Abstract
The natural world is under unprecedented and accelerating pressure. Much work on understanding resilience to local and global environmental change has, so far, focussed on ecosystems. However, understanding a system’s behaviour requires knowledge of its component parts and their interactions. Here we call for increased efforts to understand ‘biological resilience’, or the processes that enable components across biological levels, from genes to communities, to resist or recover from perturbations. Although ecologists and evolutionary biologists have the tool-boxes to examine form and function, efforts to integrate this knowledge across biological levels and take advantage of big data (e.g. ecological and genomic) are only just beginning. We argue that combining eco-evolutionary knowledge with ecosystem-level concepts of resilience will provide the mechanistic basis necessary to improve management of human, natural and agricultural ecosystems, and outline some of the challenges in achieving an understanding of biological resilience.
Subject terms: Ecology, Biodiversity, Community ecology, Ecological modelling, Ecosystem ecology, Evolutionary ecology, Molecular ecology, Palaeoecology
Introduction
The Anthropocene is characterised by the pervasive impact of human activity on all aspects of life on earth1. Human-driven climate change and overexploitation of natural resources, as well as increasing human population densities and urbanisation, are placing progressively larger areas under human influence and disturbances such as increased and/or more variable temperatures (and associated events such as droughts and fires), direct anthropogenic alterations (e.g. pollution, land-use changes, habitat fragmentation), and introduction of invasive species2. Even the world’s topology has changed, as global movement of individuals and goods erodes biogeographical barriers3. These environmental changes put ecosystems under unprecedented and accelerating pressures, inducing regime shifts4, causing loss of ecosystem services5, and even changing the course of evolution6. There is therefore an urgent need to determine why some species, communities or ecosystems decay while others persist or adapt7, and then implement this knowledge for improved management practices that can reverse or mitigate damage8.
In ecology, ‘resilience’ has attracted great interest as a concept that describes the capacity of a system to respond to disturbance (Table 1, Fig. 1 inset, following ref. 9; see ref.s10,11 for recent in-depth reviews of definitions). Ecosystems may show strong ‘resistance’ with minimal perturbation in state or function. Or, if perturbed (i.e. low resistance), ecosystems may over time ‘recover’ and move back towards their previous state, or even benefit from the disturbance. Systems with low recovery potential, on the other hand, may shift abruptly (i.e. a tipping point) into a new and possibly stable state (i.e. a regime shift). Resilience has therefore typically been studied theoretically and empirically by considering how a system returns to its previous state (‘engineering resilience’12) or by the amount of disturbance absorbed before it tips into a different state (‘ecological resilience’13). However, translating the concept of resilience into an understanding of the mechanisms or properties that determine how much an ecosystem can absorb or resist a disturbance, or what shapes the trajectory of its recovery back to a previous or new stable state, remains challenging10,14. In part, this may be because resilience has typically been studied at the level of the ecosystem15,16 which reduces our power to identify how and why resistance and/or recovery responses occur17: understanding the behaviour and interactions of a system’s component parts is essential to understand and forecast ecology18. On the other hand, while studying lower biological levels in isolation makes it easier to measure properties that might comprise a system’s resilience (e.g. population size, individual fecundity, genetic diversity; see Fig. 1 inset), reductionist approaches can hinder detection of connections between seemingly isolated biological events19. How can we deal with this complexity to identify the critical drivers and indicators of resistance and recovery?
Table 1.
Definitions and examples of key terminology and how used across biological levels.
| Term | Definition - resilience in ecology | Definition or use at biological levels | Examples relevant for biological resilience |
|---|---|---|---|
| Disturbance event | abiotic or biotic force, process, or agent with potential to impact a system | equivalent but not often used as a defined term. Somewhat analogous to a selection event |
• broad scale e.g. Climate change & local scale e.g. introduction of invasive species • short to long duration, or pulses • multiple disturbances are possible, related directly (e.g. temperature & drought) or indirectly (e.g. eutrophication & invasive species) • can be experimentally approximated in the field and/or lab |
| Perturbation | response of a system to a disturbance, measured as change in a state variable | alteration of function |
• sometimes synonymous with ‘disturbance’ • ‘Perturbation biology’ concerns changes in proteins and cellular features, modelled using networks121 • e.g. gene knock-out studies as perturbation to multiple factors122, conservation translocations of social phenotypes87 |
| Resilience | capacity of a system to manage disturbance | somewhat analogous to homoeostasis, a self-regulating feedback process that maintains physiological stability |
• where used, resilience can be a metaphor, property of dynamic models, or a measurable quantity • broad adoption of resilience including socioecological systems, neurobiology, psychology, medicine • disruption of homoeostasis leads to disease, i.e. a state-change123 |
| Resistance | ability to persist despite a disturbance event | preventing infection or invasion by an enemy; includes physical, behavioural or cellular defences |
• cellular ‘memory’ from past exposure influences drug resistance of cancer cells124 • behavioural defences against parasites vary according to social structure125 |
| Recovery | ability to return over time towards a pre-disturbance state | somewhat analogous to tolerance, ability to maintain fitness despite e.g. infection, lack of resource |
• measured as time to recover, amount of recovery, and rate of recovery11 • physiological drought-tolerance as mechanism for resilience of grasslands to climate change126 |
| Plasticity | not commonly used, but a potentially important mechanism shaping resistance and recovery components of resilience? | variation in the expression of a gene/trait due to differences in environmental conditions |
• gene expression plasticity and stress tolerance127 • physiological plasticity and resilience to climate warming46 • behavioural plasticity and resilience of communities128 |
Fig. 1.
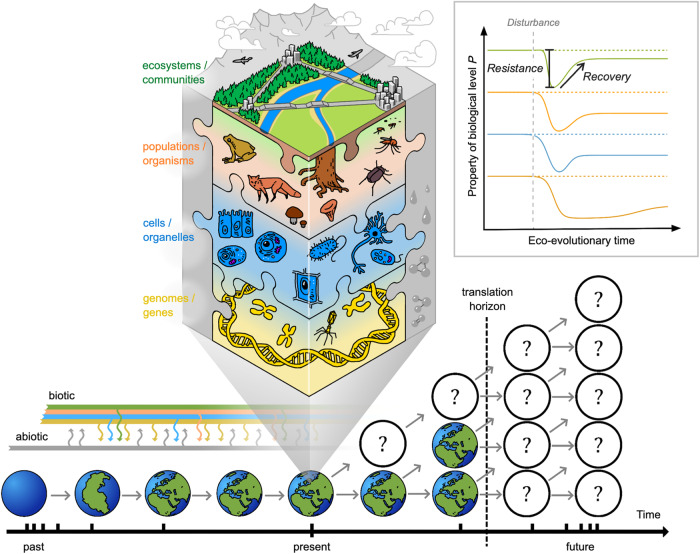
Biological resilience (mechanisms and processes across biological levels that enable systems to resist disturbance and/or recover over time back to a steady state after perturbations) is mediated by connections within and among levels of organisation (simplified to genes and genomes, cells and organelles, organisms and populations, communities and ecosystems; depicted by multi-coloured shading and lines), and recognises that the present state (expanded in centre of figure) is shaped by ecological and evolutionary responses to past biotic (multi-coloured) and abiotic (grey) disturbance and selection (note that time is represented by a log-scale). Resistance (change) and recovery (time, state and rate) can be measured using properties of different biological levels (inset) to provide a ‘common currency’ for integration, and then enhance the translation horizon (vertical dashed line, close in time) by providing more readily measurable indicators and improving accuracy of forecast outcomes (grey arrows and question marks within circles). Note that the resistance and recovery trajectories of biological levels to a disturbance event may differ in both amplitude and temporal scale (inset), and that ‘recovery’ is also sometimes referred to in the literature as a measure of resilience (e.g. refs. 11,27).
Here we propose that this can be achieved by adopting a ‘biological resilience’ framework (Fig. 1) where we: (1) test ecosystem-level resilience concepts (i.e. resistance and recovery responses, state changes) across lower levels of biological organisation; and (2) harness knowledge provided by the eco-evolutionary history of adaptation to past perturbations to better understand resilience from the bottom up. In doing so, biological resilience acknowledges that processes occurring within and between components across biological levels, from genes to communities, shape how systems resist disturbance or recover from perturbations. This framework stands out from recent calls to encourage analysis of resilience across systems and scales, and from ecosystems to populations (e.g. refs. 16,17) as we explicitly acknowledge the crucial role of eco-evolutionary history. Furthermore, investigating how biological levels themselves respond over time to a disturbance event (e.g. from changes in cellular processes to genetic adaptation via measures of gene or allelic diversity) would provide scope for a common ‘language’ and integration of data to dive deeper into uncovering the mechanisms and processes that afford resilience from individuals to communities and ecosystems. We first (i) explore how the eco-evolutionary past provides context for present and future resistance and recovery responses, and then (ii) discuss why it is necessary to consider how abiotic and biotic disturbance events can affect biological levels differently to detect mechanisms and underlying processes. Next, we (iii) outline three testable hypotheses to kick-start research into resilience across levels of biological organisation, from genes to cells, individuals, populations and communities. Collecting and integrating large amounts of data about how every biological component responds to a disturbance is often considered unrealistic. However, here we (iv) identify new opportunities emerging from the ongoing infusion of big data into ecology and evolutionary biology and stress the need to combine these data with experimental approaches to (v) enable advances in translating research into practice. Each of these steps is beginning to be investigated (examples across taxa, biological levels, and ecological context are given throughout) but they lack an overarching framework that brings all of them together. Our aim here is therefore not to cover all aspects of how resilience is, or could be, studied in-depth, but to extend recent calls to move from ecosystems to species (e.g. ref. 16) and encourage discussion of why and how ‘resilience thinking’ could be applied across biological levels.
(Re)Placing resilience into an eco-evolutionary context
When Holling introduced ecological resilience in his landmark paper13, he briefly suggested that a system’s resilience is a product of its evolutionary history (1973:p.18). Most research conducted since, however, has lacked an evolutionary perspective14,20. Therefore, much of the discussion, theory and examples of resilience in ecology lack a long time horizon and largely ignore how past environments influence current (or future) resistance and recovery responses21. Similarly, eco-evolutionary biologists rarely study how a system’s resilience might be conferred by processes that occur within or across the biological levels that form the focus of their studies22, despite research programmes often having a shared interest in determining how particular measurable traits or variables vary in response to a stressor or disturbance event (e.g. ref. 23). This disconnect among fields may be because much of the work on resilience describes patterns at the ecosystem level14, whereas studies of evolutionary processes rarely scale to complex communities24. Indeed, focusing on how ecology and evolution shape patterns and processes within individuals and populations has attracted criticism for being too narrow to address large ecological problems20,25. Nevertheless, here we argue that adopting a ‘common currency’ of studying resistance and recovery across biological levels will improve integration of eco-evolutionary theory with resilience (see Box 1) and provide information from the evolutionary past to improve our power to estimate both present and future states.
