVs as potential biomarkers for SLE by combining their phenotypic and physicochemical characterisation. It might act as an entry point for us to identify therapeutic target and explore new events in disease pathogenesis.
Introduction
A hallmark of SLE is the defective clearance of apoptotic bodies, which are considered the largest Extracellular Vesicles (EVs) and a major source of autoantigens.1 2 EVs are particles naturally secreted by cells, enclosed by a lipid bilayer, and they cannot replicate.3 Ranging in size from 30 nm to 5 µm in diameter, they are classified based on their biogenesis, composition and origin.4 EVs play pivotal roles in various physiological processes, can contribute to Immune Complexes (IC) formation, leading to systemic inflammation and tissue damage.5 6
Previous studies of EVs in the plasma of patients with SLE have indicated that they differ from healthy individuals in concentration levels, cellular origin and specific molecular composition including the existence of microRNA and autoantigenic cargoes like proteins and nucleic acids that contribute to IC formation.7,9 Furthermore, elevated levels of EVs have been detected in the urine of patients with lupus nephritis (LN),8,10 with specific EV subtypes, such as EV-HMGB1 positive.11 Although these characteristics have drawn interest in EVs as potential contributors to pathogenic processes in the context of SLE and LN,5,13 there remains a notable gap in our knowledge of their physical and biochemical composition, which is crucial for understanding their interaction with cells and the surrounding environment.8,15
Physicochemical properties can impact the mechanisms of interaction of EVs with membrane cells. In line with that, multiple approaches for its characterisation are recommended to use by the Minimal Information for Studies of Extracellular Vesicles (MISEV) guidelines including data on the physical characteristics, biochemical composition and/or descriptions of isolation conditions or cell of origin to define EV subtypes.3
Physical characteristics such as EV size can be evaluated by different techniques such as Electron Microscopy (EM) and Dynamic Light Scattering (DLS); the differences in size may reflect the vesicle itself or by the hard and soft corona of proteins, meaning that changes maybe influenced by alterations in the vesicle’s surface composition. These changes can impact the type of interaction and effect of these particles on other cells or their immunogenicity. Besides, other high-resolution techniques allow us to analyse the biochemical properties of EVs in more detail, including their Energy Dispersion Spectrum (EDS) and the electrical state of the sample.16 17 Zeta potential evaluates the EVs’ electric charge, and its change can affect its tendency to permeate membranes, with cationic particles generally displaying an easier interaction with cellular membranes or other structures and biomolecules.
In this work, we focus on the evaluation of physicochemical characteristics such as size, charge, opsonisation and the association of these with SLE and LN. We demonstrated that EVs are increased in patients with SLE, with large EVs mainly CD45+CD14+ and IgM+, and those characteristics help discriminating patients from healthy controls. Furthermore, SLE-EVs differ from Ctrl-EVs in their physicochemical properties, including elemental atomic distribution and surface charge. While this field is relatively unexplored in SLE patient samples, these findings suggest that plasma EV analysis could be valuable for looking biomarkers or to understand some pathogenic events in SLE disease.
Materials and methods
Research subjects
Peripheral blood samples were collected from a cohort of 38 women diagnosed with SLE who met the criteria established by the American College of Rheumatology/European Alliance of Associations for Rheumatology 2019 (ACR/EULAR 2019).18 Participants were recruited from ARTMEDICA and the Rheumatology Service at the ‘Hospital Universitario San Vicente Fundación’ in Medellín, Colombia. To assess disease activity in SLE, the Systemic Lupus Erythematosus Disease Activity Index 2K (SLEDAI) score was employed.19 Of these patients with SLE, 55% (n=21) presented with a history of LN as determined by biopsy. Additionally, blood samples were obtained from similar age and sex-matched healthy controls. All subjects did not show clinical signs of infections, and the control group had no history of cancer, diabetes, autoimmune diseases or ongoing immunosuppressive therapy. Participation in the study was voluntary, and all individuals provided informed consent approved by the Universidad de Antioquia’s Medical Research Institute.
Reagents and equipment
In online supplemental table 1, all detailed information about the reagents and equipment used is described.
Plasma sample collection and EV concentration
EVs were isolated from peripheral blood of patients with SLE (SLE-EVs) and healthy controls (Ctrl-EVs) following the MISEV 2018 guidelines.4 To control possible differences in handling, a phlebotomy-trained person collected all the samples from the same anatomical location, the left arm’s antecubital fossa with a 21-gauge needle. Samples were carefully transported within the first 20 min after collection in a biological samples transport container. Peripheral blood was collected in citrate tubes and EVs were concentrated by differential centrifugation protocol. Samples were centrifuged at 1800 g for 10 min in a Sorvall Legend microcentrifuge to separate plasma from cells. Subsequently, 4 mL of plasma was collected, followed by two spins at 3000 g for 10 min each to obtain platelet-poor plasma (PPP). PPP aliquots of 500 µL were then centrifuged at 17 000 g for 60 min. Afterwards, 480 µL of supernatant was removed, and vesicle pellet was supplemented with 100 µL of filtered Dulbecco’s phosphate-buffered saline (PBSfp). EVs pellets were stored at −70°C until further use. The freshly thawed EVs pellet was washed adding 1 mL of PBSfp and centrifuging it again at 17 000 g for 60 min. The resulting EVs pellet was resuspended in 1 mL of PBSfp for subsequent analysis.
Size distribution and surface charge
Concentrated plasma EVs size distribution, from 30 nm to 1 µm, was measured with DLS by using a Zetasizer Pro at room temperature (RT). The EVs intensity and frequency were analysed with the Gaussian function used to know the EVs diameter (nm) distribution. Briefly, before DLS measurements, each thawed sample was washed and diluted in Milli-Q water for size and ζ potential measurement to know the surface charge. The EVs were homogenised to disaggregate possible clumps. Then, 1 mL of each diluted sample was added to a cuvette Zeta DTS1070 to be evaluated. The analysis was conducted by operating at 633 nm and recording the backscattered light at an angle of 173°. The light scattering was recorded for 150 s, with three replicate measurements for each sample.
High-resolution microscopy
EVs were thawed at RT and were washed by adding 1 mL of 0.9% PBSfp (diluted with distilled water). Then, 60 µL of 1% glutaraldehyde was added, incubated at RT for 15 min and centrifuged for 1 hour at 16.000 g. Then, the supernatant was removed. Finally, 10 µL of the sample was placed onto Lacey carbon-coated grids and contrasted in 4% uranyl-acetate for 8 min at RT. Grids were immersed in distilled water 20 times to remove the excess sample and dried at RT. The EVs were visualised using Transmission Electron Microscopy mode (TEM) and Scanning Electron Microscopy (SEM) mode. Image processing was done consistently across all conditions and regions. EVs Energy Dispersive X-ray Spectroscopy spectrum (EDXS) was evaluated using SEM mode. The EVs were focused on a field of 150 000 magnification, and an electron beam was directed at different points on the surface of the EVs to stimulate the emission of X-rays typically using EDX detectors. The vesicle size was determined using ImageJ software.
