Abstract
Objectives
The aim of this study was to assess the histopathological features of the parotid glands in patients with paediatric-onset Sjögren’s disease (pedSjD) in comparison to patients with adult-onset Sjögren’s disease (adSjD).
Methods
This study was performed in Groningen, the Netherlands. Patients with pedSjD from a diagnostic paediatric cohort (n=19), patients with adSjD from a diagnostic adult cohort (n=32) and patients with adSjD who participated in a clinical trial (n=42) with a baseline parotid gland biopsy were included. Parotid gland biopsies were analysed after (immuno)histological staining for SjD-related histopathological markers and compared between groups.
Results
All characteristic histopathological features of adSjD were also observed in pedSjD. There were no significant differences in lymphoepithelial lesions or immunoglobulin A (IgA)/IgG plasma cell shift between the pedSjD and the adSjD cohorts. However, compared with the diagnostic adSjD cohort (with comparable total EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) scores), pedSjD showed more severe lymphocytic infiltration as reflected by a higher focus score (p=0.003), a higher relative surface area of CD45+ infiltrate (p=0.041), higher numbers of B and T lymphocytes/mm2 (p=0.004 and p=0.029, respectively), a higher B/T lymphocyte ratio (p=0.013), higher numbers of CD21+ follicular dendritic cell networks/mm2 (p=0.029) and germinal centres (GC)/mm2 (p=0.002). Compared with the trial adSjD cohort, with significant higher total ESSDAI scores (p=0.001), only the B/T lymphocyte ratio and numbers of GC/mm2 were significantly higher in the pedSjD cohort (p=0.023 and p=0.018, respectively).
Conclusion
Patients with pedSjD exhibit more pronounced histopathological features compared with patients with adSjD at diagnosis. Notably, the histopathology of patients with pedSjD aligns more closely with that observed in an adSjD clinical trial cohort, with even stronger B lymphocyte involvement.
Keywords: Sjogren's Syndrome, Autoimmune Diseases, B-Lymphocytes, Child, Inflammation
WHAT IS ALREADY KNOWN ON THIS TOPIC
Patients with paediatric-onset Sjögren present more often with clinical signs of major salivary gland involvement than adult patients. Detailed quantitative histopathological studies of (parotid) salivary glands are lacking.
WHAT THIS STUDY ADDS
Patients with paediatric-onset Sjögren show more severe lymphocytic infiltration (especially B lymphocytes) in parotid glands compared with adult patients.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our findings highlight the need for a different approach in the care of patients with paediatric-onset Sjögren with more specific attention to salivary gland inflammation.
Introduction
Sjögren’s disease (SjD) is a chronic, systemic autoimmune disease characterised by dysfunction of exocrine glands. In adults, mainly the lacrimal and salivary glands are involved, resulting in typical ocular and oral sicca symptoms.1 Predominantly, females are affected with a female-to-male ratio of 10:1. The disease is typically diagnosed in the fourth or fifth decade of life.2,4 While it is relatively uncommon, SjD can also emerge in children.5 The prevalence and prognosis of paediatric-onset SjD (pedSjD) still remain unclear. According to a recent study, 1.3% of adult patients with SjD have a paediatric-onset disease.5 The presenting symptoms in pedSjD differ from those in adults with SjD. Paediatric patients present more often with non-specific extraglandular manifestations like fever and arthralgias.6 Furthermore, paediatric patients present less often with sicca complaints and more often with symptoms of major salivary gland involvement, reflected by recurrent salivary gland swelling (in particular of the parotid glands), and higher salivary gland ultrasound (SGUS) scores compared with adults.5 7 For adults, the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria were developed in 2016.8 Due to a lack of child-specific criteria, the ACR/EULAR classification criteria are also used for classifying pedSjD.
In the current ACR/EULAR classification criteria for SjD, the presence of periductal lymphocytic infiltrates (foci) (focus score (FS) ≥1) is a leading histopathological parameter for classification.8 These infiltrates are dominated by T and B lymphocytes and also harbour a variety of non-lymphoid cells, including myeloid and plasmacytoid dendritic cells, macrophages and follicular dendritic cells (FDCs). Initially, these infiltrates seem to be unorganised, but they can develop towards ectopic lymphoid tissue with T and B lymphocyte compartmentalisation, presence of CD21+ FDC networks and high endothelial venules.9,11 In the B lymphocyte areas of ectopic lymphoid tissue with CD21+ FDC networks, germinal centres (GCs) can develop. Identification of GCs can be challenging in H&E stained sections.9 12 GCs characteristically express the transcription factor Bcl6 and detection of this protein by immunohistochemistry can assist in detecting (ectopic) GCs unequivocally.13 Another specific histopathological feature of SjD is infiltration of B lymphocytes into the ductal epithelium, resulting in the development of lymphoepithelial lesion (LELs).14 These LELs are composed of hyperplastic epithelial cells with high numbers of intraepithelial lymphocytes. In addition to GCs and LELs, another typical histopathological feature of SjD is an increase in the presence of immunoglobulin G (IgG) plasma cells, resulting is the so-called IgA/IgG plasma cell shift.15 Presence of these features assists in the diagnosis of SjD.16
Since patients with pedSjD present more often with major salivary gland involvement, in particular swelling of the parotid glands, compared with patients with adult-onset SjD (adSjD), we hypothesised that histopathological findings of the parotid glands differ between patients with pedSjD and adSjD. To our knowledge, detailed quantitative histopathological studies of (parotid) salivary glands in pedSjD are lacking. Therefore, the aim of this study was to assess and quantify the histopathological features of the parotid glands in patients with pedSjD in comparison to different cohorts of patients with adSjD using both H&E staining and immunohistochemical stainings. Obtaining more insight into the histopathological changes in the salivary glands of patients with pedSjD may increase our knowledge about the underlying pathophysiological mechanism in children and this might lead to a better recognition and treatment of pedSjD.
Methods
Patients and inclusion
This study was performed at the University Medical Center Groningen (UMCG), a tertiary referral hospital and an expertise centre for SjD in the Netherlands. For the paediatric cohort, baseline data were collected from consecutive patients who participated in the Registry of Pediatric Sjögren’s Disease LongiTudinal (REpSULT) study.7 The REpSULT cohort study started in 2020 and includes patients who are referred to the UMCG with a suspicion of having SjD, when the age of symptom onset is ≤16 years. Patients who visited the UMCG before 2020 were entered retrospectively into the database. Patients with a confirmed diagnosis according to the (paediatric) rheumatologist and who underwent a diagnostic parotid gland biopsy at the UMCG between January 2009 and December 2021 were included in this study.