Box 1 Integrating ecology and evolution to understand biological resilience.
Evolutionary mechanisms (mutation, drift, migration, natural selection) generate changes in allele frequencies from one generation to another (i.e. microevolution) and, given sufficient time or conditions, can lead to large-scale changes that transcend species boundaries (i.e. macroevolution). Similarly, processes that influence ecology (e.g. density, connectivity, competition, species interactions) at smaller scales (e.g. within populations, communities) give rise to large-scale macroecological patterns (e.g. biodiversity and ecosystem function). Darwin made no distinction between micro and macro scales, nor did he (or Wallace) separate ecology from evolutionary processes (see ref. 20). Over the 20th century, however, research in ecology and evolution specialised to specific scales and processes that presents a major challenge for understanding ecological patterns and processes20. Adopting a biological resilience framework necessitates reintegration. How might this be achieved?
(i) Harness existing and emerging approaches
The combination of theory, modelling and empirical approaches of eco-evolutionary dynamics provides a potential solution to reintegrate ecological and evolutionary processes across biological levels and scales85,136,137 and detect relevant responses to environmental change (e.g. refs. 23,138). Here, phenotypic and genotypic variation coupled with rapid evolution play a key role to explain how populations scale up to influence species interactions and ecological communities (including their structure, function, and dynamics), as well as influence how selection pressures are responded to and genomes are inherited. Work in this rapidly developing field is scaling up from population-level studies139 to analyse how evolutionary processes impact ecological dynamics (and vice versa) in communities and even ecosystems136, with explicit acknowledgement that interactions and feedback also occur across non-adjacent biological levels (see Fig. 1 in refs. 136,140, and see ref. 141 for a review of available models) – as we propose here for biological resilience. Studies of eco-evolutionary dynamics are possible in both the lab and the field85 and are expanding in scope towards a landscape perspective24,51,142. Taking an eco-evolutionary approach to consider feedbacks on ecosystem-level processes is also now beginning to attract attention, suggesting that evolutionary changes in the variation of traits may play an important role in shaping how and when ecosystems reach tipping points and possibly irreversible ecosystem change22.
(ii) Recognise conceptual similarities
Understanding biological resilience will require a step change to move from describing either macro- or micro- scale patterns to demonstrating how evolutionary and ecological processes shape short- and longer-term responses to environmental change. Fortunately, eco-evolutionary dynamics and resilience in ecology rely on similar landscape-based frameworks to conceptualise and mathematically explore predictions. Resilience is typically described by a ‘stability landscape’ where valleys in the landscape represent alternative stable states and disturbance events create wobbles that may push systems over the hilltops between valleys (see ref. 10). In principle, these landscapes can be described by mathematical functions and may be measured by identifying the critical state variables that describe its dimensions, although in practice it remains very challenging to identify alternative stable states available in the past or the future10. Similarly, evolutionary biology makes use of ‘adaptive landscapes’ where fitness functions are described according to phenotypic traits (or genotypes) to conceptualise and predict the strength and direction of selection. Populations or species are described as ‘climbing’ towards ‘adaptive peaks’ of trait/genotype combinations with the highest fitness, where ‘adaptive valleys’ of lower fitness inhibit movement across the landscape. Emerging topics of research include integrating environmental variables to understand past, current and future movement among adaptive peaks (e.g. refs. 143,144). Although the axes of adaptive and resilience landscapes are at different biological levels (typically populations and ecosystems, respectively) and the location of stable states are inverse (‘peaks’ in adaptive landscapes, ‘valleys’ in resilience landscapes), in both cases the population or system of interest is expected to oscillate and move across the landscape in response to ecological change. Considerable effort is now going into translating these landscapes from metaphor to useful predictive tools (e.g. resilience landscapes10, adaptive landscapes143) meaning the time is right to bridge the gap.
(iii) Identify shared terminology
A lack of common language is a widely recognised barrier to disciplinary integration, and this is further exacerbated when fields share jargon but differ in definitions, or when definitions of key terms are easily confused (e.g. ref. 145). This is a problem for integrating evolutionary biology with resilience across biological levels as ecologists and evolutionary biologists share terms but use them differently (Table 1). For example, ‘resistance’ is used in resilience (e.g. ref. 11) to describe how much a system is perturbed by a disturbance event (i.e. a rate) whereas biologists studying pathogens, parasites and antagonistic coevolution define resistance as a strategy to prevent or limit infection by an enemy (i.e. a trait, or suite of traits; e.g. ref. 146). ‘Tolerance’ on the other hand describes how much a host can prolong its survival or recover its reproductive success, given infection147. This perhaps has analogies to ‘recovery’ back to a stable state in resilience (see Introduction, Fig. 1), although time to recover is less of a focus in studies of tolerance than resilience. Providing in depth equations is beyond the scope of this conceptual overview, and even amongst existing studies of resilience, there is variation in how resistance and recovery parameters are measured (see ref. 11 for an overview of studies of resilience in forest trees, soil communities, and watersheds). The first step for comprehensive studies utilising information across disciplines is therefore to build a shared glossary, preferably in mathematical terms that relate to the landscapes outlined in (ii), with expectations of how (and when) putative state variables at the different biological levels being measured will respond to the disturbance event of interest.
Using eco-evolutionary theory to read the past from the present state
Estimating components (or attributes) of resilience such as resistance and recovery rely on measures before, during and after disturbance events (see ref. 11 for example equations used in different ecological contexts). This presents a major challenge for understanding resilience, as even if the ‘before disturbance event’ state is contemporaneous or known, it is rare that information is available about what stable states may have been like in the past. Evolutionary genetics provides an approach to help tackle this problem, as past perturbations leave their mark on the genome (i.e. ‘evolutionary memory’26) which can (i) affect an individual’s capacity to respond, (ii) influence a population’s ability to adapt to changing environmental conditions, and therefore (iii) shape ecological community interactions and potentially ecosystem function now and in the future, even if perturbations are novel to those experienced in the past27. At the level of genes, evolutionary history is manifested in variation introduced by mutation and/or migration (gene flow) as well as recombination (new combinations of genetic variation) that is filtered by natural selection or fixed by random genetic drift. Some genetic variants may provide an advantage against future disturbance events, such as through acquired resistance against a parasite, pest or antibiotic encountered in the past28. On the other hand, disturbances that result in severe population bottlenecks can result in the loss of potentially beneficial variation and/or fixation of maladapted alleles, and thus have negative effects on resilience29. Similarly, past selection that strongly favoured specific alleles may also limit future resilience due to the loss of genetic variation required for new adaptation to take place (e.g. Afrotropical butterfly experiencing climate change induced variation in seasonality30).
The principle of evolutionary parsimony states that species with a shared evolutionary history are likely to have experienced similar selection pressures (e.g. from shared disturbance events) in the past31, and therefore possibly convergent responses at different biological levels. It is perhaps not surprising, then, that a recent study harnessing evolutionary history found that current variation in demographic resilience (i.e. responses of population growth and size) was explained more by phylogenetic relatedness among species than recent (~50 years) environmental stochasticity21. Incorporating historical global temperature records, species-level functional traits, and rates of phylogenetic diversification is also helping to explain how microevolutionary history induces different macroevolutionary responses to temperature change across angiosperms32. Evolutionary history can also be harnessed to understand resilience at the cellular and molecular level, with comparisons of e.g. protein interactomes across the tree of life revealing how these complex networks of molecular interactions evolve greater resilience to a loss of network connections over time33. Moving beyond phylogenetic relatedness, there is a rich body of evolutionary theory (e.g. the Coalescent) and simulation frameworks (e.g. SLiM) available to estimate past population sizes and genetic diversity (summarised in ref. 34) or the prevalence of deleterious genetic mutations in response to dated environmental events (e.g. ref. 35). These tools could be used to model resistance and recovery of populations or species of interest to current and future disturbance scenarios (e.g. ref. 36 and see ref. 37 for a workflow to detect and predict responses to thermal disturbances), or by comparing demographic histories for interacting species (see ref. 34 for an example with great apes, malaria Plasmodium, and the Anopheles mosquito vector), it could soon be possible to gain a deeper perspective on past states of biological levels from populations and single species to communities and ecosystems.
While genetic information will underpin the capability of an organism to respond, there is now also abundant evidence from many taxa that genotypes can generate different phenotypic (including cellular, physiological, morphological, and behavioural) responses depending on environmental conditions (i.e. plasticity, Table 1). Such plasticity can enable individuals to resist negative impacts on fitness and consequently buffer (or even increase) populations from possible demographic perturbations (see ref. 38), or be maladaptive if it leads individuals to respond inappropriately to previously reliable environmental cues (e.g. ref. 39). Phenotypically plastic responses can be modified further depending on the composition, structure and spatial context of the perturbed population or ecological community40, and variation in plasticity can influence individual and species interactions and therefore feedback on community composition and ecosystem function41,42. Although often studied by measuring individual phenotypes or gene expression in response to a specific environmental condition (i.e. representing a disturbance event), it is now acknowledged that plasticity may leave heritable ‘epigenetic’ marks on the genome (i.e. not changes to DNA sequences) that influence the future regulation of gene expression and shape how subsequent generations may resist or recover (e.g. refs. 43,44). Therefore, phenotypic plasticity that evolved in the past may be ‘read’ now to explain current, and predict future, states and interactions across biological levels. While there is growing interest in testing whether current plasticity plays a significant role in resistance and recovery to e.g. climate warming (heterotrophy of corals45, physiology of ectotherms46, demographic variation of commercially-important fishes47), there have been few attempts to ‘read the past’ from current plasticity38,48. Harnessing knowledge about the past to understand biological resilience will likely require integrating phenotypic plasticity, epigenetics and genetic information (e.g. ref. 49), meaning there is potential to provide a major advance across diverse fields.
Finding the right scale: effects of disturbance events vary across biological levels
If we can uncover how elements of the system have responded to past disturbance events or state perturbations, then this information will become useful for predicting current and future changes. However, disturbances can be complex and vary in intensity, duration, frequency and spatial extent50 and the impact of disturbance events on both the degree and timing of any perturbation will vary across biological levels (Fig. 1). For example, an adaptive genetic mutation51 or socially-inherited behaviour52 enabling a species to exploit its perturbed habitat can in turn, alter community assembly through variation in demography. This may or may not occur contemporaneously with the spread of the genetic mutation, as community changes caused by past disturbances may also determine subsequent community assembly through complex cascading effects on species succession (e.g. the order in which species recolonize an area after a habitat perturbation is important for community assembly53) and potentially ecosystem function. Adopting a biological resilience framework could help to predict these events as incorporating a longer time horizon reveals resilience to be a dynamic and constantly evolving product of long term (co-) evolutionary, ecological and biogeographical processes (e.g. ref. 54).