Flow cytometry analysis
EVs were characterised by FSC-H and SSC-H parameters of the LSR Fortessa Cytometer and FACS DIVA software. EVs region was determined using mononuclear peripheral cells and reference polystyrene spheres of known sizes (0.5, 1 and 2 μm). PBSfp was used as buffer‐only control to quantify the contribution of background noise, to set the trigger threshold and to confirm whether the flow cytometer was clean. To prevent swarm detection, the sample was diluted before analysis and the flow rate was adjusted during acquisition, as recommended by van der Pol et al.20
To establish the origin of the EVs, control and patient samples were thawed and diluted in 1 mL of PBSfp, mixed and then diluted by half. Then, 200 µL of the diluted sample was used to label the EVs with the following fluorescently conjugated antibodies: CD45, CD105, CD235a, CD33, HLA-DR, IgM, IgG, CD63, CD14, HMGB1, CD16, C4d and annexin V in the presence of annexin binding buffer. The percentage of positive EVs for each surface molecule in every sample corresponded to the total counts detected inside the EVs size range using reference size spheres.
To estimate the number of EVs per sample, frozen aliquots were thawed at RT. Then, 20 μL of EVs from the same initial dilution used for phenotyping was acquired. The counts of EVs in every sample corresponded to the total counts detected inside the gating strategy used for determining EV size range using reference polystyrene spheres Gigamix (100–900 nm) with a lower limit defined by the electronic noise (according to the acquisition of PBSfp) and after the correction regarding the dilution used.
Flow cytometry data were analysed using FlowJo V.10.5.0 software, adhering to the guidelines outlined in ‘A compendium of single extracellular vesicle flow cytometry’.20
Statistical analysis
Comparisons between the two groups were conducted using the Mann-Whitney test to assess the differences in distributions statistically significant (p<0.05). The differentiation of two distributions was determined using the area under the curve (AUC) and Kolmogorov-Smirnov test, demonstrating a strong distinction between the groups with an AUC value of 0.85, which was statistically significant (p<0.01). Multiple comparisons were executed using the Kruskal-Wallis test, followed by Dunn’s post hoc test, indicating significant differences between the multiple subgroups (p<0.01), highlighting the statistical relevance of the observed variations. Correlations were assessed using Spearman’s rank correlation coefficient with a 95% CI, revealing a strong positive correlation (r=0.75, p<0.001) between the variables under study, signifying a statistically significant relationship.
The Receiver Operating Characteristic (ROC) curve, multivariate discriminant analysis and Principal Component Analysis (PCA) were performed using GraphPad Prism V.10.2 (GraphPad Software), with a likelihood ratio consistently exceeding 7.1, signifying robust predictive capabilities and statistical significance.
Results
Clinical and demographic characteristics of the patients
This study included 38 female patients who met the ACR/EULAR 2019 criteria for SLE. 18 women were selected as a control group, with ages comparable to those of the patients and without a history of autoimmune diseases. The average age of the patients was 33.4±11 years, with a mean SLEDAI of 6.9±4.8. 55% of the patients had a histological diagnosis of LN. Clinical characteristics can be found in online supplemental table 2. Paraclinical analyses performed at least 30 days before sampling showed that 39% of patients were anti-DNA positive, 44% had C3 hypocomplementaemia and 32% had low C4 levels, as detailed in table 1.
Table 1. Sociodemographic and clinical characteristics of the patients.
| Variables | Controlsn=28 | Patientsn=38 | P value |
| Age, years (mean±SD) | 31.93±9.16 | 33.44±11.14 | 0.563* |
| Gender, female, n (%) | 28 (100) | 38 (100) | 1† |
| Weight, kg (mean±SD) | 60.31±13.56 | 60.97±13.44 | 0.6906* |
| BMI, kg/m2 (mean±SD) | 23.17±3.9 | 24.29±6.8 | 0.9248* |
| SLEDAI (mean±SD) | – | 6.9±4.8 | – |
| Haematuria, n (%) | – | 7 (18) | – |
| Proteinuria, n (%) | – | 7 (18) | – |
| Anti-DNA, n (%) | – | 15 (39) | – |
| Thrombocytopenia, n (%) | – | 2 (5.2) | – |
| Leucopenia, n (%) | – | 2 (5.2) | – |
| Hypocomplementaemia | |||
| C3, n=30, n (%) | – | 17 (44) | – |
| C4, n=28, n (%) | – | 9 (32) | – |
| Lupus nephritis, n (%) | – | 21 (55) | – |
| Arthritis, n (%) | – | 8 (21) | – |
| Pyuria, n (%) | – | 5 (13) | – |
| Alopecia, n (%) | – | 3 (7) | – |
| Vasculitis, n (%) | – | 1 (2.6) | – |
| Pleuritis, n (%) | – | 1 (2.6) | – |
Mann-Whitney test.
Fisher’s exact probability test.
BMIBody Mass IndexSLEDAISystemic Lupus Erythematosus Disease Activity Index 2K
Plasma-circulating EVs from patients with SLE are heterogeneous in morphology and size
To know the size distribution and frequency of the EVs in each sample, the DLS technique was used, which works based on photon correlation spectroscopy to measure the diffusion constants of the particles (EVs) in Brownian motion and determine their hydrodynamic diameters.21 EVs were visualised by using TEM and Scanning Transmission Electron Microscopy (STEM) to confirm EVs morphology and the variability in size distribution according to the MISEV 2018 guidelines.4
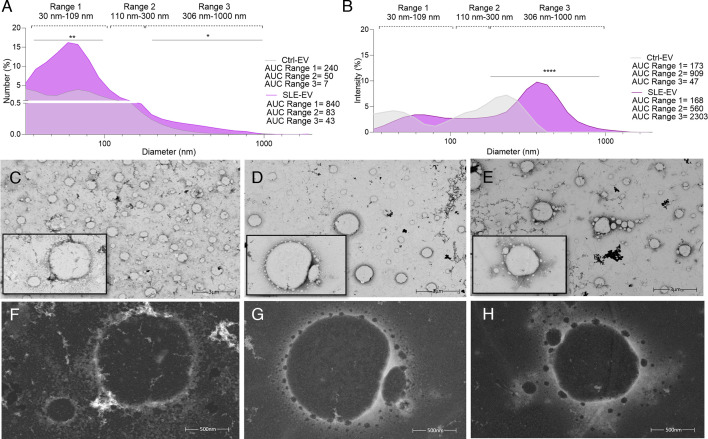
Plasma samples from patients (n=6) and controls (n=6) were analysed in a size range of 30 nm–1 µm, according to the guidelines defined by MISEV for EVs. EVs were classified into three subpopulations according to their diameter: range 1 (30–109 nm), range 2 (110–300 nm) and range 3 (306–1000 nm). Size distribution based on the relative number of EVs present in suspension showed an asymmetric distribution with an increase in the relative number of EVs in ranges 1 and 3 in the plasma of patients with SLE (figure 1A,B). Larger diameters were observed in the EVs of patients with SLE compared with the EVs of controls. Additionally, the intensity of light scattered by the EVs, which correlates with the translational diffusion coefficient and the molecular weight of the particles, showed that large EVs had higher scattered light intensity, possibly due to their size and/or the formation of aggregates.
