Abstract
Background
Oxylipin metabolism plays an essential role in glioma progression and immune modulation in the tumor microenvironment. Lipid metabolic reprogramming has been linked to macrophage remodeling, while the understanding of oxylipins and their catalyzed enzymes lipoxygenases in the regulation of glioma-associated microglia/macrophages (GAMs) remains largely unexplored.
Methods
To explore the pathophysiological relevance of oxylipin in human glioma, we performed Ultra-high performance liquid chromatography-MS/MS (UHPLC-MS/MS) analysis in human glioma and non-tumor brain tissues. To comprehensively investigate the role of arachidonate lipoxygenase 5 (ALOX5) in glioma, we performed in vivo bioluminescent imaging, immunofluorescence staining and flow cytometry analysis on tumors from orthotopic glioma-bearing mice. We developed an ALOX5-targeted nanobody, and tested its anti-glioma efficacy of combination therapy with α-programmed cell death protein-1 (PD-1).
Results
In this study, we found that ALOX5 and its oxylipin 5-hydroxyeicosatetraenoic acid (5-HETE) are upregulated in glioma, accumulating programmed death-ligand 1 (PD-L1)+ M2-GAMs and orchestrating an immunosuppressive tumor microenvironment. Mechanistically, 5-HETE derived from ALOX5-overexpressing glioma cells, promotes GAMs migration, PD-L1 expression, and M2 polarization by facilitating nuclear translocation of nuclear factor erythroid 2-related factor 2. Additionally, a nanobody targeting ALOX5 is developed that markedly suppresses 5-HETE efflux from glioma cells, attenuates M2 polarization of GAMs, and consequently ameliorates glioma progression. Furthermore, the combination therapy of the ALOX5-targeted nanobody plus α-PD-1 exhibits superior anti-glioma efficacy.
Conclusions
Our findings reveal a pivotal role of the ALOX5/5-HETE axis in regulating GAMs and highlight the ALOX5-targeted nanobody as a potential therapeutic agent, which could potentiate immune checkpoint therapy for glioma.
Keywords: Immunosuppression, Immunotherapy, Solid tumor, Macrophage
WHAT IS ALREADY KNOWN ON THIS TOPIC
Oxylipin metabolism has been considered a potential therapeutic vulnerability for glioma. Glioma-associated microglia/macrophages (GAMs), known to mediate immunosuppression within the glioma, have altered lipid composition and disrupted lipid metabolism. However, the understanding of the crosstalk between oxylipin metabolism and GAMs remodeling in glioma is still limited.
WHAT THIS STUDY ADDS
Our findings highlight that arachidonate lipoxygenase 5 (ALOX5) and its metabolite 5-hydroxyeicosatetraenoic acid (5-HETE) function as critical immune regulators for GAMs remodeling in the glioma. We provide detailed insight into the mechanism by which glioma cell-derived 5-HETE, functions as a metabolic messenger, leading to the accumulation of programmed death-ligand 1+ M2-GAMs in a nuclear factor erythroid 2-related factor 2-dependent manner. In addition, we have developed an ALOX5-targeted nanobody, which modulates the phenotypes of GAMs, thereby suppressing glioma progression.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our study indicates the ALOX5/5-HETE axis as a potential therapeutic target for the treatment of glioma, influencing both the tumor parenchyma and GAMs phenotype. Since therapeutic approaches against metabolic targets of glioma remain scarce, we have developed an ALOX5-targeted nanobody, which exhibits superior anti-glioma efficacy when combined with α-programmed cell death protein-1 therapy. These findings suggest the ALOX5-targeted nanobody as a potential therapeutic agent that could potentiate immune checkpoint therapy for glioma.
Introduction
Glioma is the most common and aggressive type of intracranial tumor, with limited treatment options and a poor prognosis. Glioblastoma (GBM), a grade IV glioma according to the WHO classification, is the most lethal and malignant brain tumor, with high resistance to conventional combination treatments.1 2 Considerable efforts have been made to understand the lipid metabolism reprogramming in glioma to discover novel therapeutic targets.3 4 As lipids account for more than 50% of the brain’s total dry weight, fatty acids are essential for the central nervous system.5 Polyunsaturated fatty acids are metabolized by cyclooxygenases (COXs), lipoxygenases (LOXs), and cytochrome P450 enzymes to form oxylipins, a group of bioactive lipid metabolites that can act as signals in a variety of pathological processes in cancers.6 Recent research has shown that disrupting the oxylipin metabolism of glioma cells by targeting LOXs, the enzymes that catalyze oxylipin formation, can effectively attenuate glioma progression, highlighting that oxylipin metabolism presents a promising therapeutic target in glioma.7,9
Our previous work, and that of others, have shown that LOXs, a family that includes arachidonate lipoxygenase (ALOX)E3, ALOX5, ALOX12, ALOX12B, ALOX15, and ALOX15B, is critically involved in the regulation of glioma. Our previous study uncovered that ALOXE3 and its related metabolite 12-hydroxyeicosatetraenoic acid (12-HETE) regulate ferroptosis and the migratory capacity of glioma cells triggering malignant progression.7 ALOX15 and its metabolites, 9-hydroxyoctadecadeinoic acid and 13-hydroxyoctadecadeinoic acid, exhibit protumorigenic effects on GBM cells by increasing cell migration and decreasing cell cycle arrest in the G2/M phase.8 Additionally, targeting ALOX5 sensitizes GBM to temozolomide treatment in a β-catenin-dependent manner.9 In addition to modulating glioma cell proliferation, cell death, and migration, oxylipin metabolism is involved in the glioma immune microenvironment. A recent study has reported that ALOX5 plays a role in inflammation-related pathways and is correlated with immune cell infiltration in glioma.10 However, the roles played by LOXs and their oxylipin products in the immune microenvironment of glioma remain unclear.
Glioma-associated microglia/macrophages (GAMs), represent 30–50% of the total cell population in the glioma microenvironment and play an essential role in glioma progression and drug resistance by creating an immunosuppressive microenvironment.11 12 A recent study has shown significant differences in the expression of oxylipin metabolism-associated enzymes between M1 and M2 macrophages. Specifically, upregulation of COX2 and downregulation of COX1 and ALOX5 were observed in macrophages after treatment with interferon (IFN)-γ and/or lipopolysaccharide (LPS), whereas expression of ALOX15 and COX1 increased markedly in response to interleukin 4 (IL-4) stimulation.13 Apoptotic cancer cells can decrease ALOX5 expression in tumor-associated macrophages (TAMs) via direct cell contacts.14 Emerging evidence suggests that lipid metabolism is involved in modulating GAMs in glioma. Glioma cell with lipid loading promotes infiltration and activation of macrophages in the glioma niche.15 In both mouse models and human glioblastoma samples, there are clusters of GAMs that exert a unique gene signature, which shows an association between phagocytosis and lipid metabolism, as revealed by the single-cell analysis.16 Despite these promising findings, the understanding of the role of LOXs and their oxylipin products in the regulation of GAMs is still limited.
Owing to their smaller size, nanobodies can penetrate the blood-brain barrier (BBB) via various mechanisms.17,20 Several studies have illuminated various aspects of preclinical assessment and therapeutic applications of nanobodies against glioma. Jovčevska et al identified differentially expressed proteins in GBM using a nanobody-based anti-proteome approach, highlighting the ability of nanobodies to traverse the BBB and their utility of nanobody in identifying biomarkers for distinguishing GBM from lower-grade glioma.21 Another study provided evidence for the overexpression of the mitochondrial translation elongation factor TUFM in GBM stem cells, and developed a GBM-specific anti-TUFM nanobody, that exhibited specificity and a significant inhibitory effect on GBM stem cell growth.22 The ability of nanobodies to cross the BBB and target GBM cells provides a promising avenue for the development of nanobodies for glioma therapy.
In this study, we found that the ALOX5/5-hydroxyeicosatetraenoic acid (5-HETE) axis is significantly activated in GBM. ALOX5 activity results in the preferential accumulation of programmed death-ligand 1 (PD-L1)+ M2-GAMs in glioma in a paracrine manner, triggering malignant glioma progression. Specifically, 5-HETE derived from glioma cells overexpressing ALOX5 promotes migration, PD-L1 expression, and M2 polarization of GAMs, thereby orchestrating an immunosuppressive glioma microenvironment. Mechanistically, 5-HETE induces PD-L1 expression and M2 polarization of GAMs via facilitating the nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2). To reverse the immunosuppressive glioma microenvironment, we develop an ALOX5-targeted nanobody and deliver it via an alternative adeno-associated virus (AAV)-based methodology. On investigation, we found that the ALOX5-targeted nanobody effectively hinders 5-HETE secretion from glioma cells and activates the M1 polarization of GAMs ameliorating glioma in vivo. The combination therapy of an α-programmed cell death protein-1 (PD-1) plus the ALOX5-targeted nanobody exhibits superior anti-glioma efficacy. These findings highlight an essential role of the ALOX5/5-HETE axis in the regulation of GAMs and suggest the ALOX5-targeted nanobody as a potential therapeutic agent, which could potentiate immune checkpoint therapy for glioma.