Understanding how these processes operate at different biological levels of organisation will be critical, as the rate of evolution for example is constrained by generation times that vary from minutes (e.g. cells and microbes) to centuries (e.g. trees), reproductive strategy influences opportunities for outcrossing and mutation, and migration can diversify or limit local genotypic and phenotypic variation. However, at present, it remains unclear whether one level in particular will be of greater importance for predicting responses to current and future disturbance, and while it is likely that responses of one level to a given disturbance event will influence how multiple other levels respond, investigations into the carry-over effects of perturbations across biological levels are few and mostly focus on adjacent levels (e.g. changes in population influence response of communities55). The composition, structure and spatial context of a perturbed population or ecological community also needs to be taken into account40. Range-edge populations, for example, can be comprised of a different set of individual response-types than those found in the range core (e.g. spatial sorting56) and potentially set up cascades of change across other biological levels (e.g. reduced genetic diversity57), and fragmented habitats influence the degree to which species can reduce their exposure to perturbations by shifting, shrinking or expanding their range via dispersal58, or by modifying physiological or behavioural responses59. Spatial context also has fundamental implications for longer-term adaptation to environmental change as it shapes gene flow60. Integrating past and present distributions and habitats is therefore likely to be a key, albeit challenging, aspect to understand biological resilience. Nevertheless, using evolutionary history as a ‘natural experiment’ and integrating information about adaptation explicitly into a resilience framework could provide a previously untapped resource for predicting how ecological systems respond to disturbance events.
A biological resilience framework generates testable hypotheses
It is clear that determining how different biological levels resist and recover and buffer other levels from perturbations will be complex, and that harnessing available information from the past is not straightforward. However, theory and mathematical models lay the foundations for identifying what to measure from experimental and empirical systems and how to extract these observations from real data (Box 1). Much of the theoretical work on resilience has made use of complex dynamic system models (e.g. ref. 61), but simpler approaches to calculate resilience are available (e.g. ref. 15), and efforts to incorporate evolutionary perspectives into models of ecosystem-level responses (e.g. tipping points22, warning signals54, species coexistence62) and model complex interactive processes across biological levels (e.g. network models63) are beginning. Furthermore, there is growing theory surrounding the ecological and evolutionary dynamics of resistance (e.g. antibiotics64) and rapid genetic adaptation to ecological change (e.g. ref. 65) that could provide useful approaches to bridge resistance and recovery responses across biological levels. A long-term problem in ecological modelling, however, is that theoretical models are good for understanding causality, but difficult to test critically with data, whereas statistical models are correlative, and thus may not identify the relevant underlying mechanisms even if they fit the present data well. Nevertheless, considering perturbations across biological levels in terms of eco-evolutionary form and function helps generate hypotheses concerning the role of past disturbances in shaping current and future resilience (i.e. resistance and recovery, Fig. 1): (i) past experience primes a biological entity to cope with future disturbances of a similar nature. Alternatively, but not necessarily mutually exclusively, (ii) populations and communities exposed to more variable environments and higher levels of disturbance over the long term are expected to be most resilient. However, even these may accrue a resilience debt if the magnitude and frequency of the disturbances differ too much from their historical disturbance regimes66. Finally, (iii) even without long-term disturbance histories, rapid adaptation may improve resilience against specific stressors. This may, however, come at the cost of decreased resilience in the longer term because of reduced pre-existing diversity after rapid adaptation or altered species interactions57,67. Aspects of these hypotheses have already begun to be tested (Table 2), but not yet across biological levels within a relevant system.
Table 2.
Three hypotheses regarding how the ecological and evolutionary past shapes current and future responses to environmental change, and the multiple study approaches required to understand this biological resilience (with examples).
| Hypotheses | Methodological approaches | Examples |
|---|---|---|
| (i) past experience primes a biological entity to cope best with future disturbances of a similar nature | ||
| Describe patterns using correlational or before-after survey data |
• Current and future responses are mediated by past infection using long-term data on Soay sheep129 • Co-occurrence of taxa before and after Holocene83 |
|
| Use modelling and simulations to generate testable predictions | • Transgenerational priming77 | |
| Perform experimental perturbations in micro- or mesocosms or field settings |
• Experimental evolution with yeast76 • Legacy effects of drought exposure on microbial communities130 • Transgenerational acquired resistance in model plants43 • Resurrection studies93 |
|
| Interrogate findings with data from natural experiments | • Captive and wild songbirds respond differently to temperature perturbations78 | |
| (ii) diversity of environments and disturbances in the past generates greater resilience in the future | ||
| Make use of long-term survey data and/or big ecological and genetic datasets (including ancient DNA) to measure past diversity |
• Paleological history131 • Ecological and evolutionary memory33,66 • Adaptive genetic diversity57 |
|
| Use modelling and simulations to generate testable predictions | • Predicting a species response to environmental change when preadaptation of community differs132 | |
| Perform experimental perturbations in micro- or mesocosms or field settings | • Resurrection studies93 | |
| Interrogate findings with real-world examples, e.g. natural experiments | • Biological invasions133 | |
| (iii) rapid adaptation to match current conditions reduces future resilience | ||
| Compare current resilience of biological entities and search for signs of rapid adaptation in the past | • Genome-wide scans in forest trees to detect adaptation to aridity134 | |
| Use modelling and simulations to generate testable predictions | • Evolutionary rescue135 | |
| Experimentally induce a novel perturbation in cases where rapid adaptation is present vs. absent | • Resurrection studies93 | |
Approaches to understand biological resilience
Understanding biological resilience will require concerted multidisciplinary research programmes where the effects of a disturbance (or multiple stressors) in terms of resistance and recovery responses are investigated across different levels, and where feedback among levels is also measured explicitly (Table 2, Fig. 1). At present, research into coral reef resilience provides a worked example: surveys and experiments have demonstrated that different coral species exhibit different degrees of resistance and recovery to similar stressors68. Comparing the species’ evolutionary history provides some insight into why: a recent study suggests Caribbean corals show lower recovery than Indo-Pacific corals due to an evolutionary bottleneck 2.8 million years ago that favoured large and long-lived species with low rates of recruitment69. Efforts to investigate genomic predictors of coral bleaching70, and even to assist evolution towards more resilient forms71, are also now attracting wide attention72. Furthermore, mapping dependencies of coral-fish species based on natural history and fitting structural equation models has recently suggested that coral loss may lead to substantial negative change in fish diversity and biomass worldwide, with effects extending beyond the fish species directly dependent on corals55. Salmonid fishes (see Box 2) could also provide a model system for similar combinations of approaches to better understand current changes in populations following disturbances (including at the ecosystem level) from fishing, find reliable indicators of the mechanisms that improve recovery, and provide more reliable forecasts of management scenarios.
There are many other studies beyond these examples that report genetic-, phenotypic-, or community-level changes along environmental gradients or responses to natural changes, but far fewer either consider more complex environmental scenarios (e.g. multiple or sequential stressors) or how the effects at one biological level may affect others. As such, much of the current work in understanding biological resilience (even if not yet couched in this terminology) relies on surveys and correlations that are carried out at one level. For example, ‘which genes contribute to more resilient phenotypes?’73, ‘which populations are more resilient to certain perturbations?’74 or, ‘which species are most affected by which particular aspects of a perturbation?’75. Furthermore, the results of experiments, particularly into resilience at the cellular76 or genetic levels77, are often not interpreted in a broader ecological context or compared to available data from natural populations78. Here we explore how we can move beyond studying the effects of single stressors or single species or levels and progress towards more complex experimental designs and assessments of more complex situations in the wild. Although this survey is not exhaustive, we hope that it provides insight into the range of methodologies used across biological levels to better enable discussion and design of multidisciplinary research.
To enable future studies to cover multiple biological levels, incorporating standardized collection of data and sample material across biological levels (e.g. genetic material, phenotype and community structure) into geographical surveys and long-term studies is a good starting point. If these standardised surveys are conducted over multiple seasons, years, or generations, this long-term monitoring has the potential to facilitate (i) detection of subtle responses and/or subtle perturbations, (ii) replication over time, and (iii) detection of ecological and evolutionary memories79. The same recommendation is relevant for “opportunistic” sampling following the (often unexpected) formation of a resilience-relevant gradient/difference. Data for multiple biological levels at sites that have experienced a heat wave for example, or an oil spill or chemical release, can either be compared to those of a nearby site that did not experience the perturbation80, or in the event that surveys of the affected sites were conducted prior to the perturbation, a ‘before vs. after’ analysis can be conducted81. Second, the prehistoric and palaeocological record is an important potential source of survey data, as it is now becoming tractable to incorporate with extant data (e.g. biotic interactions through food web analyses, process-based models of origin and extinction, and species co-occurrence matrices, ref. 82). This paleo-perspective could offer natural experiments: data are available to potentially help explain how community assembly (and disassembly) works when time spans are increased83, for example, or how genetic structure and adaptations respond to perturbations ranging from major extinctions to rapid climate change or species invasions over long time periods (e.g. ref. 84).
A major challenge for survey approaches mentioned above however is to disentangle the effects of co-varying environmental characteristics (e.g. photoperiod and temperature along a latitudinal gradient, or simultaneous drought and reduced food availability). Therefore, experiments in semi-natural (e.g. in vitro microcosms or outdoor mesocosm setups) or field settings (e.g. ponds/tanks, forest/field plots, enclosures suitable for small mammals, or free-ranging individuals and populations) are an essential third approach to test how resilience occurs across biological levels, and offer an attractive compromise where ‘real-world’ conditions are partly retained but where some manipulation and/or control is nevertheless possible, together with replicates85. These experiments can range greatly across organismal scale, geography, and biological levels (e.g. ref. 48,86), and can also be conducted alongside interventions to mitigate species decline or change in ecosystem function (e.g. conservation actions including introductions of individuals or translocations of populations87), if the selection of individuals or species to be moved is designed to test the relative resilience of different characteristics (e.g. social behaviour88, genetic diversity67). Although further removed from ‘real world’ conditions, common garden experiments (i.e. the rearing individuals in a controlled environment under common conditions) could be used to study responses to environmental or anthropogenic stressors by adding ‘treatments’ such as thermal stress, disease, or changes in community (e.g. flour beetles89, burying beetles90). Here, environmental differences can be eliminated, or specific environmental factors can be tested so that the extent of resilience that is plastic versus evolutionary (e.g. fish91, crops92) can be measured. Resurrection-type experiments (i.e. dormant propagules from ancestral populations) are also a promising approach in taxa where genotypes that have experienced varying conditions in the past are available to test responses under experimental conditions93. Experimental designs like these outlined above have been criticised for over-simplifying ecological processes, however taking an experimental approach will be essential to tease apart the relative effects of multiple stressors, either simultaneously, or sequentially, or at different stages of an organism’s life-history. Starting with experimental designs or studies at single biological levels is tractable yet will enable refining hypotheses and study designs for the future study of other biological levels in more complex conditions.