Figure 1. The SLE-EVs are increased in the plasma with greater sizes than Ctrl-EVs. Figure shows Extracellular Vesicle (EV) hydrodynamic size distribution and representative micrographs of plasma-circulating EVs evaluated by high-resolution microscopy. (A, B) SLE-EVs (n=6) and Ctrl-EVs (n=6) hydrodynamic size by Dynamic Light Scattering (DLS). Each line represents the median of the relative percentages of EVs contained in various sizes based on the number and the intensity of scattered light. Horizontal dashed lines represent range 1 (diameter range 30–109 nm), range 2 (diameter range 110–300 nm) and range 3 (diameter range 306–1000 nm). (C) Ctrl-EVs and (D, E) SLE-EVs Transmission Electron Microscopy (TEM) visualisation at low 25.000X (3000 nm) magnification in bright field mode showed the vesicular shape and heterogeneous size distribution. (F) Ctrl-EVs and (G, H) SLE-EVs visualisation at high 100.000X (500 nm) magnification in high angular annular dark field (HAADF) mode showed large vesicles surrounded by little vesicles in active SLE patient samples. Kolmogorov-Smirnov test: *p<0.05, **p<0.001. AUC, area under the curve; Ctrl, control.
High-resolution EM is considered the optimal method to detect and characterise EVs.22 In this study, peripheral blood EVs from controls (n=2), and active SLE samples (n=3), considered as having an SLEDAI greater than 4, were thawed and observed by EM, as described in the Materials and methods section. Cup-shaped and round vesicles were identified (figure 1C–E), which confirmed the morphology previously described for these structures.23 EV samples from controls (figure 1C) and patients with SLE (figure 1D,E) showed heterogeneity in size, with diameters ranging from approximately 100 nm to 1000 nm (figure 1). In addition, visualisation by EM showed small vesicles surrounding larger ones in the photomicrographs obtained by using the ‘High Angular Annular Dark Field’ technique, which allows observation with higher atomic resolution (figure 1G,H). In summary, the analysis of the size distribution of EVs in the plasma by DLS and EM evidenced that EVs are increased in the frequency of small and large EVs in patients with SLE and the heterogeneity in the sizes was confirmed by EM.
EVs from patients with SLE present changes in their EDS and they are less electronegative compared with controls
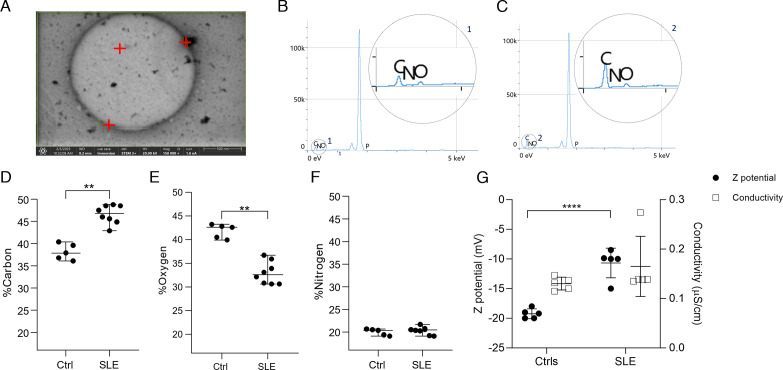
We found that SLE-EVs had more intensity of light scattered and some large EVs surrounded by small EVs were seen in SLE-EVs samples forming aggregates. Hence, these particles are heterogeneous, and they could have different properties in their chemical composition compared with the controls. To have an approach to the chemical characteristics of the EVs circulating in SLE, the EDS emitted by carbon, oxygen and nitrogen atoms in SLE was evaluated by EDXS. Three large EVs (500 nm–1 µm) per sample and at least three measurements were made in EVs considered as medium-large from patients with SLE (n=3) and controls (n=3) (figure 2A). The carbon (average, 46%) and oxygen (average, 33%) atoms present in SLE-EVs had a significantly different distribution related to Ctrl-EVs (carbon: average, 37%; oxygen: average, 43%) (figure 2B,C), while nitrogen did not have a different distribution between SLE-EVs (average, 22%) and Ctrl-EVs (average, 20%). Considering that the chemical composition of EVs varies depending on the activation state of the cell of origin,24 25 and that they can interact with the extracellular medium, for example, by forming immune complexes,26 the differences observed in the distribution spectrum of atoms in the context of SLE suggest that SLE-EVs have different chemical properties that could reflect indirectly their size complexity (large EVs). Also, it could affect their physicochemical properties such as surface charge and colloidal stability by altering their ability to interact with other structures and biomolecules as well as their biodistribution.27
Figure 2. Extracellular Vesicles (EVs) from patients present a different energy dispersion spectrum and are less electronegative than controls. Chemical characteristics of SLE-EVs and Ctrl-EVs by Energy Dispersive X-ray Spectroscopy (EDXS) and surface charge by Dynamic Light Scattering (DLS) evaluation. (A) Representative microphotograph of an EV at 150.000X magnification. Red marks indicate the points where EDXS analysis was performed. (B, C) Representative EDXS spectrum. Details 1 and 2 show the peaks for carbon, oxygen and nitrogen atoms in a control and patient sample, respectively. Two EVs per sample were evaluated and two or three points were analysed per EV; then, the mean of the lectures obtained per point was plotted. (D–F) Dot plots show the distribution (%) between carbon, oxygen and nitrogen atoms in each EV and the means of SLE-EV (n=3) and Ctrl-EV (n=3) individual measurements. (G) Charge surface of SLE-EVs (n=5) and Ctrl-EVs (n=5). SLE and control samples were analysed by DLS to obtain electrokinetic potential. The conductivity value was given by the instrument before the analysis to guarantee proper results. Each sample was analysed twice. T-test: ****p<0.0001. Mann-Whitney test: **p<0.00. Ctrls, controls; SLE, Systemic Lupus Erythematosus.
To know the electrical charge of the EVs the electrokinetic potential, or ζ potential; was measured as an indicator of the charge and colloidal stability of the surface of the EVs.28 To verify the absence of ions in the buffer suspension that could affect the analysis and ensure a more precise measurement of the ζ value deionized water without sample was analysed. Then, the EVs were thawed, washed and suspended in 1 µL of deionised water. The total sample volume was then analysed with the Zetasizer Nano, as described in the Materials and methods section. It was observed that the SLE-EVs (n=5) were less negative (mean, −9 mV) than Ctrl-EVs (n=5) (mean, −19 mV) (p<0.0001) (figure 2G). Considering that EVs are colloidal particles and their colloidal stability may depend on their electrical surface charge and size,28 the differences observed in atom distribution in large SLE-EVs compared with large Ctrl-EVs and the less electronegative surface charge may suggest that SLE-EVs have physicochemical properties such as a lower degree of electrostatic repulsion between adjacent particles compared with Ctrl-EVs that could influence their interactions.