For adult patients with SjD (age of symptom onset >16 years), data from two previously published adult cohorts were used. The first cohort was a prospective diagnostic adSjD cohort, with comparable inclusion criteria to the paediatric cohort. This cohort consists of consecutive patients, who were referred to the UMCG with suspected SjD between December 2013 and August 2016 and underwent a multidisciplinary evaluation including a parotid gland biopsy.17 From this cohort, patients who retrospectively fulfilled the ACR/EULAR classification criteria for SjD were included in the current study. This cohort is further referred to as ‘diagnostic adSjD cohort’.
The second adult cohort consists of patients who participated in a prospective clinical trial: an open-label, proof-of-concept, phase II study with abatacept (ASAP-II) or a randomised, double-blind, placebo-controlled, phase III trial with abatacept (ASAP-III).18,20 In general, these patients had active disease according to a moderate to high score on the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI).21 From these two clinical trials, patients who underwent a parotid gland biopsy within 1 year before start of the treatment were included in one cohort in the current study. This cohort is further referred to as ‘trial adSjD cohort’. From all the cohorts, patients with insufficient parotid biopsy material (≤4 mm2) and mucosa-associated lymphoid tissue (MALT) lymphoma were excluded.
Data collection
Clinical characteristics collected from medical records included gender, age at symptom onset, age at the time of the salivary gland biopsy and medication use in the 6 months before (history) and at the moment of the biopsy (current). Systemic disease activity was measured using the ESSDAI at the time of the biopsy (or within 1 year from the biopsy for the clinical trial cohort). B-mode SGUS was performed at the time of the biopsy using the MyLabTwice scanner (Esaote). All ultrasound images were scored real time by trained readers. The scoring system by Hocevar was applied (range 0–48).22
The international Outcome Measures in Rheumatology (OMERACT) working party have proposed a novel semi-quantitative and simpler scoring system that evaluates homogeneity and presence of hypoechoic areas in the parotid and submandibular glands (range 0-3). To evaluate the OMERACT scores, we have converted the Hocevar scores to OMERACT scores using a formula published by Rebel et al.23 Salivary gland function tests included unstimulated whole saliva (UWS) and stimulated whole saliva (SWS) (by paraffin-chewing) flow rates.24 In 60% of all the patients, the sum of gland-specific (parotid, submandibular and sublingual glands) saliva production (by applying 2% citric acid solution) was used for SWS flow rate. A UWS flow rate of <0.1 mL/min was considered abnormal.
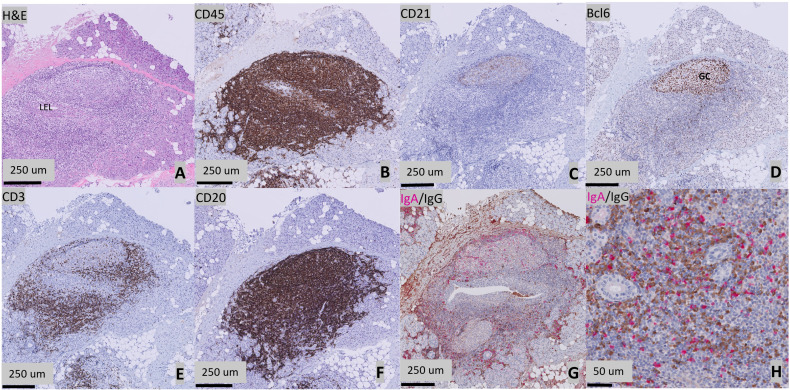
Salivary gland tissue was collected by a biopsy of the parotid gland.25 Formalin-fixed (4%), paraffin-embedded tissue samples were serially sectioned at 3 μm thickness and sections were subsequently deparaffinised. Tissue sections were stained with H&E to determine the FS (the number of periductal foci (clusters of >50 lymphocytes) per 4 mm2) and the presence and stage of LEL (stages 1–3). H&E slides were scored by two trained observers (UN and SCL) together with a senior head and neck pathologist (BvdV). A consensus meeting was performed in case of discrepancies between both observers.26,29 Tissue sections were stained immunohistochemically for CD45 (clone 2B11+PD7/26), for CD21 (clone 2G9) to detect FDC networks, for Bcl6 (clone GI19E/A8) to detect GCs, for CD3 (clone 2GV6) to detect T lymphocytes and for CD20 (clone L-26) to detect B lymphocytes. All stained slides were digitised using a Philips UFS slide scanner (Philips, Best, the Netherlands) and assessed using Philips IntelliSite Pathology Solution software. Quantitative digital image analyses of salivary gland sections stained for CD3+ T lymphocytes, CD20+ B lymphocytes and CD45+ lymphocytic infiltrates were performed using QuPath V.0.1.2.30 Identification of FDC networks was done by manually counting the number of CD21-positive stained areas. GCs were identified after staining for Bcl6. GCs were defined as a cluster of ≥5 Bcl6-positive cells. In order to estimate the IgA/IgG plasma cell shift, biopsies were dually stained for IgA and IgG and manually evaluated. A percentage of >30% IgG+ plasma cells of all IgA and IgG plasma cells in the total parenchyma were considered as a threshold for an IgA/IgG shift. Details on the assessment of FS, LELs, GCs and plasma cell shift have been previously described.16 An example of H&E and immunological staining of parotid gland sections of a representative patient with pedSjD is shown in figure 1A–H.
Figure 1. Histopathological features of a parotid gland of a 14-year-old patient with Sjögren’s disease (SjD). (A) H&E staining. Presence of a periductal infiltrate of ≥50 lymphocytes (focus) surrounding a striated duct with ductal hyperplasia (lymphoepithelial lesion, LEL). (B) CD45 staining showing lymphocytic infiltration. (C) CD21 staining showing follicular dendritic cell (FDC) networks. (D) Presence of a germinal centre, defined as a cluster of ≥5 adjacent Bcl6+ cells. (E) CD3 staining showing T-cell infiltration. (F) CD20 staining showing B-cell infiltration. (G) Presence of a plasma cell shift, as shown by a relative increase in the number of immunoglobulin G (IgG) (brown) plasma cells compared with the IgA (pink) plasma cells. (H) High magnification image of the IgA/IgG staining.