Fourth, eco-evolutionary and environmental Big Data, from the molecular to the ecosystem level, provide a broad and expanding scope, particularly when datasets span space and/or time. At the molecular level, Big Data on genes and genomes (NCBI94) and databases of their function (Gene Ontology GO95, Kyoto Encyclopaedia of Genes and Genomes KEGG96) are rapidly increasing. These databases are designed to be taxonomically comparable, or even species-neutral, to enable transfer of functional annotation (molecular function, biological role and cellular location) or gene network information derived from model organisms to inferred orthologues in newly sequenced species. If the current focus on medical science or morphological characters broadens to encompass functions in response to ecological stimuli97, then big genomic data will become an even more useful resource for studying the molecular basis of biological resilience. Similarly, finding the most potent data sources for reconstructing time series into the past still requires innovation, but this approach carries considerable promise for analyses of resilience to changes that have already occurred. For example, abiotic data from the last few decades are now openly available (e.g. CORINE98, WorldClim99, CHELSA100) and big data on species occurrences (GBIF101), traits (TRY102) and abundances through time103 are becoming available at an increasing rate. Collecting data of changes in the deeper past requires continued efforts in digitising physical collections (museum specimens104) and application and development of new techniques for data extraction and analysis82.
At present, most of the global databases (e.g. those mentioned above) at present contain (partially) non-comparable data, and experimental data are rarely combined with observational data despite potential to increase credibility of conclusions105. Leveraging big data across biological levels is challenging as it requires intensive upskilling in data integration106 and ideally coordinated platforms for e.g. different ecosystems, communities, or management areas of interest (e.g. ‘ePlant’ platform107 for data across multiple levels from Arabidopsis and crop plants, or ‘Metascape’108 for multiple -omics assays to understand molecular mechanisms). However, as the resolution and density of data increases, and new algorithms that make use of large-scale computational resources become available, the possibilities to find and match comparable drivers-to-biotic-units cases will increase. In the meantime, existing data can be analysed by taking advantage of newly developed methods that minimise biases in unrelated or uncertain data (e.g. Bayesian approaches109), or when fully comparable data are available, by using mechanistic models that allow moving beyond correlative analyses (e.g. individual-based models110). Artificial intelligence could also begin to be utilised to predict the consequences of ongoing and future change. Although ‘black-box’ neural network approaches are popular, symbolic regression (an approach that finds explicit mathematical formulas to explain linear and non-linear relationships) holds much promise for distiling previously hidden natural laws from available data as it derives simpler and more interpretable equations (e.g. in community ecology111). However, an outstanding issue is the need to incorporate measures of sampling effort as unbalanced sampling may lead to incorrect interpretations if not accounted for in analyses112 – a problem similar to discriminatory biases in social data applications of machine learning.
Box 2 Investigating biological resilience of salmonid fishes.
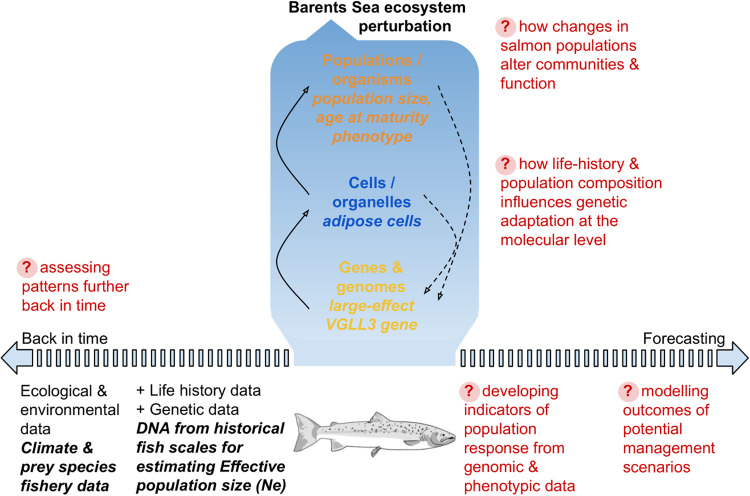
Numerous species and populations of salmonid fishes have been the focus of intensive monitoring and sampling programmes extending across many decades because of their socioeconomic significance and important ecosystem roles (including as keystone species). By combining existing research across biological levels (including genes, cells, populations and ecosystems) and evaluating the next steps within a biological resilience research framework, here we provide a worked example of the value of considering multiple biological levels when investigating an ecosystem-level perturbation.
The Barents Sea ecosystem is being perturbed by rapid increases in fishing pressure and climate change. Long-term ecological and environmental data together with life-history phenotype and genetic information from a population of Atlantic salmon (Salmo salar) from northernmost Europe is now being used to determine how this organism is responding via adaptation and shaping the overall resilience of the ecosystem (Box 2 Figure). Population genetic analyses148 using a 50-year archive of fish scales have supported the hypothesis that reductions in life-history diversity (i.e. apparent low resistance) were actually an adaptive response to the perturbation149. The potential drivers of this response have then been investigated by linking these findings with long-term environmental and salmon prey species ecosystem data. It was discovered that as the abundance of capelin, a fat rich prey, declined so too did the abundance of salmon with a large body size and late-maturing life-history strategy148. Molecular biological research has also shown that the large-effect gene linked with the late-maturing life-history strategy and body condition in salmon150 has important roles in adipocyte production regulation151, thus providing connections about biological resilience processes from genes and cells to populations and ecosystems. This example has implications for fisheries management, as prey species abundance was driven primarily by commercial fishing pressure: capelin is a common protein source in domestic animal (including aquaculture salmon) feed. Thus, research across multiple biological levels demonstrated indirect effects of (capelin) fishing on wild salmon life-history diversity.
To move closer to understanding biological resilience, the next steps include determining how selection acts on life-history traits when undergoing an ecosystem-level perturbation (including epigenetic markers on the genome from changes in cellular function, e.g. ref. 152), investigating how population-level demographic changes in salmon (including composition according to life-history traits) scale up to influence other ecological interactions within the Barents Sea ecosystem, and measuring the response curves (i.e. resistance and recovery, Fig. 1) of genetic diversity, demographic variables (i.e. effective population size), and community composition, before, during and possibly after the perturbation (i.e. depending on potential management scenarios to lessen disturbance on the ecosystem). This could be achieved by targeted experimental approaches and by using Big Data from both long-term surveys mentioned above and ancient DNA to determine response to past known ecological disturbance events (see ref. 153 for an example of herring population dynamics in response to the ‘first example of industrial fishing’ 800 years ago by the Vikings). Demo-genetic individual-based simulations that bring together data from individuals, populations, and communities (e.g. ref. 154) could then be a particularly useful method to link data across biological levels and to forecast future scenarios.
BOX FIGURE Overview of an ongoing worked example investigating biological resilience in Atlantic salmon. Text in red indicates next steps for research, see Box text for details.
Translating biological resilience from research to management and conservation
While there have been many calls to adapt management and conservation of natural resources to improve resilience to environmental change, substantial obstacles remain before this can be realised. First, managers require indicators at levels most appropriate for decision-making. Many of the indicators currently available, however, are system-wide or remain challenging to quantify15,113,114. Indicators based on species diversity and habitat connectivity, for example, allow assessment of large-scale patterns113, but they are less helpful for management of more tractable system components. Similarly, current discussions around genetic diversity are often difficult to reconcile with ecosystem health as they operate at different timescales and in many cases the links to ecosystem functioning remain unclear (e.g. see ref. 115 for a discussion of this problem in the ecological restoration of plants). Second, attempts to manage ‘for resilience’ typically focus on avoiding thresholds or tipping points. Rather, managers need to compare alternative choices, assess potential outcomes with greater certainty than is currently possible, and manage adaptively8 (Weise et al., 2020). Third, management approaches largely aim for current or recent known or assumed historical states, rather than attempting to forecast outcomes according to novel future conditions. This is especially problematic when the time horizon is long8, for example in forestry and agriculture where long or uncertain time horizons play a large part in the difficulty to translate recommendations116,117. Determining how resilience operates at different biological levels has potential to move beyond this stalemate, as the ecological and evolutionary history of components of the system82,118 can be used to better evaluate past states, identify more manageable indicators at tractable biological levels, and predict future states under different management scenarios (e.g. Boxs 2, 3).
Box 3 Applying biological resilience.
Here we highlight the broad potential for the applicability of a biological resilience approach by briefly exploring how it could influence translation and management in two divergent examples: (i) forestry and agriculture, and (ii) human health.
(i) Biological resilience in forestry and agriculture
In the past, forest managers have assumed that the climate and other associated factors will remain stable, in spite of the long generation times and individual lifespans of many forest tree species and biomes117. However, soil degradation (for example) can occur rapidly compared to the lifespan of the forest and then impact on the ability of trees to withstand other environmental perturbations155. Similarly, modern plant breeding selects for yield potential under high and stable resource supply, and generally relies on genetically uniform cultivars. A biological resilience framework, however, encourages a different approach. For example, studies of local adaptation at the population level would help to understand how we can best buffer food and/or timber production against perturbations, perhaps by combining long-term data series and targeted experiments informed by historical farming practices or evolutionary processes117. In a context with clear applications for management, Ives and colleagues recently discovered that spatial heterogeneity in crop-harvesting is a major driver of the ecological and evolutionary feedbacks that limit resistance of pea aphids to parasitoid wasps, an important biological control agent156. Past perturbations also leave abiotic ‘stress memory’, encoded in DNA methylation and chromatin marks, which may increase resilience over multiple generations157,158 in a process of acquired transgenerational resistance43. Similarly, interactions across trophic and biological levels are well-known features of plant growth and health, with key work demonstrating that these also influence resilience (e.g. plant-microbe interactions influence resistance to climate change159). Harnessing this information could lead to improved crop plant and tree breeding programmes (e.g. ref. 160), but much of this work remains embedded in model plant systems, such as Arabidopsis. Understanding which features at what biological level are most important to manage (e.g. managing for genetic diversity of monotypic plantations versus diversity of associated mycorrhizal fungi) will require combined approaches and translation of work from model species to natural systems.