Large EVs are increased in the plasma of patients with SLE and LN
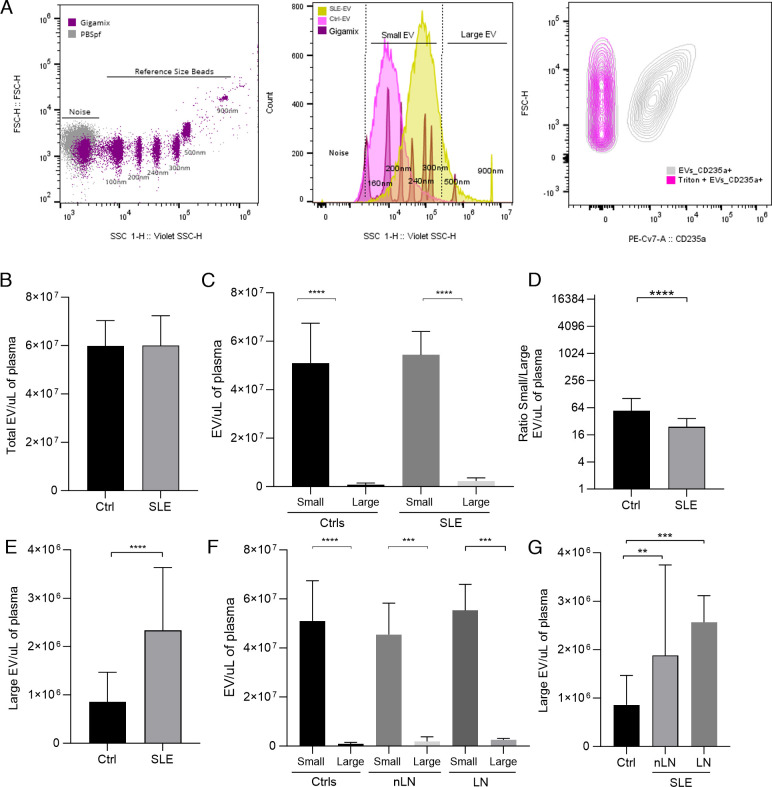
To determine the number of EVs in terms of number per millilitre of plasma, the highly sensitive flow cytometry (nano-Flow Cytometry, nFCM) was used, which allows the detection and counting of particles with a diameter up to 100 nm.20 Considering this and taking into account the limited sensitivity of the equipment to identify small particles,20 an analysis strategy similar to that of Mobarrez et al29 was used with reference beads of known size (Gigamix, 100–900 nm). Therefore, for the calculation of the EVs concentration in patients with SLE (n=38) and controls (n=18) (figure 3A), only those EVs considered small EVs (100–300 nm) and large EVs (500–900 nm) were included. Analysis of total EVs (100–900 nm) revealed no differences in concentration between SLE-EV and Ctrl-EV (p=0.790) (figure 3B). However, when comparing the concentrations of small EVs versus large EVs, it was observed that small EVs are more abundant than larger EVs in both control (median, 5.1×107 EV/µL of plasma) and patients (median, 5.4×107) (p<0.0001) (figure 3C), a finding consistent with what was initially observed by DLS (figure 1A). Furthermore, it was found that the population of larger EVs was higher in patients (median, 2.3×106 EV/µL of plasma) compared with controls (median, 858 000 EV/µL of plasma) (p<0.0001) (figure 3E), and the ratio of small/large EVs was lower in patients compared with controls (p<0.001) (figure 3D). Therefore, we show that patients with SLE have higher plasma concentrations of large EVs than controls.
Figure 3. Large Extracellular Vesicles (EVs) are increased in the plasma of patients with SLE and Lupus Nephritis (LN). EVs counts of SLE, LN and control samples by using nano-flow cytometry. (A) The left plot shows the gating strategy used to determine the EV region by using filtered Dulbecco’s phosphate-buffered saline (PBSfp) as background and Gigamix beads (size range 100–900 nm) as size reference region. The middle plot shows the region established for small (100–300 nm) and large EVs (300–900 nm) and representative histograms of a control (pink) and SLE patient (yellow) sample. The right plot shows that EVs are sensitive to detergent treatment. Representative CD235a versus FSC-H contour plot of EVs untreated and treated with 0.05% Triton X-100. CD235a positivity was rapidly altered after Triton X-100 addition. 20 µL of SLE-EVs and Ctrl-EVs were acquired by Cytoflex. (B) Barr diagram shows EVs counts in the total range of the reference beads. (C) Shows the small and large EVs counts by group. (D) Shows the small/large EV ratio and (E) shows the large EV counts for controls and SLE samples. (F) Shows the small and large EV counts by subgroups. (G) Shows the large EV counts for controls and SLE pateints with or without Lupus Nephritis. Ctrls (n=18); SLE (n=35); nLN (n=16); LN (n=19). Mann-Whitney test and Kruskal-Wallis test: **p<0.001, ***p<0.0001, ****p<0.00001. Ctrls, controls; LN, Lupus Nephritis; nLN, without Lupus Nephritis; SLE, Systemic Lupus Erythematosus.
We investigated whether there were differences in the concentration of EVs of different sizes between patients with LN (SLE-LN, n=18) and those without nephritis (SLE-nLN, n=15). Although an increase in large EVs concentrations was observed in both patients with LN (median, 2.5×106 EV/µL of plasma, p<0.0001) or nLN (median, 1.8×106 EV/µL of plasma, p<0.001) compared with controls (median, 8.5×105), no significant differences were found between patient groups. This suggests that there is an increase in the concentration of large EVs in the circulation of patients with SLE, but this increase is independent of the presence of LN.
In summary, the counts of EVs present in circulation using two populations separated by size through nFCM showed that patients with SLE, regardless of whether or not they present LN, had an increase in the number of large EVs when compared with the Ctrl-EVs (figure 3F,G).