Methods
Clinical specimens and tissue microarray construction
Glioma lesions were obtained from patients with glioma who underwent neurosurgical resection, and non-tumor brain tissues were obtained from surgical trauma patients. All clinical human samples of tissue microarray (TMA) were collected from Yijishan Hospital and Shenzhen Hospital of Southern Medical University under the surveillance of hospital Human Ethics Committees. A TMA containing human glioma lesions (n=25 for WHO II, n=26 for WHO III and n=19 for GBM) and non-tumor tissues (n=8) was generated by Servicebio company. The tumor grading of each sample was defined by two pathologists according to WHO classification. Informed consent was obtained from all subjects.
Oxylipin targeted UHPLC-MS/MS analysis
Oxylipins were extracted by using sample preparation and filtration columns (catalog #186008055; Waters) according to manufacturer’s instruction. The levels of oxylipins were determined by using UHPLC-QQQ-MS (Agilent 1290–6460).23
Immunohistochemical staining
Immunohistochemical (IHC) staining of TMA was performed as we previously described.7 24 Anti-ALOX5 antibody (1:50; catalog #MA5-38050; Invitrogen) was used. The staining results were assessed by two independent investigators blinded to WHO classification.
Animal studies
Experiments were conducted on C57 mice, having free access to sterilized water and standard chow and housing in a room with 23℃ temperature and 12 hours light/dark cycle control. Orthotopic implantation of glioma cells into mice was performed as we previously described.25 The investigators were not blinded to the experimental groups. Tumor growth was monitored by IVIS Spectrum in vivo imaging system (PerkinElmer) after intraperitoneal injection with luciferin (150 mg/kg; catalog #P1043; Promega).
Real-time quantitative PCR
TRIzol (catalog #15596026; Thermo Fisher Scientific) was employed to extract total RNA from frozen tissues and cells. After reverse transcription with the PrimeScript RT reagent kit (catalog #RR037A; Takara), quantitative PCR (QPCR) analysis was performed with SYBR Green (catalog #AQ131-02; Trans) and the specific primers using Real-time PCR System (Applied Biosystems 7500). Primer sequences are shown in online supplemental table S1.
Immunoblotting
Total protein was extracted from tissues or cell lines using a total protein extraction buffer supplemented with a protease inhibitor cocktail (catalog #DI101-01; Trans). Proteins were separated by SDS-PAGE electrophoresis and were electro-transblotted onto polyvinylidene difluoride membranes. After incubation with primary antibodies and horseradish peroxidase-conjugated secondary antibodies, protein bands were visualized by an imaging system (Bio-Rad ChemiDoc Imaging System) and quantified using ImageJ software. Antibodies for immunoblotting are shown in online supplemental table S2. Full images of immunoblotting are shown in online supplemental figure S8.
Generation of stable cell lines with ALOX5 silencing or ALOX5 overexpression
ALOX5 knockout in mouse GL261 glioma cells was achieved by using CRISPR/Cas9 approach as described previously.25 ALOX5 targeted sgRNAs were generated by Jiangsu GenScript Biotech. Sequences are shown in online supplemental table S3. Mouse CT2A and human U87 glioma cells with ALOX5 overexpression were generated using a lentiviral-mediated overexpression system as we previously described.26 The lentiviral-overexpression vectors encoding the full length of the mouse or human ALOX5 gene were constructed by Shanghai GeneChem.
Flow cytometry
Tumor tissues harvested from orthotopic glioma-bearing mice were dissociated into single-cell suspensions by filtering through a 40 µm cell strainer, and subjected to staining with indicated antibodies. The stained cells were subjected to flow cytometry (FCM) using a Sony SA3800 analyzer. Antibodies for FCM are shown in online supplemental table S4.
siRNA transfection
THP-1 cells were seeded in 12-well plates and transfected with siRNAs for 48 hours using Opi-MEM (catalog #31985062; Thermo Fisher Scientific) and lipofectamine RNAiMAX (catalog #13778150; Thermo Fisher Scientific). The siRNAs against Nrf2 were synthesized and purified by Sango Biotech. Sequences are shown in online supplemental table S5.
Biopanning of anti-ALOX5 nanobody
The library was developed at AlpaLife. Standard phage display, screening, and phage-ELISA protocols were employed for the library’s screening. Biopanning was conducted using high-binding plates coated with purified ALOX5 as we previously described.27 The ALOX5 DNA was recombined into the pFastBac plasmid. The bacmids were transfected into SF9 insect cells using a liposome packaging transfection method, with the cell culture and expression following the Bac-to-Bac Baculovirus Expression System protocol by Invitrogen. The process included blocking the coated wells with 10% Bovine serum albumin (BSA), incubating them with recombinant phage particles, and performing extensive washing with 1% Phosphate-buffered Saline with Tween 20 (PBST). We released the phage particles using 1 mg/mL trypsin (catalog #R001100; Thermo Fisher Scientific) and amplified them by infecting Escherichia coli TG1. After three rounds of biopanning, monoclonal phage particles were detected using Anti-M13-HRP (1:1,000; catalog # GE27-9421-01; GE Life Sciences) and TMB solution (catalog #T0440; Sigma). Sequencing was conducted by Sangon Biotech.
Preparation of nanobodies
Nanobody sequences were subcloned into the pET22b plasmid. An expression and purification strategy for the recombinant proteins was performed as we previously described.27 Briefly, the BL21 (DE3) E. coli strain was transformed with the expression vector and cultured until OD600 reached 0.6–0.8. Protein expression was induced at 16°C with 0.25 mM isopropyl β-D-1-thiogalactopyranoside for 20 hours. Protein extraction was performed using ultrasonic lysis, employing a Vibra Cell probe in ice for 15–30 min with alternating cycles (a 3 s pulse following every 8 s). The purification of proteins was conducted using Ni-NTA affinity chromatography.
Statistics
The sample size was determined based on previous publications and the variability observed in preliminary experiments. A sample size of each experiment is listed in the figure legends. All data were analyzed using SPSS or GraphPad Prism V.9.4.1. Data were represented as the mean±SEM, unless otherwise specified. The statistical significance of normally distributed data with equal variance was analyzed using two-tailed Student’s t-test. The Kaplan-Meier survival analysis was calculated using log-rank test. P value<0.05 was considered statistically significant in all statistical comparisons.
Results
ALOX5 expression and 5-HETE production are up-regulated in human GBM
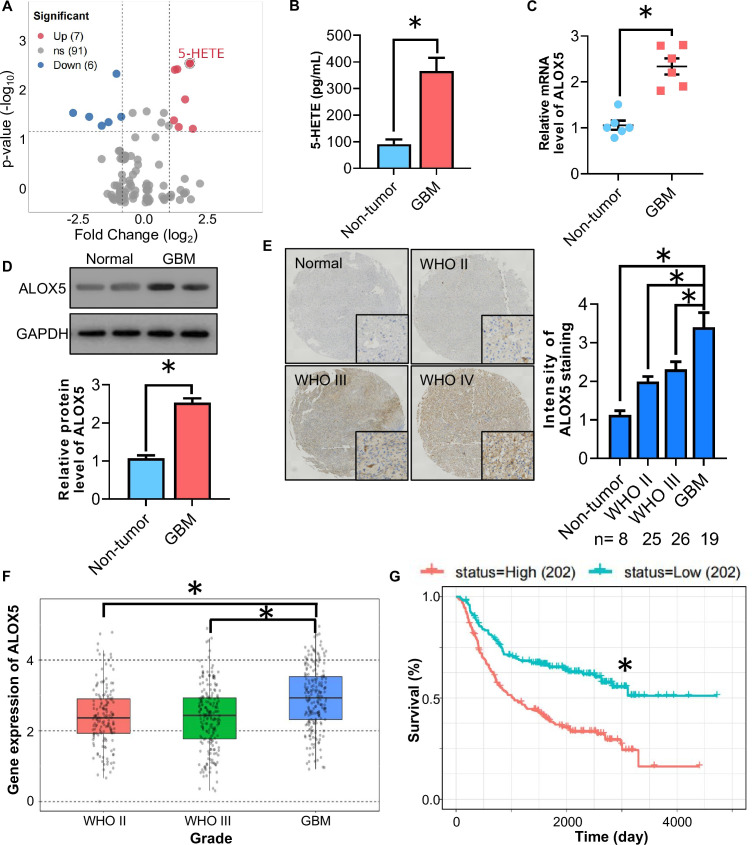
To explore the pathophysiological relevance of oxylipin in human glioma, we first investigated its expression in human GBM and non-tumor brain tissues via the targeted Ultra-high performance liquid chromatography-MS/MS (UHPLC-MS/MS) analysis. Among the 104 detected oxylipins and related metabolites, 6 oxylipins were significantly downregulated and 7 oxylipins were significantly upregulated in human GBM tissues compared with those in normal brain tissues (figure 1A). The level of 5-HETE changed significantly in GBM (figure 1B), indicating that 5-HETE may play a role in GBM progression. In addition, we observed that 12-HETE level was significantly increased in human GBM, which is consistent with our previous findings.7 ALOX5 is a dioxygenase enzyme that metabolizes arachidonic acid to 5-HETE. Next, we validated the expression of ALOX5 in GBM by analyzing the ALOX5 levels in clinical human GBM lesions and non-tumor brain tissues obtained from patients with surgical trauma. QPCR analysis showed that the messenger RNA (mRNA) abundance of ALOX5 was upregulated in human GBM tissues when compared with that in non-tumor brain tissues (figure 1C). Consistently, the protein level of ALOX5 was increased in GBM tissues, as revealed by immunoblotting analysis of GBM samples and IHC staining in a TMA (figure 1D,E). In the Chinese Glioma Genome Atlas data set, with matched RNA sequencing and survival data from 693 patients, high ALOX5 expression was shown to be associated with higher glioma WHO grades and a worse overall survival (figure 1F,G). Altogether, these findings suggested that the ALOX5/5-HETE axis is significantly activated in GBM and may play a role in GBM development.