(ii) Biological resilience in human health
While ecological systems are increasingly becoming viewed as socio-ecological systems2, the idea that the human mind and body can be viewed as a complex ecological system is only just beginning to be recognised123,161. Understanding how circadian misalignment of sleep/wake cycles leads to a mismatch between abiotic cues and internal cellular functions (e.g. impairment of beta cell function and insulin sensitivity162), and then scales up to affect system health via resilience to disease and other stressors, could help to provide more appropriate guidelines for managing shift work, for example. Recent experiences with COVID-19 also demonstrate the need to consider how resilience operates across biological levels: identifying what makes an individual more resilient to a virus at the cellular level (e.g. vaccine development) is not enough if insufficient people take up the vaccine (i.e. population level), or if the virus itself evolves resistance. Indeed, understanding the biological resilience of viral infections, or cancerous growths for example, to medical interventions could assist in progress with treatment. Genetic heterogeneity is known to negatively affect treatment success in cancer163, yet this heterogeneity reflects the selective pressures endured, and the variation accumulated, during the whole history of that cancer and can reveal vulnerabilities to therapy164. Furthermore, life-history strategies of cells, such as dormancy, can blunt the effects of therapy (e.g. tuberculosis). This suggests that diversity could be an important component of resilience in human health, but this requires testing in translational models.
Challenges of implementing a biological resilience framework
Here we have argued that understanding and managing for biological resilience requires moving away from the approach of considering function or resilience only at the level of ecosystems, or of focusing studies within a single biological level. We have also stressed how the resilience of the present state not only relies on perturbations experienced in the past (whether contemporary, transgenerational, or deeper in evolutionary time) but that we can also access information about these past responses. Nevertheless, incorporating evolutionary history and complex interactions within and across biological levels is non-trivial, and key challenges exist for modelling complexity and broadening the scope of data collection, as well as setting the temporal and spatial boundaries of the systems or components being studied.
Firstly, in both theoretical and empirical work, we need to identify which connections among what levels are most critical to study. A top-down view of ecosystems works best when considering change over a relatively short period of time, and reduces power for forecasting future responses, either to predicted environmental change or potential management interventions. In ecosystem ecology, species, for example, are normally classified into functional types that leave out valuable information about evolutionary responses to specific perturbations in the past. These responses can however be searched for by mining existing data (e.g. ref. 33) or by experiment (e.g. ref. 30). Similarly, we need to move beyond research focusing on what makes an individual, or a species, resistant or tolerant to some disturbance event without assessing its relevance to systems or communities. Research in eco-evolutionary dynamics is already beginning to tackle these interactions (see Box 1) and adapting this approach to investigate resilience provides a model for moving forwards. While it is not tractable to measure everything, well-controlled experiments can provide critical data to understand the mechanisms that drive biological resilience – or the lack of it. However, as experiments entail at least some simplification of natural complexity, results will need to be linked conceptually to surveys of the relevant organisms and ecosystems.
Considering multiple levels of biological organisation will also necessitate data collection that tracks responses and maximises phylogenetic, functional, spatial and temporal coverage with minimum monetary cost119. This is a challenging task for independent research groups as the acquisition of uninterrupted and consistent time series of ecological and environmental data depend on continued funding. Therefore, coordinated multidisciplinary research projects would enhance data collection and optimise funding streams, making it possible to expand the scope from single- to multiple levels. Some types of data are already available to inform about responses to past conditions, but if we are to make better use of existing and future available datasets, these will require high quality metadata annotations including as many potential ecological variables as possible (and not only the ones directly related to the analyses data were collected for) and easy and open access (e.g. following the FAIR principles120).
Providing the evidence necessary to make the case to policy makers is perhaps the most important challenge. For example, accumulating knowledge on ecosystem resilience is yet to change the principles of forestry or cropland management dramatically, which is alarming given that we know many current management practices compromise the ability of future generations to meet their own needs. This may be because resilience is currently difficult to quantify, and a lack of resilience is easier to recognise than a successful management practice. A biological resilience framework could improve identification of ‘resilience indicators’ at scales in which management decisions are made. Tracking genetic diversity at a species level, for example, is a feasible method to collect robust data, and could enable modelling of which actions are likely to be most successful. A critical further step, however, will be improved monitoring of the impact of potential indicators so that we are able to learn from both successful and less successful implementations. Similarly, there are still substantial gaps to bridge between scientists, policymakers and other stakeholders. For example, in commercial farming and forestry widespread adoption of science-led practices depends on short-term economic benefits, so adoption will require policy-based incentives. A deeper understanding of management practices, and co-creation of research questions with stakeholders that will apply management practices, is essential, particularly if we are to implement decisions using an experimental approach.
In summary, biological resilience requires shifting our perspective in eco-evolutionary studies towards investigating terms of resistance versus recovery (the key conceptual outcomes in ecosystem resilience) while also incorporating an eco-evolutionary perspective to better understand ecosystem-level processes (see Fig. 1, Box 1). This requires real multidisciplinary coordinated actions. But we can also begin to take small steps within existing research programmes. Researchers should consider reframing current research to test theory regarding types of responses to disturbance events under study. Or we could consider how influences from evolutionary history may impact ecological responses being detected under current conditions. Although challenging, this approach should provide the advances in data collection, modelling, and testing of hypotheses across levels that are urgently needed to understand and better support resilience in the face of current and future environmental challenges.
Acknowledgements
This manuscript is a contribution by members of the HiLIFE (Helsinki Institute for Life Science) Grand Challenge programme in Understanding Biological Resilience (BIORESILIENCE), established after external review by the HiLIFE Scientific Council and funded by the Academy of Finland funding instrument PROFI1 (awarded to the University of Helsinki). This initiative brought together 40+ researchers from diverse fields including ecology, evolution, and conservation to neurobiology, veterinary medicine, and cancer biology with the intention to spark discussion and begin novel collaborative projects—many of which were restricted by the COVID-19 pandemic. We are grateful to Jenni Villa and Unni Pulliainen for their project coordination efforts, Mikhail Shubin for his assistance with drawing figures, and helpful discussion and comments on the manuscript from Pol Capdevila. Other funding provided to: R.T.: HiLIFE start-up grant (funded by Academy of Finland PROFI1) and Academy of Finland grant no. 133803; J.C.: Jenny and Antti Wihuri Foundation; P.C.: Kone Foundation; J.K.: HiLIFE Fellows scheme (funded by Academy of Finland PROFI1) and Academy of Finland grant no. 328961; L.K.: Academy of Finland grant no. 12871741; R.M.: Academy of Finland grant no. 312912; O.O.: Academy of Finland grant no. 309581 and Norges Forskningsråd (Research Council of Norway) no. 223257; C.R.P.: HiLIFE Fellows scheme (funded by Academy of Finland PROFI1) and Academy of Finland grant no. 314254. All authors from the Research Centre for Ecological Change were funded by the Jane and Aatos Erkko Foundation.
Author contributions
All authors were involved in conceptualisation, writing—original draft preparation, and writing—review and editing. R.T. and V.M. were responsible for preparation of the final version.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Rose Thorogood, Ville Mustonen.
References
- 1.Lewis, S. L. & Maslin, M. A. Defining the Anthropocene. Nature519, 171–180 (2015). 10.1038/nature14258 [DOI] [PubMed] [Google Scholar]
- 2.Ellis, E. C. Ecology in an anthropogenic biosphere. Ecol. Monogr.85, 287–331 (2015). 10.1890/14-2274.1 [DOI] [Google Scholar]
- 3.van Kleunen, M. et al. Global exchange and accumulation of non-native plants. Nature525, 100–103 (2015). 10.1038/nature14910 [DOI] [PubMed] [Google Scholar]
- 4.Scheffer, M., Carpenter, S., Foley, J. A., Folke, C. & Walker, B. Catastrophic shifts in ecosystems. Nature413, 591–596 (2001). 10.1038/35098000 [DOI] [PubMed] [Google Scholar]
- 5.Foley, J. A. Global consequences of land use. Science309, 570–574 (2005). 10.1126/science.1111772 [DOI] [PubMed] [Google Scholar]
- 6.Sullivan, A. P., Bird, D. W. & Perry, G. H. Human behaviour as a long-term ecological driver of non-human evolution. Nat. Ecol. Evol.1, 0065 (2017). 10.1038/s41559-016-0065 [DOI] [PubMed] [Google Scholar]
- 7.Sutherland, W. J. et al. Identification of 100 fundamental ecological questions. J. Ecol.101, 58–67 (2013). 10.1111/1365-2745.12025 [DOI] [Google Scholar]