Laboratory results closest to the date of the biopsy were extracted from the medical records.
Serological parameters collected were antinuclear antibodies, anti-Sjögren’s syndrome-related antigen A (anti-SSA)/anti-SSB antibodies, rheumatoid factor (RF)-IgM level and total IgG level. Other laboratory test results included C reactive protein (CRP) level and erythrocyte sedimentation rate (ESR). IgG levels above the normal range for the age, RF-IgM >5 IU/mL, CRP >5 mg/L, ESR >15 mm/hour, C3 <0.9 g/L and C4 <0.1 g/L were considered as abnormal.
Statistical analysis
Statistical analyses were performed using IBM Statistical Packages for Social Sciences (SPSS) V.28. Results were expressed as number of patients (%) for categorical parameters and mean (SD) or median (IQR) for normally or non-normally distributed continuous parameters, respectively. To compare histopathological parameters, symptoms and characteristics between the pedSjD cohort and the diagnostic adSjD cohort and between the pedSjD cohort and the trial adSjD cohort, Fisher’s exact or χ2 tests, or Mann-Whitney U tests were used when appropriate. P values <0.05 were considered statistically significant. Spearman’s correlation coefficient was used to explore the association between histopathological and laboratory parameters in the diagnostic cohorts. A correlation coefficient of <0.2 was interpreted as poor, 0.2–0.4 as fair, 0.4–0.6 as moderate, 0.6–0.8 as good and >0.8 as excellent.31
Results
Patient inclusion
From the REpSULT cohort, 27 out of 28 patients had a confirmed diagnosis of pedSjD. From these 27 patients, eight were excluded. Reasons for exclusion were: no parotid gland biopsy (n=4), parotid MALT lymphoma (n=2) and insufficient biopsy material (n=2). Of the 19 paediatric patients included in the current study, 16 (84%) fulfilled the 2016 ACR/EULAR classification criteria and 11 (58%) fulfilled the American-European Consensus Group classification criteria.32 From the previously described diagnostic adSjD cohort,33 32 out of 37 patients who fulfilled the 2016 ACR/EULAR criteria were included in the diagnostic adSjD cohort in the current study. Reasons for exclusion were insufficient biopsy material (n=2) or MALT lymphoma (n=3).
From the clinical trial adSjD cohorts, 46 out of 95 patients underwent a parotid gland biopsy within 1 year before the baseline visit. One patient was excluded from the trial adSjD cohort and included in the pedSjD cohort, because this patient had SjD symptoms before the age of 16 years. Three patients were excluded because of insufficient biopsy material. Thus, in total, 42 patients were included in the trial adSjD cohort.
Of the included adult patients, eight were part of both the diagnostic adSjD and the trial adSjD cohort.
Differences in clinical characteristics between pedSjD and adSjD
The general patient characteristics of the three cohorts are shown in table 1. Systemic disease activity was comparable between the pedSjD cohort and the diagnostic adSjD cohort (median ESSDAI 5 vs 4, p=0.54). As expected, patients in the trial adSjD cohort had significantly higher systemic disease activity compared with the pedSjD cohort (median ESSDAI 11 vs 5, p=0.001). The score on the glandular domain of the ESSDAI was significantly higher in the pedSjD cohort compared with both the diagnostic adSjD cohort and the trial adSjD cohort (p=0.001 and p=0.001, respectively). The UWS flow rate was significantly higher in the pedSjD cohort compared with both adult cohorts (diagnostic adSjD, p=0.041 and trial adSjD, p=0.046). There were no patients who were treated with biological disease-modifying antirheumatic drugs (eg, rituximab, abatacept) or cyclophosphamide within 6 months prior to the biopsy. The laboratory results for all cohorts are shown in table 2. The pedSjD cohort comprised a relatively lower percentage of anti-SSA+ patients (diagnostic adSjD, p=0.004 and trial adSjD, p=0.12) and a lower percentage of patients with an ESR >15 mm/hour compared with the adSjD cohorts (diagnostic adSjD, p=0.010 and trial adSjD, p=0.011).
Table 1. General characteristics.
| PedSjDn=19 | Diagnostic adSjDn=32 | PedSjD versus diagnostic adSjDP value | Trial adSjDn=42 | PedSjD versus trial adSjDP value | ||||
| Gender, female, n (%) | 15 (78.9) | 31 (96.9) | 0.58 | 38 (90.5) | 0.24 | |||
| Age at start symptoms, years* | 9 (9–12) | 45 (30–58) | 0.001 | 44 (30–56) | 0.001 | |||
| Age at biopsy, years* | 14 (10–17) | 52 (46–63) | 0.001 | 51 (39–61) | 0.001 | |||
| Duration of symptoms at moment of biopsy, years* | 3 (2–5) | 5 (3–13) | 0.024 | 4 (2–8) | 0.07 | |||
| Fulfilment of ACR/EULAR criteria, n (%) | 16 (84) | 32 (100) | 0.022 | 40 (95) | 0.15 | |||
| ESSDAI total score, range* | 5 (3–11) | 4 (2–12) | 0.54 | 11 (8–16) | 0.001 | |||
| ESSDAI score glandular, range domain* | 2 (2–4) | 0 (0–2) | 0.001 | 1 (1–2) | 0.001 | |||
| ESSDAI glandular domain positive, n (%) | 18 (94.7) | 12 (37.5) | <0.001 | 33 (78.6) | 0.117 | |||
| Ultrasound | ||||||||
| Hocevar score total, range* | 23 (13–29)† | 16 (9–24)† | 0.28 | 16 (11–29)‡ | 0.33 | |||
| Hocevar score parotis, range* | 10 (8–16)† | 8 (4–12)† | 0.24 | 8 (4–14)‡ | 0.38 | |||
| Hocevar score percentage parotis/total score* | 52 (48–55)† | 52 (36–57)† | 0.82 | 50 (38–58)‡ | 0.78 | |||
| OMERACT score total* | 8 (5–10)† | 6 (1.5–8)† | 0.74 | 6 (3–11)‡ | 0.35 | |||
| Salivary flow rates | ||||||||
| UWS value, mL/min* | 0.18 (0.05–0.55)§ | 0.09 (0.03–0.19) | 0.041 | 0.10 (0.02–0.18) | 0.046 | |||
| UWS <0.1 mL/min, n (%) | 7 (38.9)§ | 19 (59.4) | 0.16 | 23 (54.8) | 0.26 | |||
| SWS value, mL/min* | 0.4 (0.22–0.59)§ | 0.65 (0.34–0.94) | 0.98 | 0.26 (0.11–0.45) | 0.033 | |||
| Medication use, n (%) | Current | History | Current | History | Current | History | ||
| Hydroxychloroquine | 5 (26) | 7 (37) | 3 (9) | 12 (38) | 1 (2) | 11 (26) | ||
| tDMARD | 1 (5) | 0 (0) | 3 (9) | 8 (25) | 0 (0) | 7 (17) | ||
| Corticosteroids | 1 (5) | 2 (11) | 3 (9) | 1 (3) | 0 (0) | 6 (14) | ||
Significant p values are italicised and bold.