Large EVs present in the plasma of patients with SLE come mainly from leucocytes and monocytes and form ICs
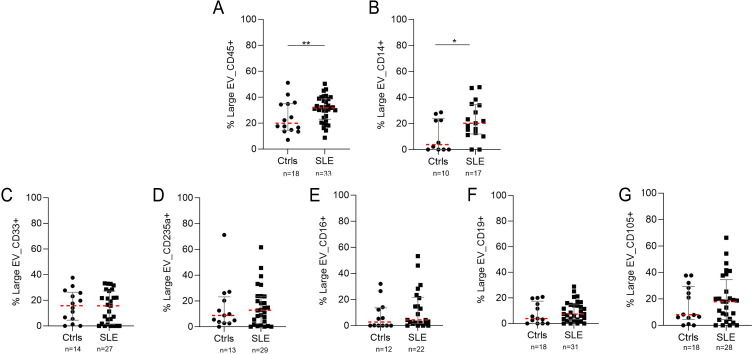
Considering that patients with SLE had an increase in the concentration of large EVs and that previous reports have shown increases in the number of EVs derived from platelets,8,12 we wanted to evaluate by flow cytometry the possible origin using cellular markers other than platelets (figure 4). Patients with SLE had a higher frequency of EV-CD45+ (median, 32%) compared with controls (median, 18%) (p<0.001) (figure 4A). Likewise, EV-CD14+ was more frequent in patients (median, 20%) than in controls (median, 5%) (p<0.05) (figure 4B). Although EVs were found positive for endothelial cell (CD105), B cell (CD19) or myeloid cell (CD33) surface markers, their number did not show differences between patients and controls (figure 4C–G). These results suggest that larger EVs are most frequently derived from leucocytes (EV-CD45+) and monocytes (EV-CD14+) in the plasma of patients with SLE.
Figure 4. Cellular markers surface expression in large extracellular vesicles (EVs) from the plasma of patients with lupus. Figure shows the percentage of EVs positive for (A) CD45, (B) CD14, (C) CD33, (D) CD235a, (E) CD16, (F) CD19 and (G) CD105. The plot shows the mean and IQR of SLE-EV (n=38) and Ctrl-EV (n=18) individual measurements. Each sample was analysed twice. Mann-Whitney test: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Ctrls, controls; SLE, Systemic Lupus Erythematosus.
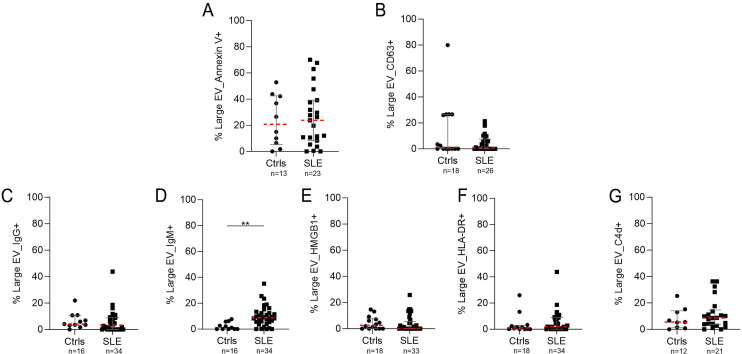
In addition to the possible cellular sources, the presence on the surface of some biomolecules with a role in the pathogenesis of SLE such as IgG, IgM, HMGB1, HLA-DR and C4d was evaluated (figure 5). Significant differences were found in the percentage of EV-IgG+ and EV-IgM+, and the latter was increased in patients with SLE (median, 10%) compared with controls (median, 2%) (p=0.001) (figure 5D). No differences were found for the presence of EVs positive for HMGB1, annexin V, HLA-DR and C4d between the groups (figure 5A,C–E).
Figure 5. Lupus-associated biomolecules in the surface of large extracellular vesicles (EVs) from the plasma of patients with lupus. Figure shows the percentage of EVs positive for (A) annexin V, (B) CD63, (C) IgG, (D) IgM, (E) HMGB1, (F) HLA-DR and (G) C4d. The plot shows the mean and IQR of SLE-EV (n=38) and Ctrl-EV (n=18) individual measurements. Each sample was analysed twice. Mann-Whitney test: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Ctrls, controls; SLE, Systemic Lupus Erythematosus.
The counts of large EVs and the percentage of EV-IgM+ allow patients with SLE to be discriminated against by control individuals
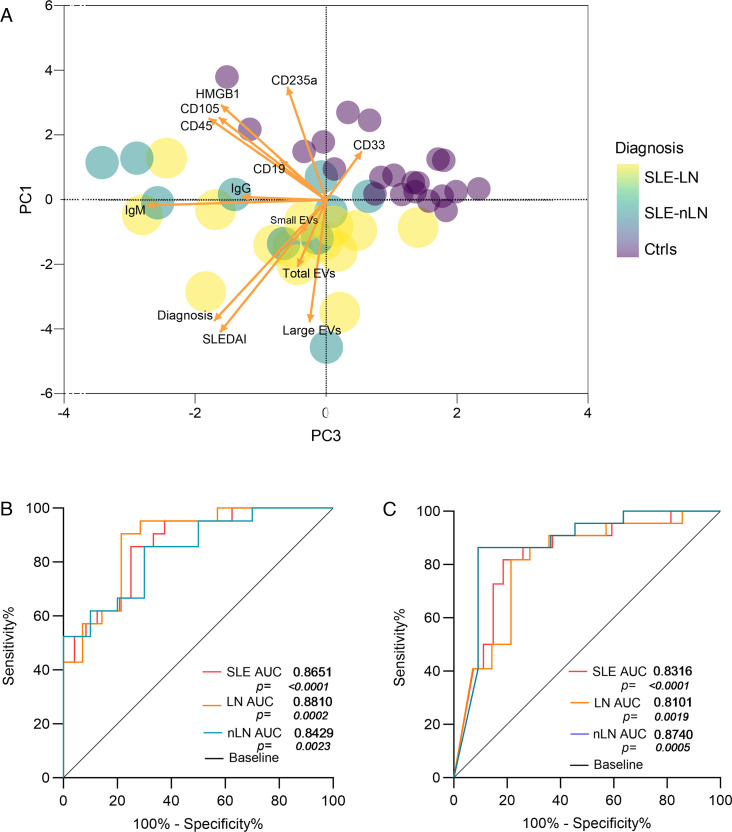
To evaluate whether some characteristics of EVs such as phenotype, associated molecules and counts help discriminate between groups of patients with SLE with or without nephritis and controls, we performed a correlation analysis between all the variables (not shown). The percentage of large EV-IgM+ and their number had a moderate correlation with SLEDAI (Spearman r=0.45–0.65, respectively). On the other hand, the PCA showed that the first three principal components PC1 (19.02%), PC2 (17.43%) and PC3 (15.92%) explained 52.38% of the variability. The variables that had the greatest influence on these components were SLEDAI, EV-IgM+ and the number of large EVs with the greatest capacity to discriminate patients with SLE from controls (figure 6A). For its part, the EVs characteristics were not able to discriminate patients with or without nephritis and active SLE from non-active SLE patients (online supplemental figure 1).
Figure 6. (A) Principal component analysis that relates the variables of Extracellular Vesicle (EV) size, phenotype and SLEDAI in patients with SLE without nephritis (SLE-nLN), Lupus Nephritis (SLE-LN) and controls (Ctrls). (B) Receiver operating characteristic (ROC) curve of large EV plasma concentrations for SLE, LN and nLN subjects. (C) ROC curve of EV-IgM+ for SLE, LN and nLN subjects. AUC, area under the curve; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index 2K.