- 8.Weise, H. et al. Resilience trinity: safeguarding ecosystem functioning and services across three different time horizons and decision contexts. Oikos129, 445–456 (2020). 10.1111/oik.07213 [DOI] [Google Scholar]
- 9.Helfgott, A. Operationalising systemic resilience. Eur. J. Oper. Res.268, 852–864 (2018). 10.1016/j.ejor.2017.11.056 [DOI] [Google Scholar]
- 10.Dakos, V. & Kéfi, S. Ecological resilience: what to measure and how. Environ. Res. Lett.17, 043003 (2022). 10.1088/1748-9326/ac5767 [DOI] [Google Scholar]
- 11.Yi, C. & Jackson, N. A review of measuring ecosystem resilience to disturbance. Environ. Res. Lett.16, 053008 (2021). 10.1088/1748-9326/abdf09 [DOI] [Google Scholar]
- 12.Pimm, S. L. The complexity and stability of ecosystems. Nature307, 321–326 (1984). 10.1038/307321a0 [DOI] [Google Scholar]
- 13.Holling, C. S. Resilience and stability of ecological systems. Ann. Rev. Ecol. Syst.4, 1–23 (1973). 10.1146/annurev.es.04.110173.000245 [DOI] [Google Scholar]
- 14.Oliver, T. H. et al. Biodiversity and resilience of ecosystem functions. Trends Ecol. Evol.30, 673–684 (2015). 10.1016/j.tree.2015.08.009 [DOI] [PubMed] [Google Scholar]
- 15.Ingrisch, J. & Bahn, M. Towards a comparable quantification of resilience. Trends Ecol. Evol.33, 251–259 (2018). 10.1016/j.tree.2018.01.013 [DOI] [PubMed] [Google Scholar]
- 16.Capdevila, P. et al. Reconciling resilience across ecological systems, species and subdisciplines. J. Ecol.109, 3102–3113 (2021). 10.1111/1365-2745.13775 [DOI] [Google Scholar]
- 17.Gladstone-Gallagher, R. V., Pilditch, C. A., Stephenson, F. & Thrush, S. F. Linking traits across ecological scales determines functional resilience. Trends Ecol. Evol.34, 1080–1091 (2019). 10.1016/j.tree.2019.07.010 [DOI] [PubMed] [Google Scholar]
- 18.Levin, S. A. The problem of pattern and scale in ecology: the Robert H. MacArthur Award lecture. Ecology73, 1943–1967 (1992). 10.2307/1941447 [DOI] [Google Scholar]
- 19.Gallagher, R. & Appenzeller, T. Beyond reductionism. Science284, 79–79 (1999). 10.1126/science.284.5411.79 [DOI] [Google Scholar]
- 20.McGill, B. J. et al. Unifying macroecology and macroevolution to answer fundamental questions about biodiversity. Global Ecol. Biogeogr.28, 1925–1936 (2019). 10.1111/geb.13020 [DOI] [Google Scholar]
- 21.Cant, J., Capdevila, P., Beger, M. & Salguero‐Gómez, R. Recent exposure to environmental stochasticity does not determine the demographic resilience of natural populations. Ecol. Lett.26, 1186–1199 (2023). 10.1111/ele.14234 [DOI] [PubMed] [Google Scholar]
- 22.Dakos, V. et al. Ecosystem tipping points in an evolving world. Nat. Ecol. Evol.3, 355–362 (2019). 10.1038/s41559-019-0797-2 [DOI] [PubMed] [Google Scholar]
- 23.Morris, D. W. Adaptation and habitat selection in the eco-evolutionary process. Proc. R. Soc. B: Biol. Sci.278, 2401–2411 (2011). 10.1098/rspb.2011.0604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tylianakis, J. M. & Maia, L. F. The patchwork of evolutionary landscapes. Nat. Ecol. Evol.4, 672–673 (2020). 10.1038/s41559-020-1184-8 [DOI] [PubMed] [Google Scholar]
- 25.Carroll, S. P. et al. Applying evolutionary biology to address global challenges. Science346, 1245993–1245993 (2014). 10.1126/science.1245993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai, M. M. Reverse evolution and evolutionary memory. Nat. Genet.41, 142–143 (2009). 10.1038/ng0209-142 [DOI] [PubMed] [Google Scholar]
- 27.Donohue, I. et al. Navigating the complexity of ecological stability. Ecol. Lett.19, 1172–1185 (2016). 10.1111/ele.12648 [DOI] [PubMed] [Google Scholar]
- 28.Bartholomé, J. et al. The genetics of exapted resistance to two exotic pathogens in pedunculate oak. New Phytol.226, 1088–1103 (2020). 10.1111/nph.16319 [DOI] [PubMed] [Google Scholar]
- 29.Donelson, J. M. et al. Understanding interactions between plasticity, adaptation and range shifts in response to marine environmental change. Philos. Trans. R. Soc. B374, 20180186 (2019). 10.1098/rstb.2018.0186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oostra, V., Saastamoinen, M., Zwaan, B. J. & Wheat, C. W. Strong phenotypic plasticity limits potential for evolutionary responses to climate change. Nat. Commun.9, 1005 (2018). 10.1038/s41467-018-03384-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hang, D., Torng, E., Ofria, C. & Schmidt, T. M. The effect of natural selection on the performance of maximum parsimony. BMC Evol. Biol.7, 1–15 (2007). 10.1186/1471-2148-7-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun, M. et al. Recent accelerated diversification in rosids occurred outside the tropics. Nat. Commun.11, 1–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zitnik, M., Sosič, R., Feldman, M. W. & Leskovec, J. Evolution of resilience in protein interactomes across the tree of life. Proc. Natl Acad. Sci. USA116, 4426–4433 (2019). 10.1073/pnas.1818013116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hecht, L. B., Thompson, P. C. & Rosenthal, B. M. Assessing the evolutionary persistence of ecological relationships: a review and preview. Infect. Genet. Evol.84, 104441 (2020). 10.1016/j.meegid.2020.104441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathur, S., Tomeček, J. M., Tarango-Arámbula, L. A., Perez, R. M. & DeWoody, J. A. An evolutionary perspective on genetic load in small, isolated populations as informed by whole genome resequencing and forward-time simulations. Evolution77, 690–704 (2023). 10.1093/evolut/qpac061 [DOI] [PubMed] [Google Scholar]
- 36.Louis, M. et al. Influence of past climate change on phylogeography and demographic history of narwhals, Monodon monoceros. Proc. R. Soc. B287, 20192964 (2020). 10.1098/rspb.2019.2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortés, A. J., López-Hernández, F. & Osorio-Rodriguez, D. Predicting thermal adaptation by looking into populations’ genomic past. Front. Genet.11, 564515 (2020). 10.3389/fgene.2020.564515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox, R. J., Donelson, J. M., Schunter, C., Ravasi, T. & Gaitán-Espitia, J. D. Beyond buying time: the role of plasticity in phenotypic adaptation to rapid environmental change. Philos. Trans. R.Soc. B374, 20180174 (2019). 10.1098/rstb.2018.0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghalambor, C. K., McKay, J. K., Carroll, S. P. & Reznick, D. N. Adaptive versus non‐adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol.21, 394–407 (2007). 10.1111/j.1365-2435.2007.01283.x [DOI] [Google Scholar]
- 40.Thebault, E. & Fontaine, C. Stability of ecological communities and the architecture of mutualistic and trophic networks. Science329, 853–856 (2010). 10.1126/science.1188321 [DOI] [PubMed] [Google Scholar]
- 41.Turcotte, M. M. & Levine, J. M. Phenotypic plasticity and species coexistence. Trends Ecol. Evol.31, 803–813 (2016). 10.1016/j.tree.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 42.Hess, C., Levine, J. M., Turcotte, M. M. & Hart, S. P. Phenotypic plasticity promotes species coexistence. Nat. Ecol. Evol.6, 1256–1261 (2022). 10.1038/s41559-022-01826-8 [DOI] [PubMed] [Google Scholar]
- 43.Holeski, L. M., Jander, G. & Agrawal, A. A. Transgenerational defense induction and epigenetic inheritance in plants. Trends Ecol. Evol.27, 618–626 (2012). 10.1016/j.tree.2012.07.011 [DOI] [PubMed] [Google Scholar]
- 44.Pazzaglia, J., Reusch, T. B. H., Terlizzi, A., Marín‐Guirao, L. & Procaccini, G. Phenotypic plasticity under rapid global changes: the intrinsic force for future seagrasses survival. Evol. Appl.14, 1181–1201 (2021). 10.1111/eva.13212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grottoli, A. G., Rodrigues, L. J. & Palardy, J. E. Heterotrophic plasticity and resilience in bleached corals. Nature440, 1186–1189 (2006). 10.1038/nature04565 [DOI] [PubMed] [Google Scholar]
- 46.Seebacher, F., White, C. R. & Franklin, C. E. Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Change5, 61–66 (2015). 10.1038/nclimate2457 [DOI] [Google Scholar]
- 47.Taylor, B. M. et al. Demographic plasticity facilitates ecological and economic resilience in a commercially important reef fish. J. Anim. Ecol.88, 1888–1900 (2019). 10.1111/1365-2656.13095 [DOI] [PubMed] [Google Scholar]
- 48.Valladares, F. et al. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett.17, 1351–1364 (2014). 10.1111/ele.12348 [DOI] [PubMed] [Google Scholar]
- 49.McNamara, J. M., Dall, S. R. X., Hammerstein, P. & Leimar, O. Detection vs. selection: integration of genetic, epigenetic and environmental cues in fluctuating environments. Ecol. Lett.19, 1267–1276 (2016). 10.1111/ele.12663 [DOI] [PubMed] [Google Scholar]
- 50.Barnosky, A. D. et al. Approaching a state shift in Earth’s biosphere. Nature486, 52–58 (2012). 10.1038/nature11018 [DOI] [PubMed] [Google Scholar]
- 51.Legrand, D. et al. Eco-evolutionary dynamics in fragmented landscapes. Ecography40, 9–25 (2017). 10.1111/ecog.02537 [DOI] [Google Scholar]
- 52.Hämäläinen, L., M. Rowland, H., Mappes, J. & Thorogood, R. Social information use by predators: expanding the information ecology of prey defences. Oikos2022, e08743 (2022). 10.1111/oik.08743 [DOI] [Google Scholar]
- 53.Fukami, T. Historical contingency in community assembly: integrating niches, species pools, and priority effects. Ann. Rev. Ecol. Evol. Syst.46, 1–23 (2015). 10.1146/annurev-ecolsys-110411-160340 [DOI] [Google Scholar]
- 54.Baruah, G., Clements, C. F. & Ozgul, A. Eco‐evolutionary processes underlying early warning signals of population declines. J. Anim. Ecol.89, 436–448 (2020). 10.1111/1365-2656.13097 [DOI] [PubMed] [Google Scholar]
- 55.Strona, G. et al. Global tropical reef fish richness could decline by around half if corals are lost. Proc. R. Soc. Lond. B288, 20210274 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Massot, M., Legendre, S., Fédérici, P. & Clobert, J. Climate warming: a loss of variation in populations can accompany reproductive shifts. Ecol. Lett.20, 1140–1147 (2017). 10.1111/ele.12811 [DOI] [PubMed] [Google Scholar]
- 57.Sgrò, C. M., Lowe, A. J. & Hoffmann, A. A. Building evolutionary resilience for conserving biodiversity under climate change. Evol. Appl.4, 326–337 (2011). 10.1111/j.1752-4571.2010.00157.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fahrig, L. Effects of habitat fragmentation on biodiversity. Ann. Rev. Ecol. Evol. Syst.34, 487–515 (2003). 10.1146/annurev.ecolsys.34.011802.132419 [DOI] [Google Scholar]
- 59.Baguette, M. & Van Dyck, H. Landscape connectivity and animal behavior: functional grain as a key determinant for dispersal. Landsc. Ecol.22, 1117–1129 (2007). 10.1007/s10980-007-9108-4 [DOI] [Google Scholar]
