Abstract
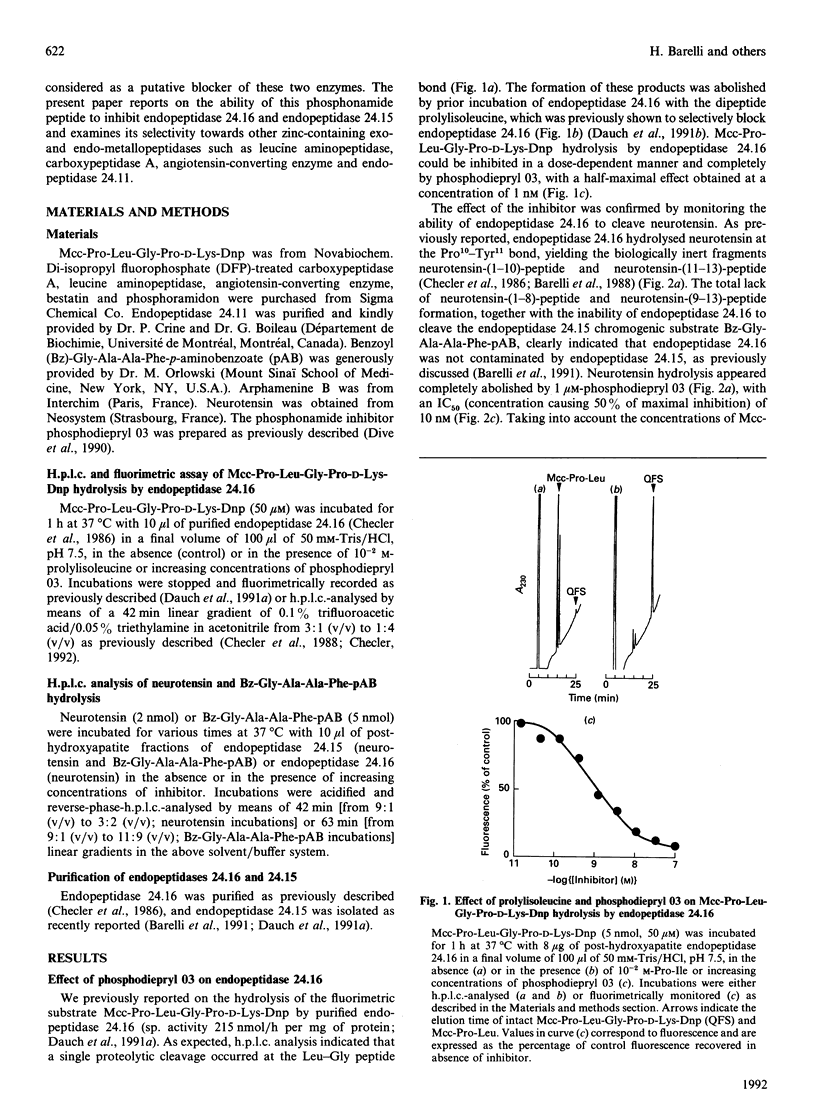
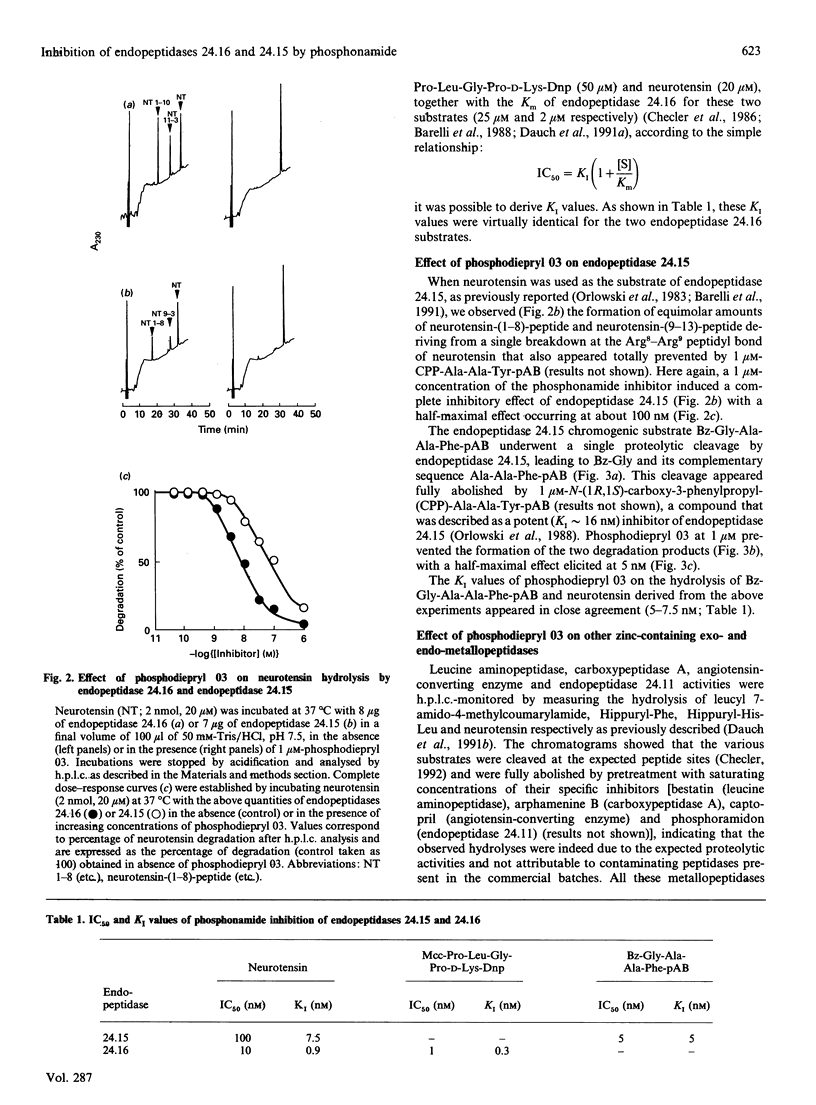
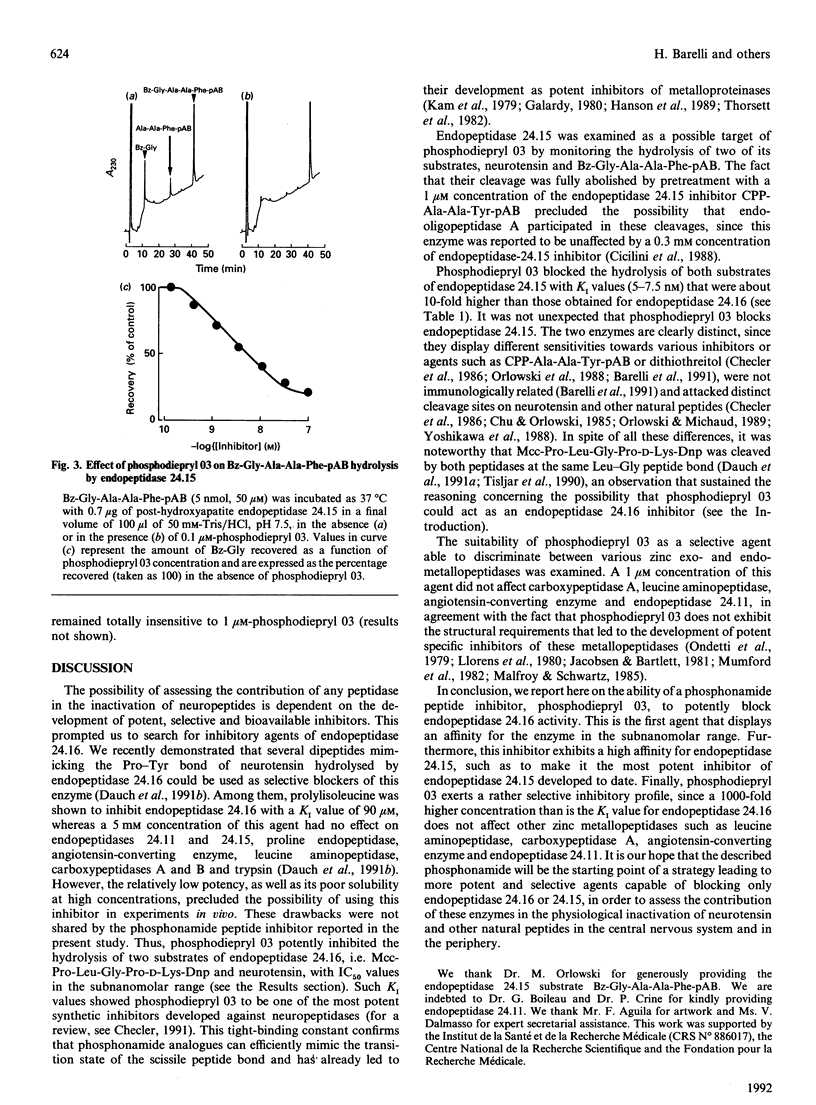
A phosphonamide peptide, N-(phenylethylphosphonyl)-Gly-L-Pro-L-aminohexanoic acid, previously shown to block Clostridium histolyticum collagenases, was examined as a putative inhibitor of endopeptidase 24.16 and endopeptidase 24.15. Hydrolysis of two endopeptidase 24.16 substrates, i.e. 3-carboxy-7-methoxycoumarin (Mcc)-Pro-Leu-Gly-Pro-D-Lys-dinitrophenyl (Dnp) and neurotensin, were completely and dose-dependently inhibited by the phosphonamide inhibitor with KI values of 0.3 and 0.9 nM respectively. In addition, the phosphonamide peptide inhibited the hydrolysis of benzoyl (Bz)-Gly-Ala-Ala-Phe-(pAB) p-aminobenzoate and neurotensin by endopeptidase 24.15 with about a 10-fold lower potency (KI values of 5 and 7.5 nM respectively). The selectivity of this inhibitor towards several exo- and endo-peptidases belonging to the zinc-containing metallopeptidase family established that a 1 microM concentration of this inhibitor was unable to affect leucine aminopeptidase, carboxypeptidase A, angiotensin-converting enzyme and endopeptidase 24.11. The present paper therefore reports on the first hydrophilic highly potent endopeptidase 24.16 inhibitor and describes the most potent inhibitory agent directed towards endopeptidase 24.15 developed to date. These tools should allow one to assess the contribution of endopeptidase 24.16 and endopeptidase 24.15 to the physiological inactivation of neurotensin as well as other neuropeptides.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barelli H., Vincent J. P., Checler F. Peripheral inactivation of neurotensin. Isolation and characterization of a metallopeptidase from rat ileum. Eur J Biochem. 1988 Aug 15;175(3):481–489. doi: 10.1111/j.1432-1033.1988.tb14220.x. [DOI] [PubMed] [Google Scholar]
- Bartlett P. A., Marlowe C. K. Possible role for water dissociation in the slow binding of phosphorus-containing transition-state-analogue inhibitors of thermolysin. Biochemistry. 1987 Dec 29;26(26):8553–8561. doi: 10.1021/bi00400a009. [DOI] [PubMed] [Google Scholar]