Figure 1. ALOX5/5-HETE axis is upregulated in human GBM specimen. (A) Volcano plot of oxylipin between human GBM and non-tumor brain tissues detected by targeted UHPLC-MS/MS analysis (n=10). (B) 5-HETE levels in human GBM and non-tumor brain tissues detected by targeted LC-MS/MS analysis (n=6). (C–D) Human GBM tissues and normal brain tissues were used. (C) Relative mRNA level of ALOX5 normalized with GAPDH in human GBM tissues and non-tumor brain tissues. The mRNA level is expressed as fold change over non-tumor brain tissues (n=6). (D) Immunoblotting analysis of ALOX5 and GAPDH in human GBM tissues and non-tumor brain tissues. Representative immunoblot images are shown. The bar chart is a relative expression level of ALOX5 normalized with GAPDH (n=8). (E) Immunohistochemistry staining of ALOX5 in human GBM tissue microarray. All data are represented as the mean±SEM *p<0.05 (Student’s t-test). (F) CGGA analysis of ALOX5 mRNA level in open access data sets. (G) Comparison of survival prognosis between ALOX5 high and ALOX5 low *p<0.05 (log-rank test). ALOX5, arachidonate lipoxygenase 5; GBM, glioblastoma; mRNA, messenger RNA; UHPLC-MS/MS, Ultra-high performance liquid chromatography-MS/MS; 5-HETE, 5-hydroxyeicosatetraenoic acid.
ALOX5 promotes orthotopic glioma growth in vivo
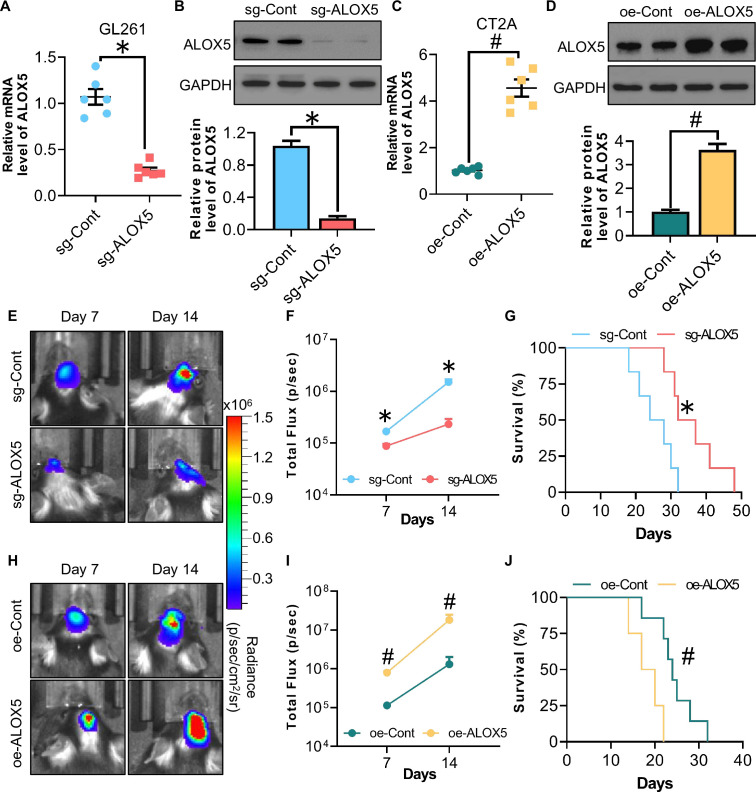
To determine the role of ALOX5 in the development of glioma, we employed the CRISPR-Cas9 system to generate a GL261 murine glioma cell line with ALOX5 knockout (sg-ALOX5) and lentiviral-mediated overexpression system to generate a CT2A murine glioma stable cell line with ALOX5 overexpression (oe-ALOX5). The ALOX5 expression level was significantly downregulated or upregulated in sg-ALOX5 and oe-ALOX5 cells, respectively, when compared with that in control cells (sg-Cont or oe-Cont cells), as revealed by QPCR and western blotting analysis (figure 2A–D). Subsequently, we generated orthotopic glioma-bearing mice by orthotopically implanting glioma cells into the hippocampus of mice to observe the tumor growth and survival. The sg-ALOX5 GL261 cells suppressed, whereas the oe-ALOX5 CT2A cells promoted tumor growth in mice as revealed by the in vivo bioluminescent imaging (figure 2E–J), suggesting a tumor-promoting role for ALOX5 in glioma. Consistently, sg-ALOX5 GL261 cells extended, whereas the oe-ALOX5 CT2A cells shortened the lifespan of mice, respectively (figure 2G,J). Hence, ALOX5 promotes orthotopic glioma growth and shortens the lifespan of mice in vivo.
Figure 2. ALOX5 promotes orthotopic glioma progression and shortens lifespan in mice. (A) Relative mRNA level of ALOX5 normalized with GAPDH in sg-Cont and sg-ALOX5 GL261 glioma cells (n=6). (B) Immunoblotting analysis of ALOX5 and GAPDH in sg-Cont and sg-ALOX5 GL261 glioma cells. Representative immunoblot images are shown. The bar chart is the relative expression level of ALOX5 normalized with GAPDH (n=8). (C) Relative mRNA level of ALOX5 normalized with GAPDH in oe-Cont and oe-ALOX5 CT2A glioma cells (n=6). (D) Immunoblotting analysis of ALOX5 and GAPDH in oe-Cont and oe-ALOX5 CT2A glioma cells. Representative immunoblot images are shown. The bar chart is the relative expression level of ALOX5 normalized with GAPDH (n=8). (E–G) sg-Cont and sg-ALOX5 GL261 glioma cells were implanted orthotopically into the hippocampus of C57 mice (n=10). (E) In vivo bioluminescent imaging of glioma-bearing mice at indicated time points. (F) Quantification of luminescence signal intensity. (G) Survival curve of mice. (H–J) oe-Cont and oe-ALOX5 CT2A glioma cells were implanted orthotopically into the hippocampus of C57 mice (n=10). (H) In vivo bioluminescent imaging of glioma-bearing mice at indicated time points. (I) Quantification of luminescence signal intensity. (J) Survival curve of mice. All data except figures G and J are represented as the mean±SEM *p<0.05, sg-Cont versus sg-ALOX5; #p<0.05, oe-Cont versus oe-ALOX5 (Student’s t-test). For figures G and J, *p<0.05, sg-Cont versus sg-ALOX5; #p<0.05, oe-Cont versus oe-ALOX5 (log-rank test). ALOX5, arachidonate lipoxygenase 5; mRNA, messenger RNA.
To further explore the underlying mechanism by which ALOX5 promotes glioma progression, we investigated the effects of ALOX5 manipulation on glioma cells in vitro. Cell proliferation, colony formation, apoptosis, and migration were not affected in glioma cells with ALOX5 knockout or overexpression (online supplemental figure S1). Furthermore, ALOX5 manipulation had no effect on the migration of glioma cells, as revealed by wound healing and transwell assays (online supplemental figure S2). Cumulatively, these findings indicated that the regulation of the immune microenvironment may be involved in ALOX5-mediated glioma progression.