- 60.Anderson, C. D. et al. Considering spatial and temporal scale in landscape-genetic studies of gene flow. Mol. Ecol.19, 3565–3575 (2010). 10.1111/j.1365-294X.2010.04757.x [DOI] [PubMed] [Google Scholar]
- 61.Clark, A. T. et al. General statistical scaling laws for stability in ecological systems. Ecol. Lett.24, 1474–1486 (2021). 10.1111/ele.13760 [DOI] [PubMed] [Google Scholar]
- 62.Yamamichi, M., Gibbs, T. & Levine, J. M. Integrating eco‐evolutionary dynamics and modern coexistence theory. Ecol. Lett.25, 2091–2106 (2022). 10.1111/ele.14078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cavigelli, S., Leips, J., Xiang, Q. Y., Lemke, D. & Konow, N. Next steps in integrative biology: mapping interactive processes across levels of biological organization. Integr. Comp. Biol.61, 2066–2074 (2021). 10.1093/icb/icab161 [DOI] [PubMed] [Google Scholar]
- 64.Meredith, H. R. et al. Applying ecological resistance and resilience to dissect bacterial antibiotic responses. Sci. Adv.4, eaau1873 (2018). [DOI] [PMC free article] [PubMed]
- 65.Waldvogel, A. et al. Evolutionary genomics can improve prediction of species’ responses to climate change. Evol. Lett.4, 4–18 (2020). 10.1002/evl3.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waples, R. S., Beechie, T. & Pess, G. R. Evolutionary history, habitat disturbance regimes, and Anthropogenic changes: what do these mean for resilience of Pacific salmon populations?. Ecol. Soc.14, art3 (2009). 10.5751/ES-02626-140103 [DOI] [Google Scholar]
- 67.Stange, M., Barrett, R. D. H. & Hendry, A. P. The importance of genomic variation for biodiversity, ecosystems and people. Nat. Rev. Genet.22, 89–105 (2021). 10.1038/s41576-020-00288-7 [DOI] [PubMed] [Google Scholar]
- 68.Hughes, T. P., Graham, N. A., Jackson, J. B., Mumby, P. J. & Steneck, R. S. Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evolu.25, 633–642 (2010). 10.1016/j.tree.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 69.Roff, G. Evolutionary history drives biogeographic patterns of coral reef resilience. Bioscience71, 26–39 (2021). [Google Scholar]
- 70.Fuller, Z. L. et al. Population genetics of the coral Acropora millepora: toward genomic prediction of bleaching. Science369, eaba4674 (2020). 10.1126/science.aba4674 [DOI] [PubMed] [Google Scholar]
- 71.van Oppen, M. J. H., Oliver, J. K., Putnam, H. M. & Gates, R. D. Building coral reef resilience through assisted evolution. Proc. Natl Acad. Sci. USA112, 2307–2313 (2015). 10.1073/pnas.1422301112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bay, R. A. & Guerrero, L. Can genomes predict coral bleaching? Science369, 249–250 (2020). 10.1126/science.abc9342 [DOI] [PubMed] [Google Scholar]
- 73.Papakostas, S. et al. A proteomics approach reveals divergent molecular responses to salinity in populations of European whitefish (Coregonus lavaretus). Mol. Ecol.21, 3516–3530 (2012). 10.1111/j.1365-294X.2012.05553.x [DOI] [PubMed] [Google Scholar]
- 74.Thom, D. et al. The climate sensitivity of carbon, timber, and species richness covaries with forest age in boreal–temperate North America. Glob. Change Biol.25, 2446–2458 (2019). 10.1111/gcb.14656 [DOI] [PubMed] [Google Scholar]
- 75.Strayer, D. L. Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshw. Biol.55, 152–174 (2010). 10.1111/j.1365-2427.2009.02380.x [DOI] [Google Scholar]
- 76.López-Maury, L., Marguerat, S. & Bähler, J. Tuning gene expression to changing environments: from rapid responses to evolutionary adaptation. Nat. Rev. Genet.9, 583–593 (2008). 10.1038/nrg2398 [DOI] [PubMed] [Google Scholar]
- 77.Kokko, H. et al. Can evolution supply what ecology demands? Trends Ecol. Evol.32, 187–197 (2017). 10.1016/j.tree.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 78.Verhagen, I., Tomotani, B. M., Gienapp, P. & Visser, M. E. Temperature has a causal and plastic effect on timing of breeding in a small songbird. The J. Exp. Biol.223, jeb218784 (2020). 10.1242/jeb.218784 [DOI] [PubMed] [Google Scholar]
- 79.Grant, P. R. et al. Evolution caused by extreme events. Philos. Trans. R. Soc. B: Biol. Sci.372, 20160146 (2017). 10.1098/rstb.2016.0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ellegren, H., Lindgren, G., Primmer, C. R. & Møller, A. P. Fitness loss and germline mutations in barn swallows breeding in Chernobyl. Nature389, 593–596 (1997). 10.1038/39303 [DOI] [PubMed] [Google Scholar]
- 81.Bergen, E. et al. The effect of summer drought on the predictability of local extinctions in a butterfly metapopulation. Conserv. Biol.34, 1503–1511 (2020). 10.1111/cobi.13515 [DOI] [PubMed] [Google Scholar]
- 82.Fraser, D. et al. Investigating biotic interactions in deep time. Trends Ecol. Evol.36, 61–75 (2020). 10.1016/j.tree.2020.09.001 [DOI] [PubMed] [Google Scholar]
- 83.Lyons, S. K. et al. Holocene shifts in the assembly of plant and animal communities implicate human impacts. Nature529, 80–83 (2016). 10.1038/nature16447 [DOI] [PubMed] [Google Scholar]
- 84.Frisch, D. et al. A millennial-scale chronicle of evolutionary responses to cultural eutrophication in Daphnia. Ecol. Lett.17, 360–368 (2014). 10.1111/ele.12237 [DOI] [PubMed] [Google Scholar]
- 85.Hendry, A. P. A critique for eco‐evolutionary dynamics. Funct. Ecol.33, 84–94 (2019). 10.1111/1365-2435.13244 [DOI] [Google Scholar]
- 86.Carpenter, S. R. et al. Early warnings of regime shifts: a whole-ecosystem experiment. Science332, 1079–1082 (2011). 10.1126/science.1203672 [DOI] [PubMed] [Google Scholar]
- 87.Franks, V. R. et al. Changes in social groups across reintroductions and effects on post-release survival. Anim. Conserv.23, 443–454 (2020). 10.1111/acv.12557 [DOI] [Google Scholar]
- 88.Goldenberg, S. Z. et al. Increasing conservation translocation success by building social functionality in released populations. Glob. Ecol. Conserv.18, e00604 (2019). [Google Scholar]
- 89.Koch, E. L. & Guillaume, F. Additive and mostly adaptive plastic responses of gene expression to multiple stress in Tribolium castaneum. PLoS Genet.16, e1008768 (2020). 10.1371/journal.pgen.1008768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun, S.-J. & Kilner, R. M. Temperature stress induces mites to help their carrion beetle hosts by eliminating rival blowflies. ELife9, e55649 (2020). 10.7554/eLife.55649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Papakostas, S. et al. Gene pleiotropy constrains gene expression changes in fish adapted to different thermal conditions. Nat. Commun.5, 4071 (2014). 10.1038/ncomms5071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bustos‐Korts, D. et al. Exome sequences and multi‐environment field trials elucidate the genetic basis of adaptation in barley. Plant J.99, 1172–1191 (2019). 10.1111/tpj.14414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Franks, S. J. et al. The resurrection initiative: storing ancestral genotypes to capture evolution in action. BioScience58, 870–873 (2008). 10.1641/B580913 [DOI] [Google Scholar]
- 94.National Library of Medicine (US) & National Center for Biotechnology Information. National Center for Biotechnology Information (NCBI). https://www.ncbi.nlm.nih.gov/ (2020).
- 95.Ashburner, M. et al. Gene ontology: tool for the unification of biology. Nat. Genet.25, 25–29 (2000). 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kanehisa, M., Sato, Y., Furumichi, M., Morishima, K. & Tanabe, M. New approach for understanding genome variations in KEGG. Nucleic Acids Res.47, D590–D595 (2019). 10.1093/nar/gky962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Primmer, C. R., Papakostas, S., Leder, E. H., Davis, M. J. & Ragan, M. A. Annotated genes and nonannotated genomes: cross-species use of Gene Ontology in ecology and evolution research. Mol. Ecol.22, 3216–3241 (2013). 10.1111/mec.12309 [DOI] [PubMed] [Google Scholar]
- 98.European Union, Copernicus Land Monitoring Service 2018, European Environment Agency. CORINE Land Cover. https://land.copernicus.eu/pan-european/corine-land-cover (2023).
- 99.Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. Int. J. Climatol.37, 4302–4315 (2017). 10.1002/joc.5086 [DOI] [Google Scholar]
- 100.Karger, D. N. et al. Climatologies at high resolution for the earth’s land surface areas. Sci. Data4, 170122 (2017). 10.1038/sdata.2017.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.GBIF.org. GBIF Home Page. https://www.gbif.org (2020).
- 102.Kattge, J. et al. TRY plant trait database – enhanced coverage and open access. Glob. Change Biol.26, 119–188 (2020). 10.1111/gcb.14904 [DOI] [PubMed] [Google Scholar]
- 103.Ovaskainen, O. et al. Chronicles of nature calendar, a long-term and large-scale multitaxon database on phenology. Sci. Data7, 47 (2020). 10.1038/s41597-020-0376-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.The NOW Community. NOW—New and Old Worlds: Database of fossil mammals. Zenodohttps://zenodo.org/record/4268068 (2020),
- 105.Kotta, J. et al. Integrating experimental and distribution data to predict future species patterns. Sci. Rep.9, 1821 (2019). 10.1038/s41598-018-38416-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barone, L., Williams, J. & Micklos, D. Unmet needs for analyzing biological big data: a survey of 704 NSF principal investigators. PLoS Comput. Biol.13, e1005755 (2017). 10.1371/journal.pcbi.1005755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Waese, J. et al. ePlant: visualizing and exploring multiple levels of data for hypothesis generation in plant biology. Plant Cell29, 1806–1821 (2017). 10.1105/tpc.17.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun.10, 1523 (2019). 10.1038/s41467-019-09234-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ovaskainen, O., & Abrego, N. Joint Species Distribution Modeling with Applications in R. (Cambridge University Press, 2020).
- 110.DeAngelis, D. L. & Grimm, V. Individual-based models in ecology after four decades. F1000Prime Rep.6, 39 (2014). 10.12703/P6-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cardoso, P. et al. Automated discovery of relationships, models, and principles in ecology. Front. Ecol. Evol.8, 530135 (2020). 10.3389/fevo.2020.530135 [DOI] [Google Scholar]
- 112.Foster, S. D. et al. Effects of ignoring survey design information for data reuse. Ecol. Appl.31, e02360 (2021). 10.1002/eap.2360 [DOI] [PubMed] [Google Scholar]
- 113.Dunbar, W. et al. in Managing Socio-ecological Production Landscapes and Seascapes for Sustainable Communities in Asia: Mapping and Navigating Stakeholders, Policy and Action (Saito, O., Subramanian, S. M., Hashimoto, S. & Takeuchi, K.) 93–116 (Springer Singapore, 2020).
