Abstract
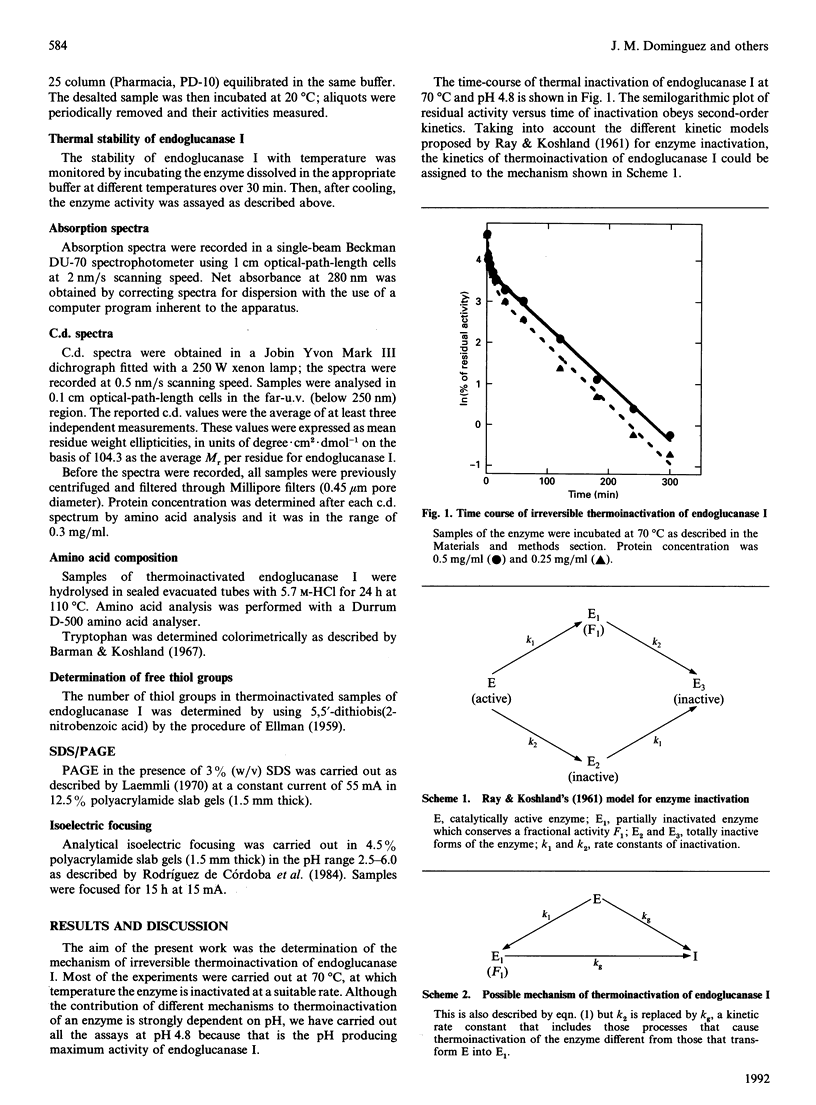
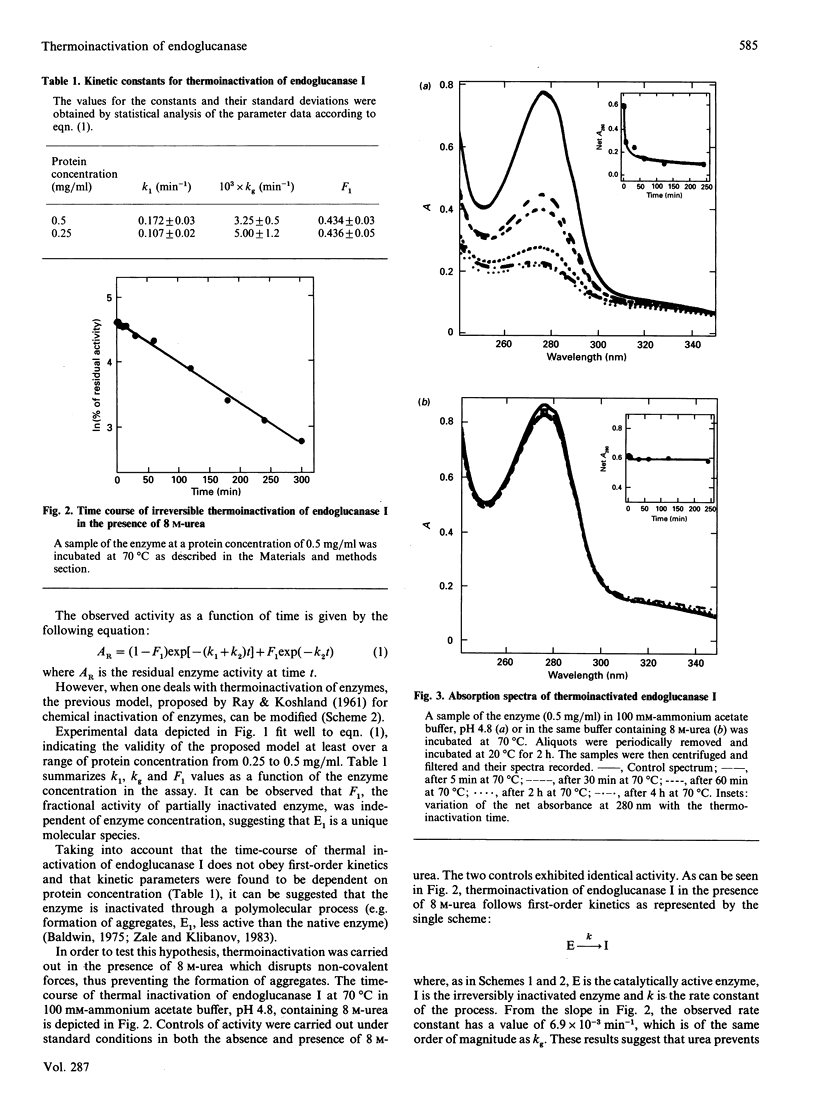
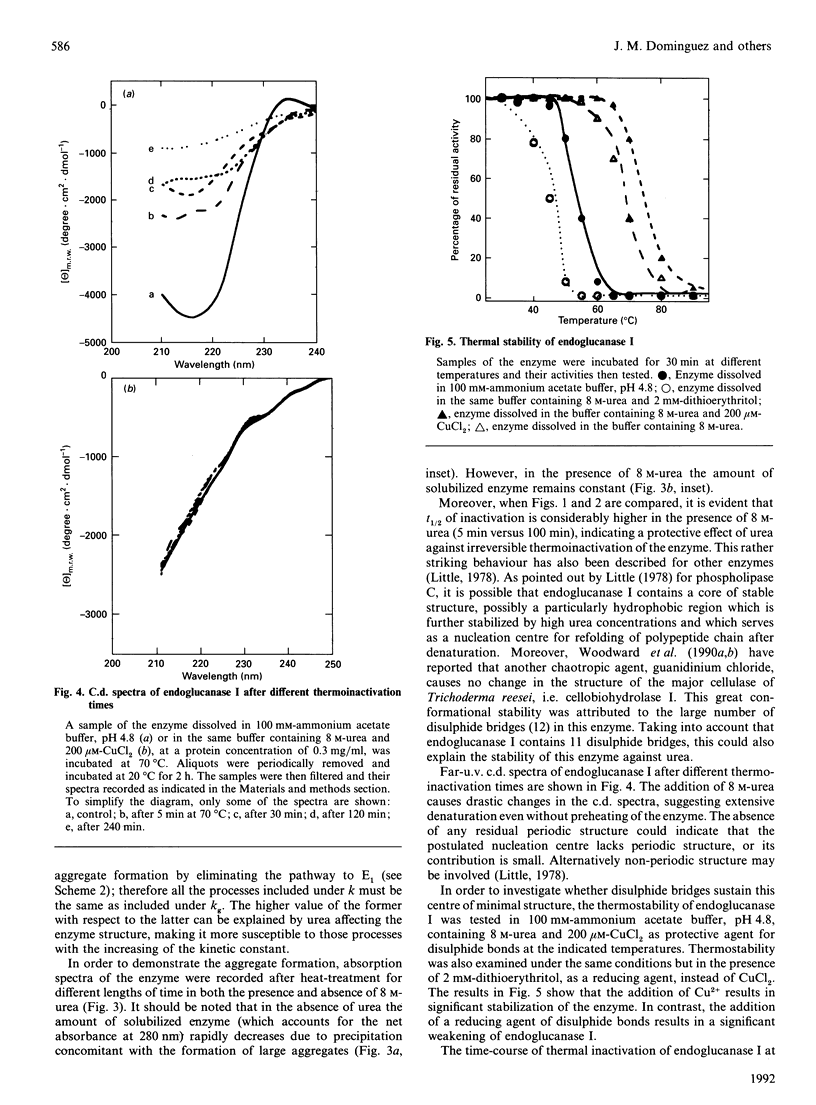
The mechanism of irreversible thermoinactivation of endoglucanase I from Trichoderma reesei has been determined at 70 degrees C at the pH of maximum enzyme activity. The time-course of thermoinactivation did not follow first-order kinetics and kinetic constants of the process were dependent on enzyme concentration, suggesting that aggregation was the main process leading to irreversible inactivation. The enzyme was extremely resistant to urea, which in fact seemed to stabilize it against temperature. Disulphide exchange, deamidation and hydrolysis of peptide bonds were also responsible for the loss of enzyme activity at 70 degrees C.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahern T. J., Klibanov A. M. The mechanisms of irreversible enzyme inactivation at 100C. Science. 1985 Jun 14;228(4705):1280–1284. doi: 10.1126/science.4001942. [DOI] [PubMed] [Google Scholar]
- Baldwin R. L. Intermediates in protein folding reactions and the mechanism of protein folding. Annu Rev Biochem. 1975;44:453–475. doi: 10.1146/annurev.bi.44.070175.002321. [DOI] [PubMed] [Google Scholar]