Because the number of larger EVs and EV-IgM+ in the plasma of patients with SLE are characteristics with the capacity to discriminate patients and controls, we decided to evaluate their sensitivity and specificity for the diagnosis of SLE by constructing ROC curves (figure 6B,C). Regarding the number of large EVs, the AUC was statistically significant in the SLE group (AUC=0.8651, p<0.0001) and the cut-off point that showed the best discrimination was 1.8×106 EV/µL of plasma with sensitivity (S) of 87% and specificity (E) of 62% (figure 6B). Similarly, differences were observed in the AUC for EV-IgM+ in the SLE group (AUC=0.8316, p<0.0001), whose cut-off point of 7.9% showed better discrimination between SLE and controls with S of 82% and E of 62% (figure 6C). In this way, it was evidenced that the counts of larger EVs and EV-IgM+ have adequate capacity to discriminate patients with SLE from controls. Although the AUC of the EVs counts was larger in the SLE-LN group (AUC=0.8810, p<0.0002) and SLE-nLN group (AUC=0.8429, p<0.0023) (figure 6B); the AUC of EV-IgM+ from SLE-LN (AUC=0.8101, p<0.0019) and SLE-nLN (AUC=0.8740, p<0.0005) showed good performance to discriminate the LN and nLN groups from the controls, an adequate discriminatory capacity was not evident between SLE-LN and SLE-nLN (data not shown).
Discussion
In this study, we employed various high-resolution techniques to investigate the physicochemical and phenotypic characteristics of circulating EVs in the plasma of patients with SLE, proposing them as potential biomarkers for the disease. Our findings revealed that EVs exhibit heterogeneity in size, with an increase in both small and large EVs in the plasma of patients with SLE. Particularly, large EVs were found to be positive for CD45+, CD14+ and IgM+ markers.
EVs isolated from the plasma of patients with Rheumatoid Arthritis (RA) were described as predominantly spherical, heterogeneity in size and containing ICs in the majority of EVs.25 More recently, another study using STEM evaluated plasma and synovial fluid samples from patients with RA, demonstrating heterogeneity in EVs sizes, in patients positive for rheumatoid factor and anticitrulline antibodies.30 Other studies in primary Sjögren syndrome31 and COVID+32 have also reported differences in the EVs size from plasma patient samples. Furthermore, in vitro inflammation conditions have shown the release of different sizes of EVs compared with non-inflammatory conditions.33 34 In line with those reports, our DLS and EM analysis showed size heterogeneity in plasma-circulating EVs from patients with SLE and controls. We classified the EVs into three subsets based on size by DLS. This strategy showed an increasing frequency of small and large circulating EVs in patients with SLE, and the size-based subsets by nFCM revealed that the concentrations of large EVs were higher in patients with SLE than in controls. Only one other study with a similar analysis strategy reported an increase in the number of large EVs containing nucleic acids and mitochondria, which were also related to the degree of disease activity.28 Additionally, DLS showed that the SLE-EVs had larger sizes and greater light dispersion (intensity) compared with controls. It is possible that the larger sizes in the SLE-EVs could correspond to apoptotic bodies, ICs and/or aggregates.21,36
A recent study characterised the elemental composition of EVs derived from a lymphocyte cell line and demonstrated that atoms of oxygen, carbon and phosphorus are part of the energy spectrum emitted by these structures.37 Although there are no previous reports on this type of analysis in EVs from the plasma of patients with SLE, the EDXS showed differences in the distribution spectrum of carbon and oxygen atoms in SLE-EVs compared with Ctrl-EVs. EVs are considered colloidal particles and changes in their composition can affect their interactions with the environment.28
The EVs from patients with SLE had a negative charge but their potential was less electronegative than what was observed in Ctrl-EVs, which suggests that the SLE-EVs are hydrodynamically more unstable and with a greater tendency to aggregate because their less negative value would indicate lower repulsive forces.28 Under physiological conditions, the surface of a plasma membrane has a network of negatively charged glycosylated proteins intercalated within the lipid bilayer which also contribute to the net negative surface charge of EVs.38 Although the magnitude of the ζ potential fluctuates depending on the electrochemical characteristics at the particle-medium interface and can be affected by factors such as surface chemistry, pH and ionic strength of the medium,28 patient and control samples were processed and analysed under the same conditions to reduce these biases. Although we did not characterise the molecular composition of EVs in detail, it is possible that in addition to the content of carbohydrates, lipids and proteins, the side chains of their different ligands and receptors are contributing to the changes seen in the ζ potential.39 Therefore, it is feasible that the less electronegative potential of SLE-EVs could be explained by the complexity of these structures.
In patients with SLE there are defects in the clearance of apoptotic bodies, included in the definition of larger EVs (50 nm–5 µm).40 Phosphatidylserine (PS) is used to identify apoptotic cells by flow cytometry staining with annexin V as well as the identification of EVs populations and has shown differences between patients with SLE and healthy controls.15 Our results showed that there were not differences between annexin V+ SLE-EVs compared with controls, but there were different levels of annexin V+ in SLE-EVs samples. In line with this, some studies in the blood of patients with SLE and healthy controls reported that subgrouped PS-negative microparticles predominated and were approximately three times more common than PS-positive microparticles compared with controls.41 42 Large EVs become a source of autoantigens.43,45 For example, the presence of double-stranded DNA can induce the production of type 1 interferon through the cGAS-STING pathway.46 Likewise, nuclear autoantigens like HMGB1 and antigenic presentation molecules like HLA-DR can be found on the surface of EVs, but we did not find significant differences between patients and controls.
Recent studies have implicated EVs in the pathophysiology of LN and their potential clinical significance.10 We found that variables such as the number of large EVs and the frequency of EV-IgM+ showed a good capacity to discriminate patients with SLE from controls in PCA and ROC curves. In contrast, the discrimination capacity of large EVs and EV-IgM+ between patients with and without nephritis was lost. This suggests that these characteristics could be due to events associated with SLE as a heterogeneous disease and not exclusively to renal involvement.
A previous study demonstrated an increase in plasma levels of EV-CD14+ in patients with active SLE and a close correlation with the severity of the disease.47 Our analysis strategy focusing on the large EV population showed an increase in EVs positive for CD45 and CD14. This finding is in line with other reports based on the plasma of patients with SLE that showed those EV-C1q+ that formed IgG or IgM ICs came mainly from leucocytes (EV-CD45+).48 49 Although our study does not explain the specific role of EV-CD14+, under ex vivo conditions, it has been reported that changes in the surface thiols in circulating CD14+ cells and EVs derived from CD14+ cells isolated from patients with RA are an event that has been associated with the activation of inflammatory cells.50 Although there are no similar studies in patients with SLE, we could propose that the increase in EV-CD14+ in the circulation of patients with SLE may be the product of greater activation of these cells.47
Regarding the limitations of this study, we demonstrated a greater presence of large EVs in patients with SLE, but DLS is highly sensitive to medium temperature and viscosity. Therefore, the results could not strictly reflect what happens in vivo in the plasma of patients with SLE. Another main limitation is the small number of samples analysed in the different techniques and the clinical heterogeneity of the patients.