ALOX5 preferentially accumulates PD-L1+ M2-GAMs in the glioma microenvironment
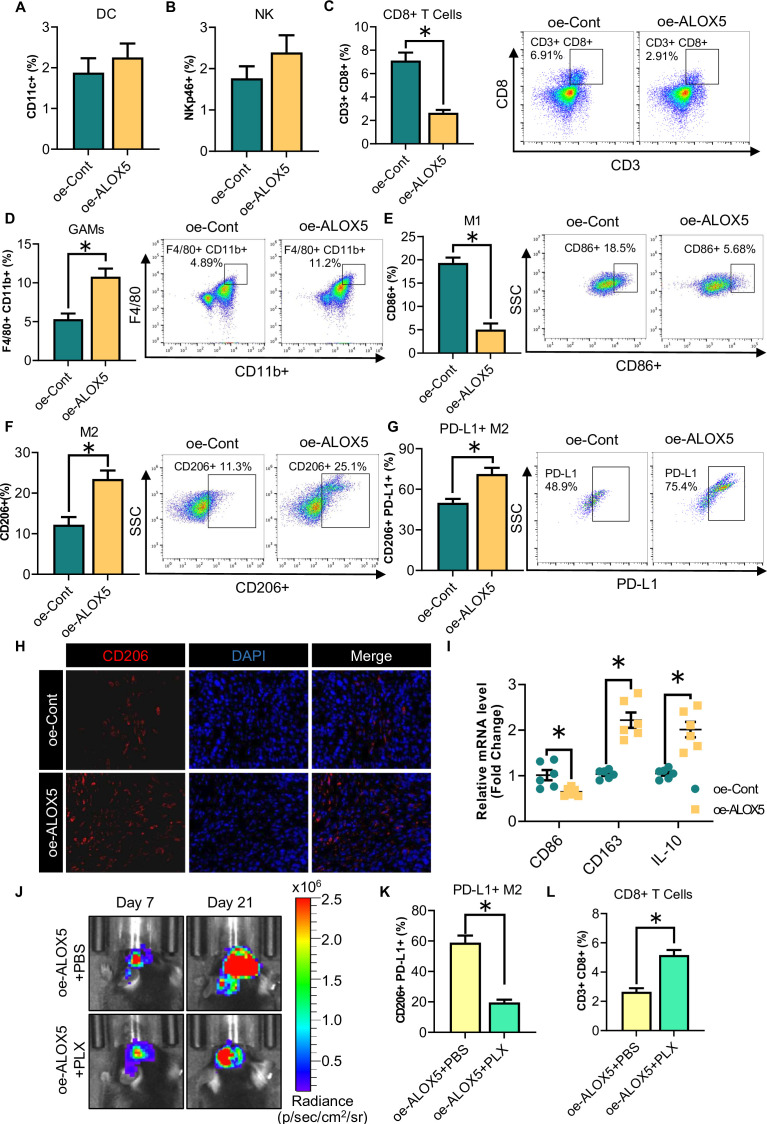
We next investigated whether tumor progression induced by ALOX5 was mediated through the regulation of the immune microenvironment. To this end, we analyzed the tumor immune microenvironment of orthotopic glioma using FCM. Immunophenotyping of orthotopic tumors using FCM showed that CD8+ T cells were decreased while GAMs were increased in the tumors of mice orthotopically implanted with oe-ALOX5 CT2A cells (figure 3A–D). In addition to an increase in the overall percentage of GAMs, a decrease in M1-GAMs and an increase in M2-GAMs were observed in the tumors (figure 3D–F). We also noted a significant increase in PD-L1+ cell subsets among CD206+ M2-GAMs (figure 3G). Consistently, immunofluorescence (IF) staining revealed an accumulation of CD206+ M2-GAMs in the tumor of mice orthotopically implanted with oe-ALOX5 CT2A cells (figure 3H). In oe-ALOX5 tumors, QPCR analysis showed that the mRNA abundance of the M1 marker CD86 and the M2 markers CD163 and IL-10 were downregulated and upregulated, respectively (figure 3I). To further explore the immune microenvironment regulation of ALOX5 on glioma, we comprehensively analyzed the diversity and landscape of tumor-infiltrating immune cells in glioma using CIBERSORT analysis. The results showed that M2-GAMs were the most enriched immune cells in the ALOX5 high group (online supplemental figure S3A), which was consistent with the status of immune cell infiltration in the glioma of mice orthotopically implanted with oe-ALOX5 CT2A cells (figure 3F). Furthermore, we performed a correlative analysis in the 163 GBM human subjects via the Gene Expression Profile Interactive Analysis28 (online supplemental figure S3A). Results showed that the mRNA expression of M2 markers (CD163, CD206 and IL-10R) was strongly and positively correlated with ALOX5 (online supplemental figure S3B–D). Thus, these findings suggested that ALOX5 enhances the tumor infiltration of PD-L1+ M2-GAMs.
Figure 3. ALOX5 increases PD-L1+ M2-GAMs accumulation in glioma. (A–I) Orthotopic tumors were harvested from oe-Cont or oe-ALOX5 CT2A glioma-bearing mice and subjected to the following experiments (n=6). (A–G) FCM for (A) DC, (B) NK, (C) CD3+CD8+ T cells and (D–G) GAMs (F4/80+CD11b+, F4/80+CD11b+CD86+, F4/80+CD11b+CD206+ and F4/80+CD11b+CD206+PD-L1+) population. (H) Immunofluorescence staining of CD206+ M2-GAMs in the tumors. (I) Relative mRNA levels of CD86, CD163 and IL-10 normalized with GAPDH in tumors. (J–L) oe-ALOX5 CT2A glioma-bearing mice were subjected to PLX or phosphate-buffered saline treatment. (J) In vivo bioluminescent imaging of glioma-bearing mice at indicated time points. (K–L) FCM for (K) F4/80+CD11b+CD206+PD-L1+ GAMs and (L) CD3+CD8+ T cells population of orthotopic tumors. All data are represented as the mean±SEM *p<0.05 (student’s t-test). ALOX5, arachidonate lipoxygenase 5; DC, Dendritic cells; FCM, flow cytometry; GAM, glioma-associated microglia/macrophage; IL, interleukin; mRNA, messenger RNA; NK, natural killer; PD-L1, programmed death-ligand 1.
To further demonstrate the effect of PD-L1+ M2-GAMs on CD8+ T cells, a Food and Drug Administration (FDA)-approved colony-stimulating factor 1 receptor inhibitor PLX3397 was employed to ablate GAMs. Tumor growth of mice orthotopically implanted with oe-ALOX5 CT2A cells was attenuated on PLX treatment when compared with that in the phosphate-buffered saline control group (figure 3J). The proportion of PD-L1+ M2-GAMs was dramatically decreased in the orthotopic gliomas obtained from the PLX group (figure 3K). In terms of lymphocytes, the intratumoral CD8+ T-cell infiltration level was increased in the tumor microenvironment of the PLX group (figure 3L). On the basis of these observations, we speculated that more GAMs infiltrating oe-ALOX5 tumors would display an M2-like phenotype and have a high PD-L1 expression, and these PD-L1+ M2-GAMs might act as the culprit in suppressing the infiltration of CD8+ T cells, promoting glioma progression.
Glioma cell-derived 5-HETE promotes microglia/macrophages migration in a paracrine manner
Knowing that oe-ALOX5 CT2A cells promoted GAMs accumulation in glioma, we aimed to ascertain whether ALOX5 could induce migration of the GAMs in a paracrine manner. To this end, conditioned medium (CM) from oe-ALOX5 U87 and CT2A glioma cells was harvested and subjected to a transwell migration assay. We found that the migration of mouse microglial BV2 cells was significantly enhanced by CM derived from oe-ALOX5 CT2A cells than that by CM derived from oe-Cont CT2A cells (online supplemental figure S4A). Additionally, human monocyte THP-1 cells and human HMC3 microglial cells exerted increased migration ability when treated with CM derived from oe-ALOX5 U87 cells (online supplemental figure S4B,C). Since UHPLC-MS analysis uncovered that 5-HETE was enriched in the CM from oe-ALOX5 U87 and CT2A glioma cells when compared with that in the CM from control cells (online supplemental figure S4D,E), we next examined whether 5-HETE is responsible for the induction of microglia/macrophages migration. To investigate this assumption, THP-1, HMC3 and BV2 cells were incubated with 5-HETE, and followed by transwell migration assays. The migration capacity of THP-1, HMC3 and BV2 cells was stimulated by 5-HETE when compared with those pretreated with vehicle (online supplemental figure S4F–H). Taken together, these observations suggested that glioma cells promote the migration of microglia/macrophages in a paracrine fashion by secreting 5-HETE.