- Chabry J., Checler F., Vincent J. P., Mazella J. Colocalization of neurotensin receptors and of the neurotensin-degrading enzyme endopeptidase 24-16 in primary cultures of neurons. J Neurosci. 1990 Dec;10(12):3916–3921. doi: 10.1523/JNEUROSCI.10-12-03916.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checler F., Barelli H., Kitabgi P., Vincent J. P. Neurotensin metabolism in various tissues of central and peripheral origins: ubiquitous involvement of a novel neurotensin degrading metalloendopeptidase. Biochimie. 1988 Jan;70(1):75–82. doi: 10.1016/0300-9084(88)90161-7. [DOI] [PubMed] [Google Scholar]
- Checler F., Vincent J. P., Kitabgi P. Purification and characterization of a novel neurotensin-degrading peptidase from rat brain synaptic membranes. J Biol Chem. 1986 Aug 25;261(24):11274–11281. [PubMed] [Google Scholar]
- Chu T. G., Orlowski M. Soluble metalloendopeptidase from rat brain: action on enkephalin-containing peptides and other bioactive peptides. Endocrinology. 1985 Apr;116(4):1418–1425. doi: 10.1210/endo-116-4-1418. [DOI] [PubMed] [Google Scholar]
- Cicilini M. A., Ribeiro M. J., de Oliveira E. B., Mortara R. A., de Camargo A. C. Endooligopeptidase A activity in rabbit heart: generation of enkephalin from enkephalin containing peptides. Peptides. 1988 Sep-Oct;9(5):945–955. doi: 10.1016/0196-9781(88)90072-1. [DOI] [PubMed] [Google Scholar]
- Dauch P., Barelli H., Vincent J. P., Checler F. Fluorimetric assay of the neurotensin-degrading metalloendopeptidase, endopeptidase 24.16. Biochem J. 1991 Dec 1;280(Pt 2):421–426. doi: 10.1042/bj2800421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauch P., Vincent J. P., Checler F. Specific inhibition of endopeptidase 24.16 by dipeptides. Eur J Biochem. 1991 Dec 5;202(2):269–276. doi: 10.1111/j.1432-1033.1991.tb16372.x. [DOI] [PubMed] [Google Scholar]
- Dive V., Yiotakis A., Nicolaou A., Toma F. Inhibition of Clostridium histolyticum collagenases by phosphonamide peptide inhibitors. Eur J Biochem. 1990 Aug 17;191(3):685–693. doi: 10.1111/j.1432-1033.1990.tb19175.x. [DOI] [PubMed] [Google Scholar]