Taking together, our findings suggest that higher counts of large EVs, originating from leucocytes and monocytes, as well as the increase in EV-IgM+ and a slightly negative electrical charge, are some of the characteristics that can be related to the role in the pathogenesis of SLE. It is noteworthy that the number of larger EVs and the frequency of EV-IgM+ can discriminate between patients with SLE and controls, but they do not discriminate between patients with or without LN, possibly because the participation of EVs as a generalised phenomenon is part of the pathogenesis of SLE and not exclusively of the organ injury.2 11 Finally, this study provides a new perspective on the physicochemical and phenotypic characteristics of circulating EVs in the plasma of patients with SLE and proposes them as potential biomarkers of the disease.
supplementary material
Acknowledgements
We acknowledged the volunteers who participated in this study, the Advanced Microscopy Center and the Polymer Research Group at the University of Antioquia.
Footnotes
Funding: This work was supported by grant 844-2019 from the Ministry of Science, Technology, and Innovation of Colombia.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by the Ethics Committee of the University of Antioquia School of Medicine. Participants gave informed consent to participate in the study before taking part.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Paula X Losada, Email: paula.losada@udea.edu.co.
Lina Serrato, Email: linam.serrato@udea.edu.co.
Ana María Daza, Email: amaria.daza@udea.edu.co.
Adriana Vanegas-García, Email: adrianavanegas@gmail.com.
Carlos H Muñoz, Email: carlmuvahos@gmail.com.
Daniel Rodriguez, Email: danielefrenrodriguezariza@gmail.com.
Juan Camilo Diaz, Email: jdiazc@ces.edu.co.
Ricardo Pineda, Email: ricardopinedatt@hotmail.com.
Mauricio Rojas Lopez, Email: mauricio.rojas@udea.edu.co.
Gloria Vásquez, Email: glomavas@gmail.com.
Data availability statement
Data are available upon reasonable request.
References
- 1.Crow MK, Mary Crow PK. Pathogenesis of systemic lupus erythematosus: risks, mechanisms and therapeutic targets. Ann Rheum Dis. 2023;82:999–1014. doi: 10.1136/ard-2022-223741. [DOI] [PubMed] [Google Scholar]
- 2.Caielli S, Wan Z, Pascual V. Systemic Lupus Erythematosus Pathogenesis: Interferon and Beyond. Annu Rev Immunol. 2023;41:533–60. doi: 10.1146/annurev-immunol-101921-042422. [DOI] [PubMed] [Google Scholar]
- 3.Witwer KW, Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J Extracell Vesicles. 2019;8:1648167. doi: 10.1080/20013078.2019.1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen NS, Jacobsen S. Microparticles - culprits in the pathogenesis of systemic lupus erythematosus? Expert Rev Clin Immunol. 2018;14:443–5. doi: 10.1080/1744666X.2018.1474100. [DOI] [PubMed] [Google Scholar]
- 6.Yu L, Zhu G, Zhang Z, et al. Apoptotic bodies: bioactive treasure left behind by the dying cells with robust diagnostic and therapeutic application potentials. J Nanobiotechnol. 2023;21:218. doi: 10.1186/s12951-023-01969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omran RN, El Shebini EM, Zahran ES, et al. Extracellular vesicles and systemic lupus erythromatosus. Egypt J Intern Med. 2019;31:389–96. doi: 10.4103/ejim.ejim_67_19. [DOI] [Google Scholar]
- 8.Zhao Y, Wei W, Liu ML. Extracellular vesicles and lupus nephritis - New insights into pathophysiology and clinical implications. J Autoimmun. 2020;115:102540. doi: 10.1016/j.jaut.2020.102540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karlsson J, Wetterö J, Potempa LA, et al. Extracellular vesicles opsonized by monomeric C-reactive protein (CRP) are accessible as autoantigens in patients with systemic lupus erythematosus and associate with autoantibodies against CRP. J Autoimmun. 2023;139:103073. doi: 10.1016/j.jaut.2023.103073. [DOI] [PubMed] [Google Scholar]
- 10.Parikh SV, Almaani S, Brodsky S, et al. Update on Lupus Nephritis: Core Curriculum 2020. Am J Kidney Dis. 2020;76:265–81. doi: 10.1053/j.ajkd.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Burbano C, Gómez-Puerta JA, Muñoz-Vahos C, et al. HMGB1+ microparticles present in urine are hallmarks of nephritis in patients with systemic lupus erythematosus. Eur J Immunol. 2019;49:323–35. doi: 10.1002/eji.201847747. [DOI] [PubMed] [Google Scholar]
- 12.Lu J, Hu ZB, Chen PP, et al. Urinary podocyte microparticles are associated with disease activity and renal injury in systemic lupus erythematosus. BMC Nephrol. 2019;20:303. doi: 10.1186/s12882-019-1482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng C, Xie L, Qin H, et al. The Role of Extracellular Vesicles in Systemic Lupus Erythematosus. Front Cell Dev Biol. 2022;10:835566. doi: 10.3389/fcell.2022.835566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen J, Zhang M, Peng M. Progress of exosome research in systemic lupus erythematosus. Cytokine X. 2022;4:100066. doi: 10.1016/j.cytox.2022.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu YJ, Wang C. A review of the regulatory mechanisms of extracellular vesicles-mediated intercellular communication. Cell Commun Signal. 2023;21:77. doi: 10.1186/s12964-023-01103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhattacharjee S. DLS and zeta potential - What they are and what they are not? J Control Release. 2016;235:337–51. doi: 10.1016/j.jconrel.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Ponce A, Mejía-Rosales S, José-Yacamán M. Scanning transmission electron microscopy methods for the analysis of nanoparticles. Methods Mol Biol. 2012;906:453–71. doi: 10.1007/978-1-61779-953-2_37. [DOI] [PubMed] [Google Scholar]
- 18.Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019;71:1400–12. doi: 10.1002/art.40930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 20.Welsh JA, Arkesteijn GJA, Bremer M, et al. A compendium of single extracellular vesicle flow cytometry. J Extracell Vesicles. 2023;12:e12299. doi: 10.1002/jev2.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmieri V, Lucchetti D, Gatto I, et al. Dynamic light scattering for the characterization and counting of extracellular vesicles: a powerful noninvasive tool. J Nanopart Res. 2014;16:1–8. doi: 10.1007/s11051-014-2583-z. [DOI] [Google Scholar]
- 22.Kwon Y, Park J. Methods to analyze extracellular vesicles at single particle level. Micro Nano Syst Lett. 2022;10:1–13. doi: 10.1186/s40486-022-00156-5. [DOI] [Google Scholar]