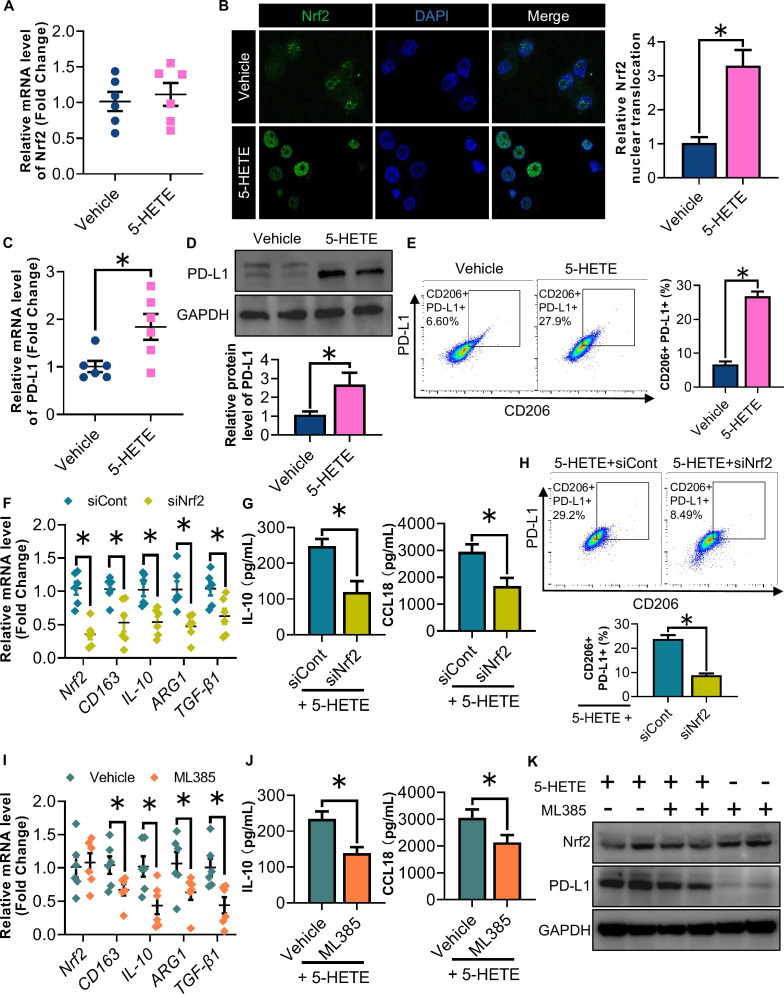
5-HETE induces the nuclear translocation of Nrf2 promoting PD-L1 expression and M2 polarization of GAMs
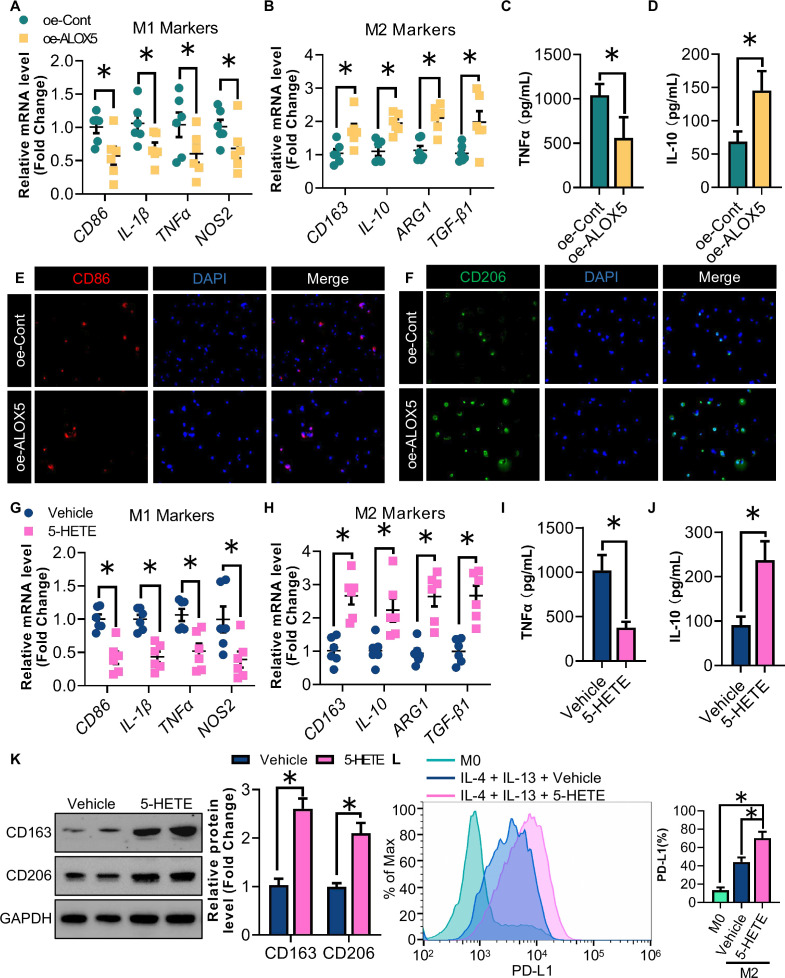
Knowing that glioma cells enhance microglia/macrophages migration by secreting 5-HETE, we aimed to determine whether oe-ALOX5 U87 glioma cells can also promote M2 polarization of the macrophages in a paracrine manner. M2 rather than M1 polarization of THP-1 cells was induced in response to stimulation with CM harvested from oe-ALOX5 U87 cells, as revealed by the mRNA abundance of M1 markers (CD86, IL-1β, TNF-α and NOS2) and M2 markers (CD163, IL-10, ARG1 and TGF-β1; figure 4A,B). ELISA further confirmed that tumor necrosis factor (TNF)-α and IL-10 levels were correspondingly decreased and elevated in the culture medium from polarized macrophages, respectively (figure 4C,D). Similarly, incubation with oe-ALOX5 CM enhanced the protein signal of the M2 marker CD206 of THP-1-derived macrophages stimulated with IL-4 and IL-10, but not of CD86 when the macrophages were stimulated with IFN-γ and LPS (figure 4E,F). We next examined whether oe-ALOX5 U87 glioma cells promoted the M2 polarization of THP-1 derived macrophages in a 5-HETE-dependent manner. Following the 5-HETE treatment, M2 rather than M1 polarization of THP-1 cells was increased as revealed by QPCR and ELISA (figure 4G–J). Western blotting analysis uncovered an increase in the expression of CD163 and CD206 in IL-4-induced and IL-10-induced macrophages following the 5-HETE treatment during M2 macrophage differentiation (figure 4K). Additionally, the PD-L1 expression was upregulated in M2 macrophages on 5-HETE stimulation (figure 4L).
Figure 4. 5-HETE promotes M2 polarization and PD-L1 expression of GAMs. (A) Relative mRNA levels of CD86, IL-1β, TNF-α and NOS2 normalized with GAPDH of M1 THP-1 macrophages stimulated with CM from oe-ALOX5 or oe-Cont U87 cells (n=6). (B) Relative mRNA levels of CD163, IL-10, ARG1 and TGF-β1 normalized with GAPDH of M2 THP-1 macrophages stimulated with CM from oe-ALOX5 or oe-Cont U87 cells (n=6). (C) ELISA analysis of TNF-α of M1 THP-1 macrophages stimulated with CM from oe-ALOX5 or oe-Cont U87 cells (n=6). (D) ELISA analysis of IL-10 of M2 THP-1 macrophages stimulated with CM from oe-ALOX5 or oe-Cont U87 cells (n=6). (E) IF staining of CD86 of M1 THP-1 macrophages stimulated with CM from oe-ALOX5 or oe-Cont U87 cells. (F) IF staining of CD206 of M2 THP-1 macrophages stimulated with CM from oe-ALOX5 or oe-Cont U87 cells. (G–L) PMA-induced THP-1 macrophages stimulated with 5-HETE or vehicle (n=6). (G) Relative mRNA levels of CD86, IL-1β, TNF-α and NOS2 normalized with GAPDH in M1 THP-1 macrophages. (H) Relative mRNA levels of CD163, IL-10, ARG1 and TGF-β1 normalized with GAPDH in M2 THP-1 macrophages. (I) ELISA analysis of TNF-α of M1 THP-1 macrophages. (J) ELISA analysis of IL-10 of M2 THP-1 macrophages. (K) Immunoblotting analysis of CD163, CD206 and GAPDH in M2 THP-1 macrophages. Representative immunoblot images are shown. The bar chart is a relative expression level of CD163 and CD206 normalized with GAPDH. (L) Flow cytometry analysis for the proportion of PD-L1+ subsets among CD206+ M2 macrophages. All data are represented as the mean±SEM *p<0.05 (Student’s t-test). ALOX5, arachidonate lipoxygenase 5; CM, conditioned medium; GAM, glioma-associated microglia/macrophage; IF, immunofluorescence; IL, interleukin; mRNA, messenger RNA; PD-L1, programmed death-ligand 1; TNF, tumor necrosis factor; 5-HETE, 5-hydroxyeicosatetraenoic acid.
The transcription factor Nrf2 is well-known for its anti-inflammatory role through inducing M2 polarization of macrophages. Recently, the Nrf2 has been shown to be activated by 5-HETE.29 5-HETE had no effect on the mRNA level of Nrf2 (figure 5A). However, the 5-HETE treatment significantly induced the nuclear translation of Nrf2 as compared with that of the vehicle control (figure 5B), suggesting that 5-HETE induces the nuclear translocation of Nrf2 activating the expression of downstream target genes. Both PD-L1 expression and M2 polarization of macrophages were induced in response to 5-HETE stimulation (figure 5C–E). To further investigate the role of Nrf2 in regulating the 5-HETE-mediated induction of PD-L1 and M2 polarization, we knocked down the Nrf2 using siRNAs. 5-HETE-mediated induction of M2 polarization of macrophages was abrogated by the Nrf2 knockdown, as revealed by the QPCR and ELISA analysis for M2 markers (figure 5F,G). We also noted that Nrf2 knockdown largely reversed the proportion of PD-L1+ M2 macrophages increased by 5-HETE stimulation (figure 5H). Furthermore, the 5-HETE-mediated induction of M2 polarization of macrophages was abolished on the treatment of ML385, an Nrf2 inhibitor that can block its transcriptional activity (figure 5I,J). ML385 showed no effect on the protein level of Nrf2, but significantly downregulated the PD-L1 protein expression level (figure 5K). Taken together, these results indicated that glioma cell-derived 5-HETE induces the nuclear translocation of Nrf2 promoting PD-L1 expression and M2 polarization of GAMs.