*Data are reported as median (IQR) or n (%).
§Missing data: 0–5; †Missing data: 6–10; ‡Missing data: 11–15
ACR/EULARAmerican College of Rheumatology/EULARadSjDadult-onset Sjögren’s diseaseESSDAIEULAR Sjogren’s Syndrome Disease Activity IndexNSAIDnon-steroidal anti-inflammatory drugOMERACT scoreOutcome Measures in Rheumatolgy ultrasound scorePedSjDpaediatric-onset Sjögren’s diseaseSWSstimulated whole salivatDMARDtraditional disease-modifying antirheumatic drugUWSunstimulated whole saliva
Table 2. Laboratory results.
| PedSjDn=19 | Diagnostic adSjDn=32 | PedSjD versus diagnostic adSjDP value | Trial adSjDn=42 | PedSjD versus trial adSjDP value | |
| IgG value, median (IQR), g/L | 16.1 (13.7–20.0)* | 16.6 (12.1–19.7) | 0.82 | 17.9 (13.2–23.5) | 0.39 |
| IgG elevated†, n (%) | 11 (64.7)* | 17 (53.1) | 0.44 | 27 (64.3) | 0.98 |
| RF-IgM value, median (IQR), IU/mL | 18 (1.7–73) | 23 (4–65)* | 0.67 | 23 (11.8–58.3) | 0.52 |
| RF+ (>5 IU/mL), n (%) | 12 (63.2) | 23 (74.2)* | 0.41 | 35 (83.3) | 0.08 |
| Anti-SSA+, n (%) | 13 (68.4) | 31 (96.9) | 0.004 | 36 (85.7) | 0.12 |
| Anti-SSB+, n (%) | 9 (47.4) | 15 (46.9) | 0.97 | 22 (52.4) | 0.72 |
| CRP>5, n (%), mg/L | 2 (10.5) | 9 (28.1) | 0.18 | 7 (16.7) | 0.71 |
| ESR>15, n (%), mm/hour | 6 (31.6) | 22 (68.8) | 0.010 | 28 (66.7) | 0.011 |
| C3<0.9, n (%), g/L | 0 (0) | 2 (6.5)* | 0.52 | 6 (14.3) | 0.16 |
| C4<0.1, n (%), g/L | 1 (5.3)* | 0 (0)* | 0.38 | 2 (4.8) | 1.00 |
| ANA+, n (%) | 16 (88.9) | 29 (90.6) | 1.00 | 29 (85.3) | 1.00 |
Significant p values are italicised and bold.
Missing data: 0–5.
IgG value elevated for age-related normal range.
adSjDadult-onset Sjögren’s diseaseANAantinuclear antibodiesC3complement 3C4complement 4CRPC reactive proteinESRerythrocyte sedimentation rateIgGimmunoglobulin GPedSjDpaediatric-onset Sjögren’s diseaseRFrheumatoid factorSSASjögren’s syndrome-related antigen ASSBSjögren’s syndrome-related antigen B
More severe histopathology in parotid glands of patients with pedSjD compared with a diagnostic adSjD cohort
First, we compared the diagnostic pedSjD cohort to the diagnostic adSjD cohort to study the differences in histopathology between pedSjD and adSjD at the time of diagnosis. The results of the histopathological analysis of the biopsies are shown in table 3. Patients with pedSjD showed more lymphocytic infiltration/4 mm2 reflected by a higher FS (2.6 vs 1.1, p=0.003) and a higher relative surface area of CD45+ lymphocytic infiltrate compared with the diagnostic adSjD cohort (p=0.041). Both T and B lymphocyte subpopulations contributed to the higher level of infiltrate seen in the glandular tissue of patients with pedSjD. The number of B lymphocytes was, however, relatively higher than the number of T lymphocytes as mirrored by the increased B/T lymphocyte ratio in biopsies from patients with pedSjD (p=0.013). When looking at the presence of ectopic lymphoid tissue and the formation of GCs within these structures, we found that both the median numbers of CD21+ FDC networks and Bcl6+ GCs per mm2 were significantly higher in the pedSjD cohort (p=0.029 and p=0.002 respectively).
Table 3. Parotid gland biopsy results.