- Galardy R. E. Inhibition of angiotensin converting enzyme with N alpha-phosphoryl-L-alanyl-L-proline and N alpha-L-valyl-L-tryptophan. Biochem Biophys Res Commun. 1980 Nov 17;97(1):94–99. doi: 10.1016/s0006-291x(80)80139-2. [DOI] [PubMed] [Google Scholar]
- Hanson J. E., Kaplan A. P., Bartlett P. A. Phosphonate analogues of carboxypeptidase A substrates are potent transition-state analogue inhibitors. Biochemistry. 1989 Jul 25;28(15):6294–6305. doi: 10.1021/bi00441a022. [DOI] [PubMed] [Google Scholar]
- Kam C. M., Nishino N., Powers J. C. Inhibition of thermolysin and carboxypeptidase A by phosphoramidates. Biochemistry. 1979 Jul 10;18(14):3032–3038. doi: 10.1021/bi00581a019. [DOI] [PubMed] [Google Scholar]
- Llorens C., Gacel G., Swerts J. P., Perdrisot R., Fournie-Zaluski M. C., Schwartz J. C., Roques B. P. Rational design of enkephalinase inhibitors: substrate specificity of enkephalinase studied from inhibitory potency of various dipeptides. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1710–1716. doi: 10.1016/0006-291x(80)91371-6. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Schwartz J. C. Comparison of dipeptidyl carboxypeptidase and endopeptidase activities in the three enkephalin-hydrolysing metallopeptidases: "angiotensin-converting enzyme", thermolysin and "enkephalinase". Biochem Biophys Res Commun. 1985 Jul 16;130(1):372–378. doi: 10.1016/0006-291x(85)90427-9. [DOI] [PubMed] [Google Scholar]
- Mumford R. A., Pierzchala P. A., Strauss A. W., Zimmerman M. Purification of a membrane-bound metalloendopeptidase from porcine kidney that degrades peptide hormones. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6623–6627. doi: 10.1073/pnas.78.11.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford R. A., Zimmerman M., ten Broeke J., Taub D., Joshua H., Rothrock J. W., Hirshfield J. M., Springer J. P., Patchett A. A. Inhibition of porcine kidney "enkephalinase" by substituted-N-carboxymethyl dipeptides. Biochem Biophys Res Commun. 1982 Dec 31;109(4):1303–1309. doi: 10.1016/0006-291x(82)91919-2. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Condon M. E., Reid J., Sabo E. F., Cheung H. S., Cushman D. W. Design of potent and specific inhibitors of carboxypeptidases A and B. Biochemistry. 