Figure 5. 5-HETE induces Nrf2 nuclear translocation promoting PD-L1 expression and M2 polarization of glioma-associated microglia/macrophages . (A–E) PMA-induced THP-1 macrophages were stimulated with 5-HETE or vehicle. (A) Relative mRNA levels of Nrf2 normalized with GAPDH (n=6). (B) Immunofluorescence staining of Nrf2 and DAPI. (C) Relative mRNA levels of PD-L1 normalized with GAPDH (n=6). (D) Immunoblotting analysis of PD-L1 and GAPDH in THP-1 macrophages. Representative immunoblot images are shown. The bar chart is a relative expression level of PD-L1 normalized with GAPDH. (E) FCM analysis for the proportion of PD-L1+ CD206+ M2 macrophages of THP-1 stimulated with IL-4 and IL-10 (n=6). (F–H) PMA-induced THP-1 macrophages were transfected with siNrf2 or siCont, and followed with stimulation of 5-HETE, IL-4 and IL-10. (F) Relative mRNA levels of Nrf2, CD163, IL-10, ARG1 and TGF-β1 normalized with GAPDH (n=6). (G) ELISA analysis of IL-10 and CCL18 (n=6). (H) FCM analysis for the proportion of PD-L1+ CD206+ M2 macrophages (n=6). (I–K) PMA-induced THP-1 macrophages were stimulated with ML385 or vehicle control, and followed with treatment of 5-HETE, IL-4 and IL-10. (I) Relative mRNA levels of Nrf2, CD163, IL-10, ARG1 and TGF-β1 normalized with GAPDH (n=6). (J) ELISA analysis of IL-10 and CCL18 (n=6). (K) Immunoblotting analysis of Nrf2, PD-L1 and GAPDH. Representative immunoblot images are shown. All data are represented as the mean±SEM *p<0.05 (Student’s t-test). FCM, flow cytometry; IL, interleukin; mRNA, messenger RNA; Nrf2, nuclear factor erythroid 2-related factor 2; PD-L1, programmed death-ligand 1; 5-HETE, 5-hydroxyeicosatetraenoic acid.
Administration of an AAV vector coding for an ALOX5-targeted nanobody ameliorates glioma progression in mice
Extensive studies have delineated that zileuton, an FDA-approved ALOX5 inhibitor, has antitumor effects against a variety of tumors, but its role in glioma is still unknown. Notably, the 5-HETE levels in the media of GL261 glioma cells were dramatically downregulated in response to zileuton treatment (online supplemental figure S5A). Administration of zileuton by intraperitoneal injection significantly reduced the tumor burden in subcutaneous glioma-bearing mice (online supplemental figure S5B). However, intravenous administration of zileuton had no effect on the tumor burden in orthotopic glioma-bearing mice (online supplemental figure S5C), suggesting that bioavailability issues limit the drug’s ability to reach orthotopic glioma.
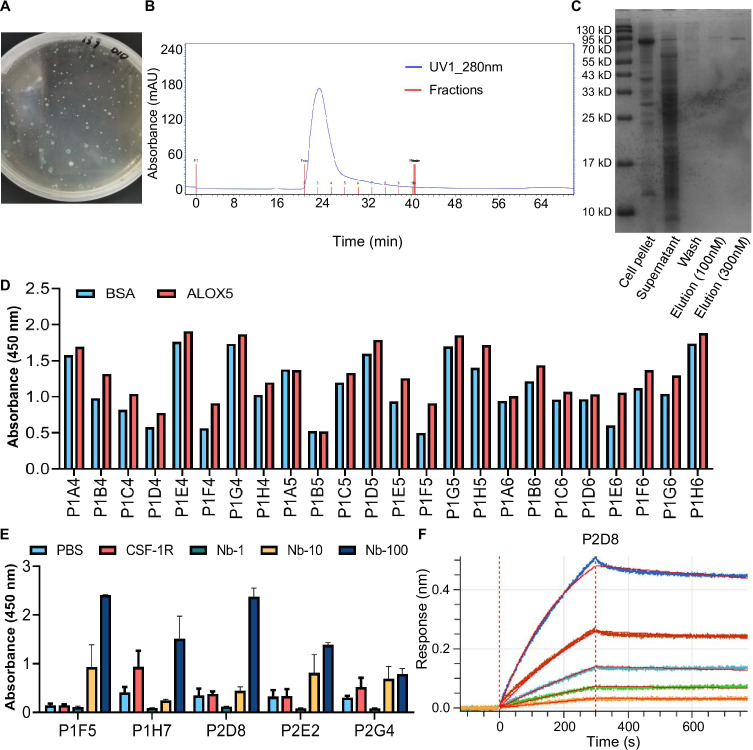
In light of the advantages of nanobodies, we have developed ALOX5 targeting nanobodies. A pFastBac-ALOX5 antigen protein recombinant expression plasmid was constructed. Using blue-white screening, we successfully obtained a recombinant expression plasmid (figure 6A). Subsequently, high-purity ALOX5 protein was expressed by transfecting a plasmid into SF9 cells, and obtained by a molecular sieve purifying system (figure 6B,C). After conducting three rounds of biopanning, we randomly selected 192 positive clones for the phage-ELISA (figure 6D). The top five clones exhibiting favorable responses in the positive phage-ELISA were then reinserted into the pET22b vector to enable soluble expression. Using ELISA validation, we verified the binding capacity of the five candidate nanobodies to the antigen (figure 6E). Finally, we assessed the affinity of the nanobodies using bio-layer interferometry analysis. Among these five candidate nanobodies, P2D8 (termed ALOX5-targeted nanobody) had the highest affinity and was selected for all the subsequent experiments (figure 6F).
Figure 6. Development and validation of an ALOX5-targeted nanobody. (A) Blue-white screening of the pFastBac-Alox5 antigen protein recombinant expression plasmid. (B) Molecular sieve purifying system obtained high-purity ALOX5 protein. (C) Coomassie Brilliant Blue stained for ALOX5 protein. (D) Phage-ELISA of selected 192 clones, 24 representative results were shown. (E) ELISA validation of five selected nanobodies. Data are represented as the mean±SEM *p<0.05 (Student’s t-test). (F) Bio-layer interferometry analysis for P2D8 nanobody (KD=4.35×10−8M). ALOX5, arachidonate lipoxygenase 5.
To deliver the ALOX5-targeted nanobody to orthotopic glioma, an AAV-based methodology was employed. The AAV vectors coding for ALOX5-targeted nanobody (AAV-ALOX5-Nb) potentiated ALOX5 function in vitro, as revealed by the decreased 5-HETE levels in the media of GL261 and CT2A cells upon AAV-ALOX5-Nb administration (figure 7A). To determine the effects of ALOX5-targeted nanobody on orthotopic glioma in vivo, we initially confirmed that the AAV-ALOX5-Nb drove strong expression of ALOX5-targeted nanobody in head site with little expression in other tissues of orthotopic glioma-bearing mice (online supplemental figure S6A,B). In addition, glioma cells, but not myeloid cells, were shown to be the main target of ALOX5-targeted nanobody (online supplemental figure S6C–E). Orthotopic CT2A glioma-bearing mice intravenously injected with AAV-ALOX5-Nb exhibited attenuated glioma growth and a prolonged lifespan as compared with the mice in the control group (figure 7B,C). Decreased 5-HETE levels in orthotopic glioma harvested from mice injected with AAV-ALOX5-Nb, further supported a notion that the AAV-ALOX5-Nb can suppress ALOX5 function in vivo (figure 7D). IF staining and FCM analysis showed a decrease in the number of M2-GAMs in the tumors of mice injected with AAV-ALOX5-Nb (figure 7E–H). In addition, the PD-L1 expression was significantly downregulated in CD206+ M2-GAMs (figure 7I), thus providing further evidence to indicate that the immunosuppressive tumor microenvironment has been overcome. Finally, we aimed to validate whether a combination therapy of an α-PD-1 plus the ALOX5-targeted nanobody exhibits superior antitumor efficacy. The results showed that, compared with the orthotopic CT2A glioma-bearing mice with monotherapy of the ALOX5-targeted nanobody or α-PD-1, the tumor growth was retarded and lifespan was prolonged of mice with the α-PD-1 plus ALOX5-targeted nanobody combination therapy (online supplemental figure S7). Consistently, similar treatment effects were also observed in mice bearing orthotopic GL261 glioma cells (figure 7J,K). Overall, these findings indicate that ALOX5-targeted nanobody effectively ameliorates glioma and could further potentiate immune checkpoint therapy for glioma (figure 7L).
Figure 7. Administration of an AAV vector coding for an ALOX5-targeted nanobody ameliorates glioma in mice. (A) 5-HETE levels in media of GL261 and CT2A cells stimulated with AAV-ALOX5-Nb or AAV-Cont-Nb (n=6). (B–I) CT2A glioma cells were implanted orthotopically into the hippocampus of C57 mice and subjected to a single intravenous injection of AAV-ALOX5-Nb or AAV-Cont-Nb. (B) In vivo bioluminescent imaging of CT2A glioma-bearing mice at indicated time points. (C) Survival curve of mice (n=10). (D) 5-HETE levels in intratumoral tumors (n=6). (E) Immunofluorescence staining of CD206+ M2-GAMs in the tumors. (F–I) Tumors were harvested and subjected to flow cytometry for GAMs (F4/80+CD11b+, F4/80+CD11b+CD86+, F4/80+CD11b+CD206+ and F4/80+CD11b+CD206+PD-L1+) population (n=6). (J–K) GL261 glioma cells were implanted orthotopically into the hippocampus of C57 mice and subjected to indicated treatment via intravenous injection. (J) In vivo bioluminescent imaging of GL261 glioma-bearing mice at indicated time points. (K) Survival curve of mice (n=10). (L) Graphical abstract: 5-HETE derived from glioma cells, promotes an immunosuppressive microenvironment and restrains the efficacy of immune checkpoint therapy for glioma through ALOX5-5-HETE-Nrf2 signaling pathway. A combination therapy of α-PD-1 plus ALOX5-targeted nanobody exhibits superior anti-glioma efficacy. All data except figure C and K are represented as the mean±SEM *p<0.05 (Student’s t-test). As for figure C, *p<0.05 (log-rank test). As for figure K, *p<0.05, ALOX5 Nb+α-PD-1 versus ALOX5 Nb+PBS; #p<0.05, ALOX5 Nb+α-PD-1 versus α-PD-1 (log-rank test). AAV, adeno-associated virus; ALOX5, arachidonate lipoxygenase 5; GAM, glioma-associated microglia/macrophage; Nrf2, nuclear factor erythroid 2-related factor 2; PBS, phosphate-buffered saline; PD-1, programmed cell death protein-1; PD-L1, programmed death-ligand 1; 5-HETE, 5-hydroxyeicosatetraenoic acid.