| PedSjDn=19 | Diagnostic adSjDn=32 | PedSjD versus diagnostic adSjDP value | Trial adSjDn=42 | PedSjD versus trial adSjDP value | |
| Focus score* | 2.6 (1.3–3.4) | 1.1 (0.3–1.8)† | 0.003 | 1.82 (1.2–4.67) | 0.79 |
| Focus score ≥1, n (%) | 16 (84.2) | 21 (65.6) | 0.20 | 36 (85.7) | 1.00 |
| CD45+ cells* (% of parenchyma) | 20.4 (9.3–35) | 10.35 (2.26–24.01) | 0.041 | 16.3 (4.9–28.9) | 0.46 |
| LELs/mm2* | 0.00 (0.00–0.29) | 0.14 (0.00–0.26) | 0.94 | 0.08 (0.00–0.35) | 0.79 |
| Presence of LELs, n (%) | 9 (48) | 18 (56) | 0.54 | 23 (56) | 0.53 |
| LEL severity (1–3)* | 2 (1–3) | 2 (1–3) | 0.87 | 1 (1–2) | 0.27 |
| CD21+ FDC networks/mm2* | 0.21 (0.06–0.79)† | 0.00 (0.00–0.35) | 0.029 | 0.21 (0.00–0.46) | 0.28 |
| Bcl6+ ectopic GC/mm2* | 0.14 (0.04–0.33) | 0.00 (0.00–0.12) | 0.002 | 0.00 (0.00–0.24) | 0.018 |
| CD3+ cells/mm2* | 1384 (841–1883) | 470 (177–1406) | 0.029 | 715 (256–1917) | 0.15 |
| CD20+ cells/mm2* | 1679 (428–2811) | 376 (61–1003) | 0.004 | 624 (108–1300) | 0.023 |
| CD20/(CD20+CD3) ratio* | 0.51 (0.32–0.58) | 0.39 (0.25–0.48) | 0.013 | 0.40 (0.23–0.50) | 0.023 |
| CD3/CD20 segregation, n (%) | 14 (74) | 18 (56) | 0.21 | 24 (57) | 0.22 |
| IgA/IgG plasma cell shift, n (%) | 9 (47) | 18 (56) | 0.54 | 24 (57) | 0.48 |
| IgM+plasma cells/mm2* | 68 (25–156) | 42 (10–93) | 0.09 | 29 (6–93) | 0.007 |
Significant p values are italicised and bold.
Data are reported as median (IQR) or n (%).
Missing data: 0–5.
adSjDadult-onset Sjögren’s diseaseFDCfollicular dendritic cellGCgerminal centreIgAimmunoglobulin ALELlymphoepithelial lesionmm2square millimetrePedSjDpaediatric-onset Sjögren’s disease
Since patients in our pedSjD cohort present more often with major salivary gland swelling compared with the diagnostic adSjD cohort, supported by a significant difference in the ESSDAI glandular domain score (table 1), we performed a subanalysis comparing patients with pedSjD to patients with adSjD who scored positive on the ESSDAI glandular domain. Although only 12 patients (38%) of the diagnostic adSjD cohort had a positive score and could be included in this subanalysis, we still found a significant difference in the FS, amount of Bcl6+ ectopic GCs/mm2, CD20+ cells/mm2 and B/T cell ratio between the pedSjD cohort and the diagnostic adSjD cohort (online supplemental table 1). These results suggest that more frequent parotid gland swelling cannot fully explain the histopathological differences between patients with pedSjD and adSjD.
To exclude potential bias in our results due to a lower frequency of anti-SSA positivity in the pedSjD cohort compared with the adSjD cohorts, we have also compared the biopsy results between anti-SSA+ patients with pedSjD and anti-SSA+ patients with adSjD (online supplemental table 2). In the subanalysis of anti-SSA-positive patients, we observed the same significant differences between the paediatric and adult patients with SjD as we observed in the total cohort.
To evaluate whether the amount of inflammation in the parotid gland of patients with pedSjD was related to clinical/serological parameters, we tested the correlations between the percentage of CD45+ cells and FS and the following key parameters: levels of ESR, IgG, RF, C3 and C4 in the blood, salivary flow rates and total Hocevar scores. Only a moderate correlation between RF and the percentage of CD45+ cells (r=0.54, p=0.017) and a good correlation between RF and total Hocevar score were seen (r=0.78, p=0.015).
Parotid gland biopsies of patients with pedSjD harbour higher numbers of B lymphocytes and GCs compared with adult patients with SjD with high systemic disease activity
Second, we compared the diagnostic pedSjD cohort with the trial adSjD cohort characterised by a higher systemic disease activity. The results of the histopathological analysis of the biopsies are shown in table 3. The histopathology of the parotid glands of patients with pedSjD was more comparable to the trial adSjD cohort than to the diagnostic adSjD cohort. Remarkably, the number of CD20+ B lymphocytes was higher in the pedSjD cohort compared with the trial adSjD cohort (p=0.023), resulting in a higher B/T lymphocyte ratio (p=0.023). The number of GCs was also significantly higher in the pedSjD cohort (p=0.018).
Discussion
In this study, we assessed in detail key histopathological features in parotid gland biopsies from patients with pedSjD and adSjD. We found that the glandular tissue of patients with pedSjD exhibited all the characteristic histopathological features of SjD that are also seen in adult patients with SjD, including: periductal infiltrates, development of ectopic lymphoid tissue, presence of LELs, GCs and the IgA/IgG plasma cell shift. However, when comparing parotid gland biopsies from the pedSjD cohort to adult-onset patients, the histopathological findings appeared to be more severe and were characterised by stronger B lymphocyte involvement in patients with pedSjD.
There were several observations supporting our notion that in patients with pedSjD the histopathological findings in the parotid glands are more severe than in adults.
First, patients with pedSjD present with a higher FS and a higher relative surface area of CD45+ infiltrate, when compared with a diagnostic cohort of adult patients. In our pedSjD cohort, 84.2% of the patients had an FS ≥1 in the parotid gland. This percentage is somewhat lower than in published data from other paediatric SjD cohorts, where percentages between 94 and 100 were reported.7 34 35 In contrast, only 65.6% of patients in our diagnostic adSjD cohort had an FS ≥1, in line with previous observations from labial and parotid gland biopsies in adults.26 36 In fact, the amount of infiltrate in patients with pedSjD, as reflected by both FS and area of CD45+ infiltrate, was similar to the amount seen in adult patients of the trial adSjD cohort, which is characterised by a relatively high systemic disease activity.
Second, B lymphocytes seem to contribute more to the periductal infiltrates than T lymphocytes in paediatric patients as mirrored by the increased B/T lymphocyte ratio compared with both cohorts of adult patients with SjD. In adults, the relative number of B lymphocytes in minor (labial) glands appears to increase with the severity of the lesions, namely the FS and amount of infiltrate.37 Thus, the higher B/T lymphocyte ratio seen in glandular tissues of paediatric patients with SjD underpins the more severe histopathology in patients with pedSjD.
