Abstract
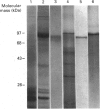
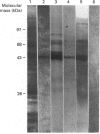
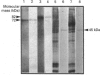
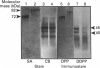
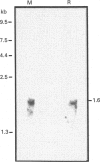
Heterogeneity among the odontoblast-specific, highly phosphorylated acidic protein dentine phosphoprotein (DPP) obtained from different species has been reported by several investigators. In the present study, the apparent molecular-mass variations in rabbit and mouse DPP were investigated. Extracellular matrix (ECM) DPPs were isolated and characterized. Primary gene products, before post-translational phosphorylation, were analysed based upon translation products produced in a rabbit reticulocyte lysate cell-free system using a polyclonal mouse anti-DPP antibody. Nascent non-phosphorylated DPPs were also identified from intracellular protein extracts. Mouse and rabbit ECM phosphoproteins exhibited a 10 kDa difference in size. However, nascent intracellular or translation products from both species showed the same lower molecular mass (approx. 45 kDa). Furthermore, Northern-blot analysis showed a single mRNA of the same size in both species (approx. 1.6 kb) which contains information for a protein no larger than 50 kDa. Our results indicate that the difference in molecular mass (or electrophoretic behaviour) among DPPs from different species is due to post-translational modifications, in this case phosphorylation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler W. T., Bhown M., DiMuzio M. T., Cothran W. C., Linde A. Multiple forms of rat dentin phosphoproteins. Arch Biochem Biophys. 1983 Aug;225(1):178–186. doi: 10.1016/0003-9861(83)90021-8. [DOI] [PubMed] [Google Scholar]
- Butler W. T., Bhown M., Dimuzio M. T., Linde A. Nonocollagenous proteins of dentin. Isolation and partial characterization of rat dentin proteins and proteoglycans using a three-step preparative method. Coll Relat Res. 1981 Feb;1(2):187–199. doi: 10.1016/s0174-173x(81)80019-2. [DOI] [PubMed] [Google Scholar]
- Dimuzio M. T., Veis A. Phosphophoryns-major noncollagenous proteins of rat incisor dentin. Calcif Tissue Res. 1978 May 26;25(2):169–178. doi: 10.1007/BF02010765. [DOI] [PubMed] [Google Scholar]
- Jonsson M., Fredriksson S., Jontell M., Linde A. Isoelectric focusing of the phosphoprotein of rat-incisor dentin in ampholine and acid pH gradients. Evidence for carrier ampholyte-protein complexes. J Chromatogr. 1978 Sep 21;157:235–242. doi: 10.1016/s0021-9673(00)92338-0. [DOI] [PubMed] [Google Scholar]
- Jontell M., Linde A., Lundvik L. Comparative studies of phosphoprotein preparations from rat incisor dentin. Prep Biochem. 1980;10(3):235–253. doi: 10.1080/10826068009412827. [DOI] [PubMed] [Google Scholar]
- Jontell M., Pertoft H., Linde A. Disagreement in molecular weight determinations of dentin phosphoprotein. Biochim Biophys Acta. 1982 Aug 10;705(3):315–320. doi: 10.1016/0167-4838(82)90253-9. [DOI] [PubMed] [Google Scholar]
- Kuboki Y., Fujisawa R., Aoyama K., Sasaki S. Calcium-specific precipitation of dentin phosphoprotein: a new method of purification and the significance for the mechanism of calcification. J Dent Res. 1979 Sep;58(9):1926–1932. doi: 10.1177/00220345790580092001. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Veis A., Glonek T. Dentin phosphoprotein: an extracellular calcium-binding protein. Biochemistry. 1977 Jun 28;16(13):2971–2979. doi: 10.1021/bi00632a026. [DOI] [PubMed] [Google Scholar]
- Linde A., Bhown M., Butler W. T. Noncollagenous proteins of dentin. A re-examination of proteins from rat incisor dentin utilizing techniques to avoid artifacts. J Biol Chem. 1980 Jun 25;255(12):5931–5942. [PubMed] [Google Scholar]
- MacDougall M., Zeichner-David M., Slavkin H. C. Production and characterization of antibodies against murine dentine phosphoprotein. Biochem J. 1985 Dec 1;232(2):493–500. doi: 10.1042/bj2320493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier G. D., Evans J. S., Veis A. Characterization of the primary gene product of rat incisor alpha-phosphophoryn. Biochemistry. 1985 Nov 5;24(23):6370–6374. doi: 10.1021/bi00344a008. [DOI] [PubMed] [Google Scholar]
- Rahima M., Veis A. Two classes of dentin phosphophoryns, from a wide range of species, contain immunologically cross-reactive epitope regions. Calcif Tissue Int. 1988 Feb;42(2):104–112. doi: 10.1007/BF02556342. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Veis A. Bovine dentin phosphophoryn: composition and molecular weight. Biochemistry. 1983 Aug 30;22(18):4326–4335. doi: 10.1021/bi00287a025. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Weinstock M., Leblond C. P. Radioautographic visualization of the deposition of a phosphoprotein at the mineralization front in the dentin of the rat incisor. J Cell Biol. 1973 Mar;56(3):838–845. doi: 10.1083/jcb.56.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeichner-David M., MacDougall M., Slavkin H. C. Enamelin gene expression during fetal and neonatal rabbit tooth organogenesis. Differentiation. 1983;25(2):148–155. doi: 10.1111/j.1432-0436.1984.tb01350.x. [DOI] [PubMed] [Google Scholar]
- Zeichner-David M., Weliky B. G., Slavkin H. C. Isolation and preliminary characterization of epithelial-specific messenger ribonucleic acids and their products during embryonic tooth development. Biochem J. 1980 Feb 1;185(2):489–496. doi: 10.1042/bj1850489. [DOI] [PMC free article] [PubMed] [Google Scholar]