Discussion
Oxylipin metabolism has been considered a potential therapeutic vulnerability for glioma. However, targeting oxylipin metabolism in glioma remains challenging due to the limited number of studies and unknown mechanisms. In this study, we demonstrated that ALOX5 and its metabolite 5-HETE, are upregulated in glioma. ALOX5 exerts no effects on the proliferation and migration of glioma cells in vitro, but preferentially accumulates PD-L1+ M2-GAMs whereas decreases the infiltration of CD8+ T cells triggering glioma malignant progression in vivo. But precisely how ALOX5 regulates oxylipin metabolism and how metabolic reprogramming regulates GAMs, requires further investigation. On investigation, we showed that glioma cells overexpressing ALOX5 enhance 5-HETE production and secretion, promoting GAMs migration, PD-L1 expression, and M2 polarization in a paracrine fashion. Mechanistic insights revealed that 5-HETE derived from glioma cells directly induces Nrf2 nuclear translocation in GAMs, which in turn leads to PD-L1 upregulation and M2 polarization. Since therapeutic approaches against metabolic targets of glioma remain scarce, we developed an ALOX5-targeted nanobody. By delivering it via an AAV-based approach, we verified a profound anti-glioma effect of the ALOX5-targeted nanobody, which could be a novel targeted approach for modulating the phenotypes of GAMs and suppressing glioma progression. Additionally, the combination therapy of the α-PD-1 plus ALOX5-targeted Nb exhibits superior anti-glioma efficacy.
As a major component of immune cells in the tumor microenvironment, TAMs support an immunosuppressive microenvironment and tumor progression. Emerging studies have shown that LOXs promote the secretion of immunosuppressive factors from TAMs triggering the immune escape of tumors. TAMs with enhanced ALOX15B activity, produce large amounts of oxylipin 15-HETE promoting IL-10 secretion and immunosuppression in renal cancer.30 In addition, the ALOX5-related metabolites, 5-HETE and leukotriene B4 are upregulated in response to an hypoxic tumor microenvironment, in turn leading to TAMs infiltration in ovarian cancer.31 Zileuton effectively inhibits the migration and tumor infiltration of TAMs by disrupting ALOX5-mediated lipid metabolism.31 32 Targeting the lipid metabolism of TAMs to reverse the immunosuppressive microenvironment provides a new direction.13 However, the therapeutic effects of these metabolic targets are still not ideal and researches on GAMs remain scarce, indicating an urgent need for new effective therapies targeting the oxylipin metabolism of GAMs in glioma treatment. To our knowledge, this is the first study uncovering that metabolic reprogramming of GAMs by targeting the ALOX5/5-HETE metabolic pathway is a potential approach for glioma therapy.
Zileuton has been reported to be able to cross the BBB.33 34 However, our findings demonstrated that intravenous injection of zileuton had no effect on the tumor burden in orthotopic glioma-bearing mice, suggesting that its bioavailability may be an issue. Nanobodies, the smallest antibodies to date, possess a straightforward structure and show better BBB and tumor penetration.35,37 In addition, nanobodies exhibit a more streamlined structure and can be stably and massively expressed in E. coli, which is a primary advantage. Considering these advantages, we pioneered the development of an ALOX5-targeted nanobody to interfere with the ALOX5/5-HETE axis, which could be used for a variety of potential applications.
Targeting intracellular ALOX5 using nanobodies remains a cutting-edge area and poses challenges.38 Successful targeting requires that the nanobodies must execute multiple steps, including identifying and entering target cells and inhibiting the target antigen. Here, we report an alternative AAV-based approach for the intracellular delivery of an ALOX5-targeted nanobody. Recombinant AAV vectors have emerged as an effective method for generating therapeutic proteins in both animal models and humans. This has been highlighted in the literatures discussing the development of optimized AAV vectors for human gene therapy and the significant strides in recombinant AAV biomanufacturing. Employing AAV vectors to deliver transgenes in an episomal form that does not integrate into the chromosomes and can maintain stability for several months, greatly enhances therapeutic safety.39 AAVs, such as AAV1, AAV8, and AAV9 are known for their adaptability and tissue-specificity, thereby paving the way for a broader scope of AAV-nanobody therapies. AAV1, for example, exhibits superior transduction efficiency and selectivity in skeletal muscle. Similarly, AAV8 shows high transduction propensity in muscle and liver tissues, while AAV9 is particularly effective in the brain. Notably, AAV-mediated gene transfer has proven to be effective in inducing the systemic secretion of broad-spectrum neutralizing antibodies against HIV in muscles, which in turn, offers protection to humanized mice against HIV infection. Several studies have demonstrated the appeal of systemic delivery of AAV9 as a potential therapeutic strategy for invasive and multifocal GBM.40 In pursuit of this, Dwijit et al used a systemic infusion of an scAAV9-hIFNβ vector to induce complete regression of established GBM8 tumors in a dose-dependent manner.41Ken et al presented two capsids, namely AAV-PHP.eB and AAV-PHP.S, which efficiently traverse the central and peripheral nervous systems, respectively.42 Termed “vector immunoprophylaxis” (VIP), this approach, via a single intramuscular injection of an AAV1 vector encoding a chosen antibody, induces and sustains anti-HIV antibodies in the mouse’s circulation over several months.43 In our study, we adopted the VIP method, starting with a single intravenous injection of AAV9. This AAV-based methodology was instrumental in establishing the significance of ALOX5 as a therapeutic target in glioma. Our study focused on innovative biologics derived from nanobodies. These biologics, in contrast to small molecule drugs, exhibit enhanced target specificity, effectively circumventing several limitations associated with small molecules and engineered proteins.44 Our study used an AAV-based gene transfer method, which is under evaluation that did not elicit any significant adverse reactions or side effects. These findings substantiate the notable therapeutic efficacy and safety profile of this approach, laying a solid foundation for the future development of treatments harnessing nanobody-based biologics.
Our findings elucidated that ALOX5 and its metabolite 5-HETE function as critical immune regulators for GAMs in the glioma microenvironment. In addition, we reported a novel mechanism by which glioma cell-derived 5-HETE, functions as a metabolic messenger, attracting GAMs infiltration, promoting PD-L1 expression, and triggering M2 polarization of GAMs in an Nrf2-dependent manner. Further investigation revealed the beneficial effects of the ALOX5-targeted nanobody on glioma, with attenuated tumor growth and increased survival. Collectively, our findings underscore the ALOX5/5-HETE axis as a potential therapeutic target for the treatment of glioma, influencing both the tumor parenchyma and GAMs phenotype.
supplementary material
Footnotes
Funding: This research was funded by National Natural Science Foundation of China (grant number: 82372890 and 82273341), Shenzhen Medical Research Funds (grant number: A2303056), Shenzhen Science and Technology Program (grant number: JCYJ20230807142211023, JCYJ20220530154001003, JCYJ20230807142213027 and JCYJ20210324122214040), NHC Key Laboratory of Nuclear Technology Medical Transformation (MIANYANG CENTRAL HOSPITAL) (2023HYX024) and Research Foundation of Shenzhen Hospital of Southern Medical University (22H3ATF02).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: All clinical human samples for targeted LC-MS/MS analysis were collected from the Department of Neurosurgery, Shenzhen Hospital of Southern Medical University with the approval of hospital Human Ethics Committees (NO. SZYYEC2020R001). Participants gave informed consent to participate in the study before taking part.
Contributor Information
Tao Chen, Email: tedc@foxmail.com.
Jiangang Liu, Email: liujiangang0605@126.com.
Chenci Wang, Email: 865626402@qq.com.
Zhengwei Wang, Email: 1079311652@qq.com.
Jiayi Zhou, Email: zhoujiayi2018@126.com.
Jiani Lin, Email: linjiani0122@163.com.
Jie Mao, Email: myw921@yahoo.com.
Tingzheng Pan, Email: girmann@163.com.
Jianwei Wang, Email: dadaowang627@126.com.
Hongchao Xu, Email: xhc092@163.com.
Xiaosheng He, Email: hexiaosheng03752@126.com.
Dinglan Wu, Email: wudinglan123@smu.edu.cn.