Third, our study showed a more pronounced formation of ectopic lymphoid tissue in parotid glands from patients with pedSjD, compared with the adult diagnostic cohort, indicated by elevated numbers of CD21+ FDC networks in the periductal infiltrates and an increased number of GCs. Intriguingly, the number of GCs in the pedSjD cohort was also higher compared with the trial adSjD cohort. Development of ectopic lymphoid tissue from organised lymphoid infiltrates occurs at sites of chronic inflammation under the influence of chemokines and cytokines.38 Thus, they can be considered as a later, more mature step in the development of the chronic infiltrate.39 Continued antigenic stimulation may finally result in GC development within the ectopic lymphoid tissue. In adult patients with SjD, presence of GCs in salivary glands is associated with increased FS and more severe disease.40
The higher level of inflammation in the salivary glands of paediatric patients is reflected in the histopathological observations and in the clinical disease activity scores. The median score on the glandular domain of the ESSDAI was significantly higher in the pedSjD cohort compared with the diagnostic adSjD cohort. The median score on the glandular domain of the ESSDAI in the pedSjD cohort was even significantly higher compared with the trial adSjD cohort, in which the total ESSDAI score was higher compared with the pedSjD cohort. These findings are in line with previous reports, showing that patients with pedSjD more often present with signs of parotid gland swelling and (recurrent) parotitis compared with patients with adSjD.7 35 Remarkably, the higher degree of inflammation in patients with pedSjD seems restricted to the salivary glands and is not reflected by signs of more active systemic disease. When looking at the total ESSDAI scores, we found no difference between patients with pedSjD and the diagnostic adSjD cohort. Total ESSDAI scores were lower in patients with pedSjD compared with the trial adSjD cohort. Furthermore, the percentage of patients with an ESR >15 mm/hour was significantly lower in the pedSjD cohort compared with both adSjD cohorts. Serological parameters, such as IgG, RF and complement levels, were comparable among all groups. Interestingly, the more severe histopathological findings in the parotid glands in paediatric patients were not reflected by a lower salivary gland function. The UWS flow rate was even significantly higher in the pedSjD cohort compared with both adult cohorts, and no significant differences were found in the percentages of patients with a UWS flow rate <0.1 mL/min. Saliva production declines with age,41 which may lead to a lower reserve capacity in older patients, explaining at least part of these differences. A higher saliva production rate in children in general could also explain the lower percentage of patients with pedSjD presenting with sicca complaints compared with patients with adSjD. No correlation between salivary flow rates and the severity of the lesions (FS, area of CD45+ infiltrate) was seen in paediatric patients, in line with previous studies in adult SjD.42 43 Thus, also in children, more severe histopathology is not directly linked to salivary gland dysfunction.
Why children present with more severe histopathology in salivary gland tissue at diagnosis is not clear. An explanation might be that the immune system in humans is in continuous development with age-related patterns of T and B lymphocyte subsets in peripheral blood and tissue, which may impact immunoregulation at different life stages.44 45 Cellular immune responses at local sites of infection also seem to change throughout life. For example, during respiratory tract infections in infants, a more preferential generation of terminally differentiated effector T lymphocytes over tissue resident memory cells is observed, which changes during the early years of childhood.46 These differences in the immune system in combination with the fact that children are exposed to more diverse external triggers, like recurrent (salivary gland) infections, than adults may result in a different cellular and humoral immune response in children. Other factors that may contribute to the more severe histopathological findings are genetic factors in the form of underlying monogenic immunodeficiencies, like c1q deficiency, but also polymorphisms in genes involved in B lymphocyte homeostasis or other important immunological pathways.47,49
Limitations
This study has some limitations. First, pedSjD is a rare disease, and as a result the number of patients is small. Despite the limited power of this study, we did find significant differences between the cohorts. Second, there are limited data about the (immuno)histopathology of the parotid gland of children without SjD. A part of the differences between the histopathological findings might be caused by age-related differences. Third, the paediatric cohort is composed based on expert diagnosis, since there are currently no child-specific classification criteria for SjD. The lack of international classification criteria may affect the generalisability of our findings.
Conclusion
To our knowledge, this is the first study to compare quantitatively all key histopathological features of salivary gland biopsies between patients with pedSjD and adSjD. We have found significant differences between the diagnostic pedSjD and adSjD cohorts and even between the pedSjD cohort and the adSjD cohort with higher systemic disease activity. Our findings suggest that high disease activity in patients with pedSjD is mainly reflected by signs of glandular inflammation, whereas high disease activity in patients with adSjD is mostly reflected by systemic inflammation. Our findings highlight the need for a different approach in the care of patients with pedSjD with more specific attention to salivary gland inflammation. Our advice is to take the histopathological findings of the salivary gland biopsies into account when evaluating the disease activity in patients with pedSjD and to consider adjusting treatment strategies according to these findings.
supplementary material
Acknowledgements
Several authors of this publication are members of the European Reference Network ERN ReCONNET.
Footnotes
Funding: This work was supported by unrestricted grants from Bristol-Myers Squibb for the ASAP-II and ASAP-III studies to HB and is part of the Veni research programme of GV (project number: 09150162010166), which is financed by the Dutch Research Council (NWO).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: All data were obtained as part of the REpSULT cohort, diagnostic sicca cohort or ASAP studies, which were approved by the local ethics committee of the University Medical Center Groningen (UMCG) (REpSULT: METc2019.541, ASAP-II: METc2009.371, ASAP-III: METc2014.118, diagnostic sicca cohort: METc2013.066). All patients provided written informed consent according to the Declaration of Helsinki.
Data availability free text: The data underlying this article will be shared on reasonable request to the corresponding author.
Contributor Information
Geertje Elizabeth Legger, Email: g.e.legger@umcg.nl.
Uzma Nakshbandi, Email: u.nakshbandi@umcg.nl.
Martha S van Ginkel, Email: m.s.van.ginkel@umcg.nl.
Silvia C Liefers, Email: s.c.liefers@umcg.nl.
Lisette de Wolff, Email: l.de.wolff01@umcg.nl.
Alja J Stel, Email: a.j.stel@umcg.nl.
Wineke Armbrust, Email: w.armbrust@umcg.nl.
Fred K L Spijkervet, Email: f.k.l.spijkervet@umcg.nl.
Arjan Vissink, Email: a.vissink@umcg.nl.
Suzanne Arends, Email: s.arends@umcg.nl.
Hendrika Bootsma, Email: h.bootsma@umcg.nl.
Bert van der Vegt, Email: b.van.der.vegt@umcg.nl.
Gwenny M Verstappen, Email: g.m.p.j.verstappen@umcg.nl.
Frans G M Kroese, Email: f.g.m.kroese@umcg.nl.
Data availability statement
Data are available upon reasonable request.