1979 Apr 17;18(8):1427–1430. doi: 10.1021/bi00575a006. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Michaud C., Chu T. G. A soluble metalloendopeptidase from rat brain. Purification of the enzyme and determination of specificity with synthetic and natural peptides. Eur J Biochem. 1983 Sep 1;135(1):81–88. doi: 10.1111/j.1432-1033.1983.tb07620.x. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Michaud C., Molineaux C. J. Substrate-related potent inhibitors of brain metalloendopeptidase. Biochemistry. 1988 Jan 26;27(2):597–602. doi: 10.1021/bi00402a015. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Michaud C. Pituitary multicatalytic proteinase complex. Specificity of components and aspects of proteolytic activity. Biochemistry. 1989 Nov 28;28(24):9270–9278. doi: 10.1021/bi00450a006. [DOI] [PubMed] [Google Scholar]
- Suda H., Aoyagi T., Takeuchi T., Umezawa H. Letter: A thermolysin inhibitor produced by Actinomycetes: phospholamidon. J Antibiot (Tokyo) 1973 Oct;26(10):621–623. doi: 10.7164/antibiotics.26.621. [DOI] [PubMed] [Google Scholar]
- Thorsett E. D., Harris E. E., Peterson E. R., Greenlee W. J., Patchett A. A., Ulm E. H., Vassil T. C. Phosphorus-containing inhibitors of angiotensin-converting enzyme. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2176–2180. doi: 10.1073/pnas.79.7.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisljar U., Barrett A. J. Thiol-dependent metallo-endopeptidase characteristics of Pz-peptidase in rat and rabbit. Biochem J. 1990 Apr 15;267(2):531–533. doi: 10.1042/bj2670531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisljar U., Knight C. G., Barrett A. J. An alternative quenched fluorescence substrate for Pz-peptidase. Anal Biochem. 1990 Apr;186(1):112–115. doi: 10.1016/0003-2697(90)90582-t. [DOI] [PubMed] [Google Scholar]
- Yoshikawa S., Tashiro T., Takahashi K. Specificity of action on neuropeptides of an endopeptidase from the synaptosomal membranes of guinea pig brain. J Biochem. 1988 Dec;104(6):1007–1010. doi: 10.1093/oxfordjournals.jbchem.a122566. [DOI] [PubMed] [Google Scholar]