- Barman T. E., Koshland D. E., Jr A colorimetric procedure for the quantitative determination of tryptophan residues in proteins. J Biol Chem. 1967 Dec 25;242(23):5771–5776. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Inglis A. S. Cleavage at aspartic acid. Methods Enzymol. 1983;91:324–332. doi: 10.1016/s0076-6879(83)91030-3. [DOI] [PubMed] [Google Scholar]
- Klibanov A. M. Stabilization of enzymes against thermal inactivation. Adv Appl Microbiol. 1983;29:1–28. doi: 10.1016/s0065-2164(08)70352-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Little C. Conformational studies on phospholipase C from Bacillus cereus. The effect of urea on the enzyme. Biochem J. 1978 Dec 1;175(3):977–986. doi: 10.1042/bj1750977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttilä M., Lehtovaara P., Nevalainen H., Bhikhabhai R., Knowles J. Homology between cellulase genes of Trichoderma reesei: complete nucleotide sequence of the endoglucanase I gene. Gene. 1986;45(3):253–263. doi: 10.1016/0378-1119(86)90023-5. [DOI] [PubMed] [Google Scholar]
- RAY W. J., Jr, KOSHLAND D. E., Jr A method for characterizing the type and numbers of groups involved in enzyme action. J Biol Chem. 1961 Jul;236:1973–1979. [PubMed] [Google Scholar]