References
- 1.Mariette X, Criswell LA. Primary Sjögren’s syndrome. N Engl J Med. 2018;378:931–9. doi: 10.1056/NEJMcp1702514. [DOI] [PubMed] [Google Scholar]
- 2.Parisis D, Chivasso C, Perret J, et al. Current state of knowledge on primary Sjögren’s syndrome, an autoimmune exocrinopathy. J Clin Med. 2020;9:2299. doi: 10.3390/jcm9072299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox RI. Sjögren’s syndrome. Lancet. 2005;366:321–31. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 4.Brito-Zerón P, Baldini C, Bootsma H, et al. Sjögren syndrome. Nat Rev Dis Primers. 2016;2:16047. doi: 10.1038/nrdp.2016.47. [DOI] [PubMed] [Google Scholar]
- 5.Ramos-Casals M, Acar-Denizli N, Vissink A, et al. Childhood-onset of primary Sjögren’s syndrome: phenotypic characterization at diagnosis of 158 children. Rheumatol (Sunnyvale) 2021;60:4558–67. doi: 10.1093/rheumatology/keab032. [DOI] [PubMed] [Google Scholar]
- 6.Ciurtin C, Cho Y, Al-Obaidi M, et al. Barriers to translational research in Sjögren’s syndrome with childhood onset: challenges of recognising and diagnosing an orphan rheumatic disease. Lancet Rheumatol. 2021;3:e138–48. doi: 10.1016/S2665-9913(20)30393-3. [DOI] [PubMed] [Google Scholar]
- 7.Legger GE, Erdtsieck MB, de Wolff L, et al. Differences in presentation between paediatric- and adult-onset primary Sjögren’s syndrome patients. Clin Exp Rheumatol. 2021;39 Suppl 133:85–92. doi: 10.55563/clinexprheumatol/vxe6h0. [DOI] [PubMed] [Google Scholar]
- 8.Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome. Ann Rheum Dis. 2017;76:9–16. doi: 10.1136/annrheumdis-2016-210571. [DOI] [PubMed] [Google Scholar]
- 9.Fisher BA, Jonsson R, Daniels T, et al. Standardisation of labial salivary gland histopathology in clinical trials in primary Sjögren’s syndrome. Ann Rheum Dis. 2017;76:1161–8. doi: 10.1136/annrheumdis-2016-210448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kroese FGM, Abdulahad WH, Haacke E, et al. B-cell hyperactivity in primary Sjögren’s syndrome. Expert Rev Clin Immunol. 2014;10:483–99. doi: 10.1586/1744666X.2014.891439. [DOI] [PubMed] [Google Scholar]
- 11.Salomonsson S, Jonsson MV, Skarstein K, et al. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren’s syndrome. Arthritis Rheum. 2003;48:3187–201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- 12.Haacke EA, van der Vegt B, Vissink A, et al. Germinal centres in diagnostic labial gland biopsies of patients with primary Sjögren’s syndrome are not predictive for parotid MALT lymphoma development. Ann Rheum Dis. 2017;76:1781–4. doi: 10.1136/annrheumdis-2017-211290. [DOI] [PubMed] [Google Scholar]
- 13.Haacke EA, van der Vegt B, Vissink A, et al. Standardisation of the detection of germinal centres in salivary gland biopsies of patients with primary Sjögren’s syndrome is needed to assess their clinical relevance. Ann Rheum Dis. 2018;77:e32. doi: 10.1136/annrheumdis-2017-212164. [DOI] [PubMed] [Google Scholar]
- 14.Ihrler S, Zietz C, Sendelhofert A, et al. Lymphoepithelial duct lesions in Sjögren-type sialadenitis. Virchows Arch. 1999;434:315–23. doi: 10.1007/s004280050347. [DOI] [PubMed] [Google Scholar]
- 15.Zandbelt MM, Wentink JRM, de Wilde PCM, et al. The synergistic value of focus score and IgA% score of sublabial salivary gland biopsy for the accuracy of the diagnosis of Sjögren’s syndrome: a 10-year comparison. Rheumatology (Oxford) 2002;41:819–23. doi: 10.1093/rheumatology/41.7.819. [DOI] [PubMed] [Google Scholar]
- 16.van Ginkel MS, Nakshbandi U, Arends S, et al. Increased diagnostic accuracy of the labial gland biopsy in primary sjögren syndrome when multiple histopathological features are included. Arthritis Rheumatol. 2024;76:421–8. doi: 10.1002/art.42723. [DOI] [PubMed] [Google Scholar]
- 17.van Ginkel MS, van der Sluis T, Bulthuis MLC, et al. Digital image analysis of intraepithelial B-lymphocytes to assess lymphoepithelial lesions in salivary glands of Sjögren’s syndrome patients. Rheumatology (Oxford) 2022;62:428–38. doi: 10.1093/rheumatology/keac212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meiners PM, Vissink A, Kroese FGM, et al. Abatacept treatment reduces disease activity in early primary Sjögren’s syndrome (open-label proof of concept ASAP study) Ann Rheum Dis. 2014;73:1393–6. doi: 10.1136/annrheumdis-2013-204653. [DOI] [PubMed] [Google Scholar]
- 19.van Nimwegen JF, Mossel E, van Zuiden GS, et al. Abatacept treatment for patients with early active primary Sjögren’s syndrome: a single-centre, randomised, double-blind, placebo-controlled, phase 3 trial (ASAP-III study) Lancet Rheumatol. 2020;2:e153–63. doi: 10.1016/S2665-9913(19)30160-2. [DOI] [PubMed] [Google Scholar]
- 20.Haacke EA, van der Vegt B, Meiners PM, et al. Abatacept treatment of patients with primary Sjögren’s syndrome results in a decrease of germinal centres in salivary gland tissue. Clin Exp Rheumatol. 2017;35:317–20. [PubMed] [Google Scholar]
- 21.Seror R, Ravaud P, Bowman SJ, et al. EULAR Sjogren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann Rheum Dis. 2010;69:1103–9. doi: 10.1136/ard.2009.110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hocevar A, Ambrozic A, Rozman B, et al. Ultrasonographic changes of major salivary glands in primary Sjogren’s syndrome. Diagnostic value of a novel scoring system. Rheumatol (Oxford) 2005;44:768–72. doi: 10.1093/rheumatology/keh588. [DOI] [PubMed] [Google Scholar]
- 23.Rebel D, de Wolff L, Delli K, et al. Added value of the salivary gland ultrasonography OMERACT score in the ACR/EULAR classification criteria for Sjögren’s disease. Semin Arthritis Rheum. 2024;67:152473. doi: 10.1016/j.semarthrit.2024.152473. [DOI] [PubMed] [Google Scholar]