- 23.Kurtjak M, Kereïche S, Klepac D, et al. Unveiling the Native Morphology of Extracellular Vesicles from Human Cerebrospinal Fluid by Atomic Force and Cryogenic Electron Microscopy. Biomedicines. 2022;10:1251. doi: 10.3390/biomedicines10061251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatischeff I, Larquet E, Falcón-Pérez JM, et al. Fast characterisation of cell-derived extracellular vesicles by nanoparticles tracking analysis, cryo-electron microscopy, and Raman tweezers microspectroscopy. J Extracell Vesicles. 2012;1 doi: 10.3402/jev.v1i0.19179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cloutier N, Tan S, Boudreau LH, et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol Med. 2013;5:235–49. doi: 10.1002/emmm.201201846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen CT, Østergaard O, Stener L, et al. Increased IgG on cell-derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation. Arthritis Rheum. 2012;64:1227–36. doi: 10.1002/art.34381. [DOI] [PubMed] [Google Scholar]
- 27.Tamrin SH, Phelps J, Nezhad AS, et al. Critical considerations in determining the surface charge of small extracellular vesicles. J Extracell Vesicles. 2023;12:e12353. doi: 10.1002/jev2.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Midekessa G, Godakumara K, Ord J, et al. Zeta Potential of Extracellular Vesicles: Toward Understanding the Attributes that Determine Colloidal Stability. ACS Omega. 2020;5:16701–10. doi: 10.1021/acsomega.0c01582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mobarrez F, Fuzzi E, Gunnarsson I, et al. Microparticles in the blood of patients with SLE: Size, content of mitochondria and role in circulating immune complexes. J Autoimmun. 2019;102:142–9. doi: 10.1016/j.jaut.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Burbano C, Rojas M, Muñoz-Vahos C, et al. Extracellular vesicles are associated with the systemic inflammation of patients with seropositive rheumatoid arthritis. Sci Rep. 2018;8:17917. doi: 10.1038/s41598-018-36335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrant J, Pontis A, Zimmermann F, et al. Phenotypic and proteomic analysis of plasma extracellular vesicles highlights them as potential biomarkers of primary Sjögren syndrome. Front Immunol. 2023;14:1207545. doi: 10.3389/fimmu.2023.1207545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setua S, Thangaraju K, Dzieciatkowska M, et al. Coagulation potential and the integrated omics of extracellular vesicles from COVID-19 positive patient plasma. Sci Rep. 2022;12:22191. doi: 10.1038/s41598-022-26473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Boza-Serrano A, Dunning CJR, et al. Inflammation leads to distinct populations of extracellular vesicles from microglia. J Neuroinflamm. 2018;15:168. doi: 10.1186/s12974-018-1204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosseinkhani B, van den Akker NMS, Molin DGM, et al. (Sub)populations of extracellular vesicles released by TNF-α -triggered human endothelial cells promote vascular inflammation and monocyte migration. J Extracell Vesicles. 2020;9:1801153. doi: 10.1080/20013078.2020.1801153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasse S, Julien A-S, Duchez A-C, et al. Red blood cell-derived phosphatidylserine positive extracellular vesicles are associated with past thrombotic events in patients with systemic erythematous lupus. Lupus Sci Med. 2022;9:e000605. doi: 10.1136/lupus-2021-000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M, Shen J, Thomas JC, et al. Particle Size Measurement Using Dynamic Light Scattering at Ultra-Low Concentration Accounting for Particle Number Fluctuations. Materials (Basel) 2021;14:5683. doi: 10.3390/ma14195683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dumontel B, Susa F, Limongi T, et al. Nanotechnological engineering of extracellular vesicles for the development of actively targeted hybrid nanodevices. Cell Biosci. 2022;12:61. doi: 10.1186/s13578-022-00784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tóth EÁ, Turiák L, Visnovitz T, et al. Formation of a protein corona on the surface of extracellular vesicles in blood plasma. J Extracell Vesicles. 2021;10:e12140. doi: 10.1002/jev2.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buzás EI, Tóth EÁ, Sódar BW, et al. Molecular interactions at the surface of extracellular vesicles. Semin Immunopathol. 2018;40:453–64. doi: 10.1007/s00281-018-0682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalra H, Drummen GPC, Mathivanan S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int J Mol Sci. 2016;17:170. doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielsen CT, Østergaard O, Johnsen C, et al. Distinct features of circulating microparticles and their relationship to clinical manifestations in systemic lupus erythematosus. Arthritis Rheum. 2011;63:3067–77. doi: 10.1002/art.30499. [DOI] [PubMed] [Google Scholar]
- 42.Mobarrez F, Vikerfors A, Gustafsson JT, et al. Microparticles in the blood of patients with systemic lupus erythematosus (SLE): phenotypic characterization and clinical associations. Sci Rep. 2016;6:36025. doi: 10.1038/srep36025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pieterse E, van der Vlag J. Breaking immunological tolerance in systemic lupus erythematosus. Front Immunol. 2014;5:164. doi: 10.3389/fimmu.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ullal AJ, Reich CF, 3rd, Clowse M, et al. Microparticles as antigenic targets of antibodies to DNA and nucleosomes in systemic lupus erythematosus. J Autoimmun. 2011;36:173–80. doi: 10.1016/j.jaut.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Pisetsky DS, Gauley J, Ullal AJ. Microparticles as a source of extracellular DNA. Immunol Res. 2011;49:227–34. doi: 10.1007/s12026-010-8184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kato Y, Park J, Takamatsu H, et al. Apoptosis-derived membrane vesicles drive the cGAS-STING pathway and enhance type I IFN production in systemic lupus erythematosus. Ann Rheum Dis. 2018;77:1507–15. doi: 10.1136/annrheumdis-2018-212988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viñuela-Berni V, Doníz-Padilla L, Figueroa-Vega N, et al. Proportions of several types of plasma and urine microparticles are increased in patients with rheumatoid arthritis with active disease. Clin Exp Immunol. 2015;180:442–51. doi: 10.1111/cei.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burbano C, Villar-Vesga J, Orejuela J, et al. Potential Involvement of Platelet-Derived Microparticles and Microparticles Forming Immune Complexes during Monocyte Activation in Patients with Systemic Lupus Erythematosus. Front Immunol. 2018;9:322. doi: 10.3389/fimmu.2018.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fortin PR, Cloutier N, Bissonnette V, et al. Distinct Subtypes of Microparticle-containing Immune Complexes Are Associated with Disease Activity, Damage, and Carotid Intima-media Thickness in Systemic Lupus Erythematosus. J Rheumatol. 2016;43:2019–25. doi: 10.3899/jrheum.160050. [DOI] [PubMed] [Google Scholar]
- 50.Szabó-Taylor KÉ, Tóth EÁ, Balogh AM, et al. Monocyte activation drives preservation of membrane thiols by promoting release of oxidised membrane moieties via extracellular vesicles. Free Radic Biol Med. 2017;108:56–65. doi: 10.1016/j.freeradbiomed.2017.03.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.