- 114.Standish, R. J. et al. Resilience in ecology: abstraction, distraction, or where the action is? Biol. Conserv.177, 43–51 (2014). 10.1016/j.biocon.2014.06.008 [DOI] [Google Scholar]
- 115.Kettenring, K. M., Mercer, K. L., Reinhardt Adams, C. & Hines, J. Application of genetic diversity-ecosystem function research to ecological restoration. J. Appl. Ecol.51, 339–348 (2014). 10.1111/1365-2664.12202 [DOI] [Google Scholar]
- 116.Dhankher, O. P. & Foyer, C. H. Climate resilient crops for improving global food security and safety. Plant Cell Environ.41, 877–884 (2018). 10.1111/pce.13207 [DOI] [PubMed] [Google Scholar]
- 117.Millar, C. I., Stephenson, N. L. & Stephens, S. L. Climate change and forests of the future: managing in the face of uncertainty. Ecol. Appl.17, 2145–2151 (2007). 10.1890/06-1715.1 [DOI] [PubMed] [Google Scholar]
- 118.Yang, Q., Fowler, M. S., Jackson, A. L. & Donohue, I. The predictability of ecological stability in a noisy world. Nat. Ecol. Evol.3, 251–259 (2019). 10.1038/s41559-018-0794-x [DOI] [PubMed] [Google Scholar]
- 119.Cardoso, P. & Leather, S. R. Predicting a global insect apocalypse. Insect Conser. Divers.12, 263–267 (2019). 10.1111/icad.12367 [DOI] [Google Scholar]
- 120.Wilkinson, M. D. et al. The FAIR guiding principles for scientific data management and stewardship. Sci. Data3, 160018 (2016). 10.1038/sdata.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Molinelli, E. J. et al. Perturbation biology: inferring signaling networks in cellular systems. PLoS Comput. Biol.9, e1003290 (2013). 10.1371/journal.pcbi.1003290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jansen, R. C. Studying complex biological systems using multifactorial perturbation. Nat. Rev. Genet.4, 145–151 (2003). 10.1038/nrg996 [DOI] [PubMed] [Google Scholar]
- 123.Billman, G. E. Homeostasis: the underappreciated and far too often ignored central organizing principle of physiology. Front. Physiol.11, 200 (2020). 10.3389/fphys.2020.00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Harmange, G., et al. Disrupting cellular memory to overcome drug resistance. Preprint at bioRxiv10.1101/2022.06.16.496161 (2022).
- 125.Stockmaier, S., Ulrich, Y., Albery, G. F., Cremer, S. & Lopes, P. C. Behavioural defences against parasites across host social structures. Funct. Ecol.37, 809–820 (2023). 10.1111/1365-2435.14310 [DOI] [Google Scholar]
- 126.Craine, J. M. et al. Global diversity of drought tolerance and grassland climate-change resilience. Nat. Clim. Change3, 63–67 (2013). 10.1038/nclimate1634 [DOI] [Google Scholar]
- 127.Rivera, H. E. et al. A framework for understanding gene expression plasticity and its influence on stress tolerance. Mol. Ecol.30, 1381–1397 (2021). 10.1111/mec.15820 [DOI] [PubMed] [Google Scholar]
- 128.Júnior, E. C. B., Rios, V. P., Dodonov, P., Vilela, B. & Japyassú, H. F. Effect of behavioural plasticity and environmental properties on the resilience of communities under habitat loss and fragmentation. Ecol. Modell.472, 110071 (2022). 10.1016/j.ecolmodel.2022.110071 [DOI] [Google Scholar]
- 129.Leivesley, J. A. et al. Survival costs of reproduction are mediated by parasite infection in wild Soay sheep. Ecol. Lett.22, ele.13275 (2019). 10.1111/ele.13275 [DOI] [PubMed] [Google Scholar]
- 130.Krause, S. M. B. et al. Environmental legacy contributes to the resilience of methane consumption in a laboratory microcosm system. Sci. Rep.8, 8862 (2018). 10.1038/s41598-018-27168-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Barnosky, A. D. et al. Merging paleobiology with conservation biology to guide the future of terrestrial ecosystems. Science355, eaah4787 (2017). 10.1126/science.aah4787 [DOI] [PubMed] [Google Scholar]
- 132.de Mazancourt, C., Johnson, E. & Barraclough, T. G. Biodiversity inhibits species’ evolutionary responses to changing environments. Ecol. Lett.11, 380–388 (2008). 10.1111/j.1461-0248.2008.01152.x [DOI] [PubMed] [Google Scholar]
- 133.Simberloff, D. et al. Impacts of biological invasions: what’s what and the way forward. Trends Ecol. Evol.28, 58–66 (2013). 10.1016/j.tree.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 134.Steane, D. A. et al. Genome-wide scans detect adaptation to aridity in a widespread forest tree species. Mol. Ecol.23, 2500–2513 (2014). 10.1111/mec.12751 [DOI] [PubMed] [Google Scholar]
- 135.Bell, G. Evolutionary rescue and the limits of adaptation. Philos. Trans. R. Soc. B: Biol. Sci.368, 20120080 (2013). 10.1098/rstb.2012.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ware, I. M. et al. Feedbacks link ecosystem ecology and evolution across spatial and temporal scales: empirical evidence and future directions. Funct. Ecol.33, 31–42 (2019). 10.1111/1365-2435.13267 [DOI] [Google Scholar]
- 137.Bassar, R. D., Coulson, T., Travis, J. & Reznick, D. N. Towards a more precise—and accurate—view of eco‐evolution. Ecol. Lett.24, 623–625 (2021). 10.1111/ele.13712 [DOI] [PubMed] [Google Scholar]
- 138.Hanski, I. Eco‐evolutionary dynamics in a changing world. Ann. N. Y. Acad. Sci.1249, 1–17 (2012). 10.1111/j.1749-6632.2011.06419.x [DOI] [PubMed] [Google Scholar]
- 139.Schoener, T. W. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science331, 426–429 (2011). 10.1126/science.1193954 [DOI] [PubMed] [Google Scholar]
- 140.Palkovacs, E. P. & Hendry, A. P. Eco-evolutionary dynamics: Intertwining ecological and evolutionary processes in contemporary time. F1000 Biol. Rep.2, 1 (2010). 10.3410/B2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Govaert, L. et al. Eco‐evolutionary feedbacks—theoretical models and perspectives. Funct. Ecol.33, 13–30 (2019). 10.1111/1365-2435.13241 [DOI] [Google Scholar]
- 142.Nadeau, C. P. & Urban, M. C. Eco-evolution on the edge during climate change. Ecography42, 1280–1297 (2019). 10.1111/ecog.04404 [DOI] [Google Scholar]
- 143.Coblentz, K. E., & DeLong, J. P. Ecological boundaries and constraints on viable eco‐evolutionary pathways. Oikos10.1111/oik.09893 (2023).
- 144.Yanniris, C. & Frankel, V. M. Bridging valleys: expanding the adaptive landscape concept beyond theoretical space: with applications in ecology and evolution. Ideas Ecol. Evol.11, 10–18 (2018). [Google Scholar]
- 145.Kelly, R. et al. Ten tips for developing interdisciplinary socio-ecological researchers. Socio-Ecol. Pract. Res.1, 149–161 (2019). 10.1007/s42532-019-00018-2 [DOI] [Google Scholar]
- 146.Svensson, E. I. & Råberg, L. Resistance and tolerance in animal enemy–victim coevolution. Trends Ecol. Evol.25, 267–274 (2010). 10.1016/j.tree.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 147.Roy, B. A. & Kirchner, J. W. Evolutionary dynamics of pathogen resistance and tolerance. Evolution54, 51–63 (2000). [DOI] [PubMed] [Google Scholar]
- 148.Czorlich, Y., Aykanat, T., Erkinaro, J., Orell, P. & Primmer, C. R. Rapid evolution in salmon life history induced by direct and indirect effects of fishing. Science376, 420–423 (2022). 10.1126/science.abg5980 [DOI] [PubMed] [Google Scholar]
- 149.Czorlich, Y., Aykanat, T., Erkinaro, J., Orell, P. & Primmer, C. R. Rapid sex-specific evolution of age at maturity is shaped by genetic architecture in Atlantic salmon. Nat. Ecol. Evol.2, 1800–1807 (2018). 10.1038/s41559-018-0681-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Debes, P. V. et al. Polygenic and major‐locus contributions to sexual maturation timing in Atlantic salmon. Mol. Ecol.30, 4505–4519 (2021). 10.1111/mec.16062 [DOI] [PubMed] [Google Scholar]
- 151.Halperin, D. S., Pan, C., Lusis, A. J. & Tontonoz, P. Vestigial-like 3 is an inhibitor of adipocyte differentiation. J. Lipid Res.54, 473–481 (2013). 10.1194/jlr.M032755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sävilammi, T. et al. Cytosine methylation patterns suggest a role of methylation in plastic and adaptive responses to temperature in European grayling (Thymallus thymallus) populations. Epigenetics16, 271–288 (2020). 10.1080/15592294.2020.1795597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Atmore, L. M. et al. Population dynamics of Baltic herring since the Viking Age revealed by ancient DNA and genomics. Proc. Natl Acad. Sci. USA119, e2208703119 (2022). 10.1073/pnas.2208703119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lamarins, A. et al. Importance of interindividual interactions in eco-evolutionary population dynamics: the rise of demo-genetic agent-based models. Evol. Appli.15, 1988–2001 (2022). 10.1111/eva.13508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Swinfield, T. et al. Imaging spectroscopy reveals the effects of topography and logging on the leaf chemistry of tropical forest canopy trees. Glob. Change Biol.26, 989–1002 (2020). 10.1111/gcb.14903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ives, A. R. et al. Self-perpetuating ecological–evolutionary dynamics in an agricultural host–parasite system. Nat. Ecol. Evol.4, 702–711 (2020). 10.1038/s41559-020-1155-0 [DOI] [PubMed] [Google Scholar]
- 157.Chang, Y. et al. Epigenetic regulation in plant abiotic stress responses. J. Int. Plant Biol.62, 563–580 (2020). 10.1111/jipb.12901 [DOI] [PubMed] [Google Scholar]
- 158.Friedrich, T., Faivre, L., Bäurle, I. & Schubert, D. Chromatin-based mechanisms of temperature memory in plants. Plant, Cell Environ.42, 762–770 (2019). 10.1111/pce.13373 [DOI] [PubMed] [Google Scholar]
- 159.Rudgers, J. A. et al. Climate disruption of plant-microbe interactions. Ann. Rev. Ecol. Evol. Syst.51, 561–586 (2020). 10.1146/annurev-ecolsys-011720-090819 [DOI] [Google Scholar]
- 160.Messier, C. et al. The functional complex network approach to foster forest resilience to global changes. For. Ecosyst.6, 21 (2019). 10.1186/s40663-019-0166-2 [DOI] [Google Scholar]
- 161.Bernstein, A. All creatures great and small. BMJ10.1136/bmj.l2385 (2019). [DOI] [PubMed]
- 162.Mason, I. C., Qian, J., Adler, G. K. & Scheer, F. A. J. L. Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia63, 462–472 (2020). 10.1007/s00125-019-05059-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Maley, C. C. et al. Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nat. Genet.38, 468–473 (2006). 10.1038/ng1768 [DOI] [PubMed] [Google Scholar]
- 164.Alexandrov, L. B., Nik-Zainal, S., Siu, H. C., Leung, S. Y. & Stratton, M. R. A mutational signature in gastric cancer suggests therapeutic strategies. Nat. Commun.6, 8683 (2015). 10.1038/ncomms9683 [DOI] [PMC free article] [PubMed] [Google Scholar]