- 24.Jensen SB, Vissink A. Salivary gland dysfunction and xerostomia in Sjögren’s syndrome. Oral Maxillofac Surg Clin North Am. 2014;26:35–53. doi: 10.1016/j.coms.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Spijkervet FKL, Haacke E, Kroese FGM, et al. Parotid gland biopsy, the alternative way to diagnose Sjögren syndrome. Rheum Dis Clin North Am. 2016;42:485–99. doi: 10.1016/j.rdc.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Pijpe J, Kalk WWI, van der Wal JE, et al. Parotid gland biopsy compared with labial biopsy in the diagnosis of patients with primary Sjogren’s syndrome. Rheumatology (Oxford) 2007;46:335–41. doi: 10.1093/rheumatology/kel266. [DOI] [PubMed] [Google Scholar]
- 27.Chisholm DM, Mason DK. Labial salivary gland biopsy in Sjögren’s disease. J Clin Pathol. 1968;21:656–60. doi: 10.1136/jcp.21.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenspan JS, Daniels TE, Talal N, et al. The histopathology of Sjögren’s syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol. 1974;37:217–29. doi: 10.1016/0030-4220(74)90417-4. [DOI] [PubMed] [Google Scholar]
- 29.Kroese FGM, Haacke EA, Bombardieri M. The role of salivary gland histopathology in primary Sjögren’s syndrome: promises and pitfalls. Clin Exp Rheumatol. 2018;36 Suppl 112:222–33. [PubMed] [Google Scholar]
- 30.Bankhead P, Loughrey MB, Fernández JA, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7:16878. doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 32.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the european criteria proposed by the american-european consensus group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Nimwegen JF, van Ginkel MS, Arends S, et al. Validation of the ACR-EULAR criteria for primary Sjögren’s syndrome in a dutch prospective diagnostic cohort. Rheumatology (Oxford) 2018;57:818–25. doi: 10.1093/rheumatology/kex495. [DOI] [PubMed] [Google Scholar]
- 34.McGuirt WF, Whang C, Moreland W. The role of parotid biopsy in the diagnosis of pediatric Sjögren syndrome. Arch Otolaryngol Head Neck Surg. 2002;128:1279–81. doi: 10.1001/archotol.128.11.1279. [DOI] [PubMed] [Google Scholar]
- 35.Basiaga ML, Stern SM, Mehta JJ, et al. Childhood Sjögren syndrome: features of an international cohort and application of the 2016 ACR/EULAR classification criteria. Rheumatol (Oxford) 2021;60:3144–55. doi: 10.1093/rheumatology/keaa757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vitali C, Moutsopoulos HM, Bombardieri S. The european community study group on diagnostic criteria for Sjögren’s syndrome. sensitivity and specificity of tests for ocular and oral involvement in Sjögren’s syndrome. Ann Rheum Dis. 1994;53:637–47. doi: 10.1136/ard.53.10.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjögren’s syndrome. J Autoimmun. 2010;34:400–7. doi: 10.1016/j.jaut.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Bombardieri M, Lewis M, Pitzalis C. Ectopic lymphoid neogenesis in rheumatic autoimmune diseases. Nat Rev Rheumatol. 2017;13:141–54. doi: 10.1038/nrrheum.2016.217. [DOI] [PubMed] [Google Scholar]
- 39.Sato Y, Silina K, van den Broek M, et al. The roles of tertiary lymphoid structures in chronic diseases. Nat Rev Nephrol. 2023;19:525–37. doi: 10.1038/s41581-023-00706-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Risselada AP, Looije MF, Kruize AA, et al. The role of ectopic germinal centers in the immunopathology of primary Sjögren’s syndrome: a systematic review. Semin Arthritis Rheum. 2013;42:368–76. doi: 10.1016/j.semarthrit.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Ben-Aryeh H, Miron D, Szargel R, et al. Whole-saliva secretion rates in old and young healthy subjects. J Dent Res. 1984;63:1147–8. doi: 10.1177/00220345840630091001. [DOI] [PubMed] [Google Scholar]
- 42.Bookman AAM, Shen H, Cook RJ, et al. Whole stimulated salivary flow: correlation with the pathology of inflammation and damage in minor salivary gland biopsy specimens from patients with primary Sjögren’s syndrome but not patients with sicca. Arthritis Rheum. 2011;63:2014–20. doi: 10.1002/art.30295. [DOI] [PubMed] [Google Scholar]
- 43.Mossel E, van Ginkel MS, Haacke EA, et al. Histopathology, salivary flow and ultrasonography of the parotid gland: three complementary measurements in primary Sjögren’s syndrome. Rheumatol (Oxford) 2022;61:2472–82. doi: 10.1093/rheumatology/keab781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blanco E, Pérez-Andrés M, Arriba-Méndez S, et al. Age-associated distribution of normal B-cell and plasma cell subsets in peripheral blood. J Allergy Clin Immunol. 2018;141:2208–19. doi: 10.1016/j.jaci.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 45.Kumar BV, Connors TJ, Farber DL. Human T cell development, localization, and function throughout life. Immunity. 2018;48:202–13. doi: 10.1016/j.immuni.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connors TJ, Baird JS, Yopes MC, et al. Developmental regulation of effector and resident memory T cell generation during pediatric viral respiratory tract infection. J Immunol. 2018;201:432–9. doi: 10.4049/jimmunol.1800396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoppenreijs EPAH. Hereditary C1q deficiency and secondary Sjogren’s syndrome. Ann Rheum Dis. 2004;63:1524–5. doi: 10.1136/ard.2003.016592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teruel M, Alarcón-Riquelme ME. Genetics of systemic lupus erythematosus and Sjögren’s syndrome: an update. Curr Opin Rheumatol. 2016;28:506–14. doi: 10.1097/BOR.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 49.Lessard CJ, Li H, Adrianto I, et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjögren’s syndrome. Nat Genet. 2013;45:1284–92. doi: 10.1038/ng.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.