Zhuohao Liu, Email: lchouhoo@gmail.com.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–50. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 2.Mohammed S, Dinesan M, Ajayakumar T. Survival and quality of life analysis in glioblastoma multiforme with adjuvant chemoradiotherapy: a retrospective study. Rep Pract Oncol Radiother. 2022;27:1026–36. doi: 10.5603/RPOR.a2022.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miska J, Chandel NS. Targeting fatty acid metabolism in glioblastoma. J Clin Invest. 2023;133:e163448. doi: 10.1172/JCI163448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao T-J, Lin C-L, Yang W-B, et al. Dysregulated lipid metabolism in TMZ-resistant glioblastoma: pathways, proteins, metabolites and therapeutic opportunities. Lipids Health Dis. 2023;22:114. doi: 10.1186/s12944-023-01881-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen LN, Ma D, Shui G, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature New Biol. 2014;509:503–6. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–93. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Liu J, Wang C, et al. miR-18a promotes glioblastoma development by down-regulating ALOXE3-mediated ferroptotic and anti-migration activities. Oncogenesis. 2021;10:15. doi: 10.1038/s41389-021-00304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souza F da C, Ferreira MT, Colquhoun A. Influence of Lipoxygenase Inhibition on Glioblastoma Cell Biology. Int J Mol Sci. 2020;21:8395. doi: 10.3390/ijms21218395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang D, Hu Y, Gao W. 5-lipoxygenase as a target to sensitize glioblastoma to temozolomide treatment via β-catenin-dependent pathway. Neurol Res. 2023;45:1026–34. doi: 10.1080/01616412.2023.2255414. [DOI] [PubMed] [Google Scholar]
- 10.Pan R-H, Zhang X, Chen Z-P, et al. Arachidonate lipoxygenases 5 is a novel prognostic biomarker and correlates with high tumor immune infiltration in low-grade glioma. Front Genet. 2023;14:1027690. doi: 10.3389/fgene.2023.1027690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venteicher AS, Tirosh I, Hebert C, et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science. 2017;355:eaai8478. doi: 10.1126/science.aai8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ochocka N, Segit P, Walentynowicz KA, et al. Single-cell RNA sequencing reveals functional heterogeneity of glioma-associated brain macrophages. Nat Commun. 2021;12:1151. doi: 10.1038/s41467-021-21407-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Yang Y, Xiong L, et al. Metabolism, metabolites, and macrophages in cancer. J Hematol Oncol. 2023;16:80. doi: 10.1186/s13045-023-01478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ringleb J, Strack E, Angioni C, et al. Apoptotic Cancer Cells Suppress 5-Lipoxygenase in Tumor-Associated Macrophages. J Immunol . 2018;200:857–68. doi: 10.4049/jimmunol.1700609. [DOI] [PubMed] [Google Scholar]
- 15.Offer S, Menard JA, Pérez JE, et al. Extracellular lipid loading augments hypoxic paracrine signaling and promotes glioma angiogenesis and macrophage infiltration. J Exp Clin Cancer Res. 2019;38:241. doi: 10.1186/s13046-019-1228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pombo Antunes AR, Scheyltjens I, Lodi F, et al. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat Neurosci. 2021;24:595–610. doi: 10.1038/s41593-020-00789-y. [DOI] [PubMed] [Google Scholar]
- 17.Abulrob A, Sprong H, Van Bergen en Henegouwen P, et al. The blood-brain barrier transmigrating single domain antibody: mechanisms of transport and antigenic epitopes in human brain endothelial cells. J Neurochem. 2005;95:1201–14. doi: 10.1111/j.1471-4159.2005.03463.x. [DOI] [PubMed] [Google Scholar]
- 18.Rotman M, Welling MM, Bunschoten A, et al. Enhanced glutathione PEGylated liposomal brain delivery of an anti-amyloid single domain antibody fragment in a mouse model for Alzheimer’s disease. J Control Release. 2015;203:40–50. doi: 10.1016/j.jconrel.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Zhu S, Huang A-G, Luo F, et al. Application of Virus Targeting Nanocarrier Drug Delivery System in Virus-Induced Central Nervous System Disease Treatment. ACS Appl Mater Interfaces. 2019;11:19006–16. doi: 10.1021/acsami.9b06365. [DOI] [PubMed] [Google Scholar]
- 20.Iqbal U, Albaghdadi H, Luo Y, et al. Molecular imaging of glioblastoma multiforme using anti-insulin-like growth factor-binding protein-7 single-domain antibodies. Br J Cancer. 2010;103:1606–16. doi: 10.1038/sj.bjc.6605937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jovčevska I, Zupanec N, Urlep Ž, et al. Differentially expressed proteins in glioblastoma multiforme identified with a nanobody-based anti-proteome approach and confirmed by OncoFinder as possible tumor-class predictive biomarker candidates. Oncotarget. 2017;8:44141–58. doi: 10.18632/oncotarget.17390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samec N, Jovcevska I, Stojan J, et al. Glioblastoma-specific anti-TUFM nanobody for in-vitro immunoimaging and cancer stem cell targeting. Oncotarget. 2018;9:17282–99. doi: 10.18632/oncotarget.24629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He J, Ma C, Tang D, et al. Absolute quantification and characterization of oxylipins in lupus nephritis and systemic lupus erythematosus. Front Immunol. 2022;13:964901. doi: 10.3389/fimmu.2022.964901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ge R, Wang C, Liu J, et al. A Novel Tumor-Promoting Role for Nuclear Factor IX in Glioblastoma Is Mediated through Transcriptional Activation of GINS1. Mol Cancer Res. 2023;21:189–98. doi: 10.1158/1541-7786.MCR-22-0504. [DOI] [PubMed] [Google Scholar]
- 25.Zhou J, Pei X, Yang Y, et al. Orphan nuclear receptor TLX promotes immunosuppression via its transcriptional activation of PD-L1 in glioma. J Immunother Cancer. 2021;9:e001937. doi: 10.1136/jitc-2020-001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Ge R, Zhou J, et al. Nuclear factor IX promotes glioblastoma development through transcriptional activation of Ezrin. Oncogenesis. 2020;9:39. doi: 10.1038/s41389-020-0223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen T, Liu X, Hong H, et al. Novel single-domain antibodies against the EGFR domain III epitope exhibit the anti-tumor effect. J Transl Med. 2020;18:376. doi: 10.1186/s12967-020-02538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagahora N, Yamada H, Kikuchi S, et al. Nrf2 Activation by 5-lipoxygenase Metabolites in Human Umbilical Vascular Endothelial Cells. Nutrients. 2017;9:1001. doi: 10.3390/nu9091001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daurkin I, Eruslanov E, Stoffs T, et al. Tumor-associated macrophages mediate immunosuppression in the renal cancer microenvironment by activating the 15-lipoxygenase-2 pathway. Cancer Res. 2011;71:6400–9. doi: 10.1158/0008-5472.CAN-11-1261. [DOI] [PubMed] [Google Scholar]
- 31.Mylonis I, Simos G, Paraskeva E. Hypoxia-Inducible Factors and the Regulation of Lipid Metabolism. Cells. 2019;8:214. doi: 10.3390/cells8030214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nosaka T, Baba T, Tanabe Y, et al. Alveolar Macrophages Drive Hepatocellular Carcinoma Lung Metastasis by Generating Leukotriene B4. J Immunol . 2018;200:1839–52. doi: 10.4049/jimmunol.1700544. [DOI] [PubMed] [Google Scholar]
- 33.Kubick N, Pajares M, Enache I, et al. Repurposing Zileuton as a Depression Drug Using an AI and In Vitro Approach. Molecules. 2020;25:2155. doi: 10.3390/molecules25092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su H-Y, Tsai Y-C, Tsai H-P, et al. Zileuton, a 5-Lipoxygenase Inhibitor, Attenuates Haemolysate-Induced BV-2 Cell Activation by Suppressing the MyD88/NF-κB Pathway. Int J Mol Sci. 2022;23:4910. doi: 10.3390/ijms23094910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frecot DI, Froehlich T, Rothbauer U. 30 years of nanobodies - an ongoing success story of small binders in biological research. J Cell Sci. 2023;136:jcs261395. doi: 10.1242/jcs.261395. [DOI] [PubMed] [Google Scholar]
- 36.Erreni M, D’Autilia F, Avigni R, et al. Size-advantage of monovalent nanobodies against the macrophage mannose receptor for deep tumor penetration and tumor-associated macrophage targeting. Theranostics. 2023;13:355–73. doi: 10.7150/thno.77560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng F, Pang Y, Li L, et al. Applications of nanobodies in brain diseases. Front Immunol. 2022;13:978513. doi: 10.3389/fimmu.2022.978513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dingus JG, Tang JCY, Amamoto R, et al. A general approach for stabilizing nanobodies for intracellular expression. Elife. 2022;11:e68253. doi: 10.7554/eLife.68253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santiago-Ortiz JL, Schaffer DV. Adeno-associated virus (AAV) vectors in cancer gene therapy. J Control Release. 2016;240:287–301. doi: 10.1016/j.jconrel.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X, Chen W, Zhu W, et al. Adeno-associated virus (AAV)-based gene therapy for glioblastoma. Cancer Cell Int. 2021;21:76. doi: 10.1186/s12935-021-01776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.GuhaSarkar D, Su Q, Gao G, et al. Systemic AAV9-IFNβ gene delivery treats highly invasive glioblastoma. Neuro Oncol. 2016;18:1508–18. doi: 10.1093/neuonc/now097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan KY, Jang MJ, Yoo BB, et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci. 2017;20:1172–9. doi: 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abad C, Demeules M, Guillou C, et al. Administration of an AAV vector coding for a P2X7-blocking nanobody-based biologic ameliorates colitis in mice. J Nanobiotechnol. 2024;22:27. doi: 10.1186/s12951-023-02285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gurevich EV, Gurevich VV. Arrestins-pharmacology and therapeutic potential. 2014. Therapeutic potential of small molecules and engineered proteins; pp. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.