- Rodriguez de Córdoba S., Rubinstein P., Ferreira A. High resolution isoelectric focusing of immunoprecipitated proteins under denaturing conditions. A simple analytical method applied to the study of complement component polymorphisms. J Immunol Methods. 1984 Apr 27;69(2):165–172. doi: 10.1016/0022-1759(84)90314-4. [DOI] [PubMed] [Google Scholar]
- Woodward J., Carmichael J. S., Capps K. M., Herrmann P. C., Lee N. E. The competitive inhibition of Trichoderma reesei C30 cellobiohydrolase I by guanidine hydrochloride. FEBS Lett. 1990 Sep 17;270(1-2):143–146. doi: 10.1016/0014-5793(90)81254-l. [DOI] [PubMed] [Google Scholar]
- Woodward J., Lee N. E., Carmichael J. S., McNair S. L., Wichert J. M. Comparison of the hydrolytic activity and fluorescence of native, guanidine hydrochloride-treated and renatured cellobiohydrolase I from Trichoderma reesei. Biochim Biophys Acta. 1990 Jan 19;1037(1):81–85. doi: 10.1016/0167-4838(90)90104-n. [DOI] [PubMed] [Google Scholar]
- Zale S. E., Klibanov A. M. Why does ribonuclease irreversibly inactivate at high temperatures? Biochemistry. 1986 Sep 23;25(19):5432–5444. doi: 10.1021/bi00367a014. [DOI] [PubMed] [Google Scholar]