Abstract
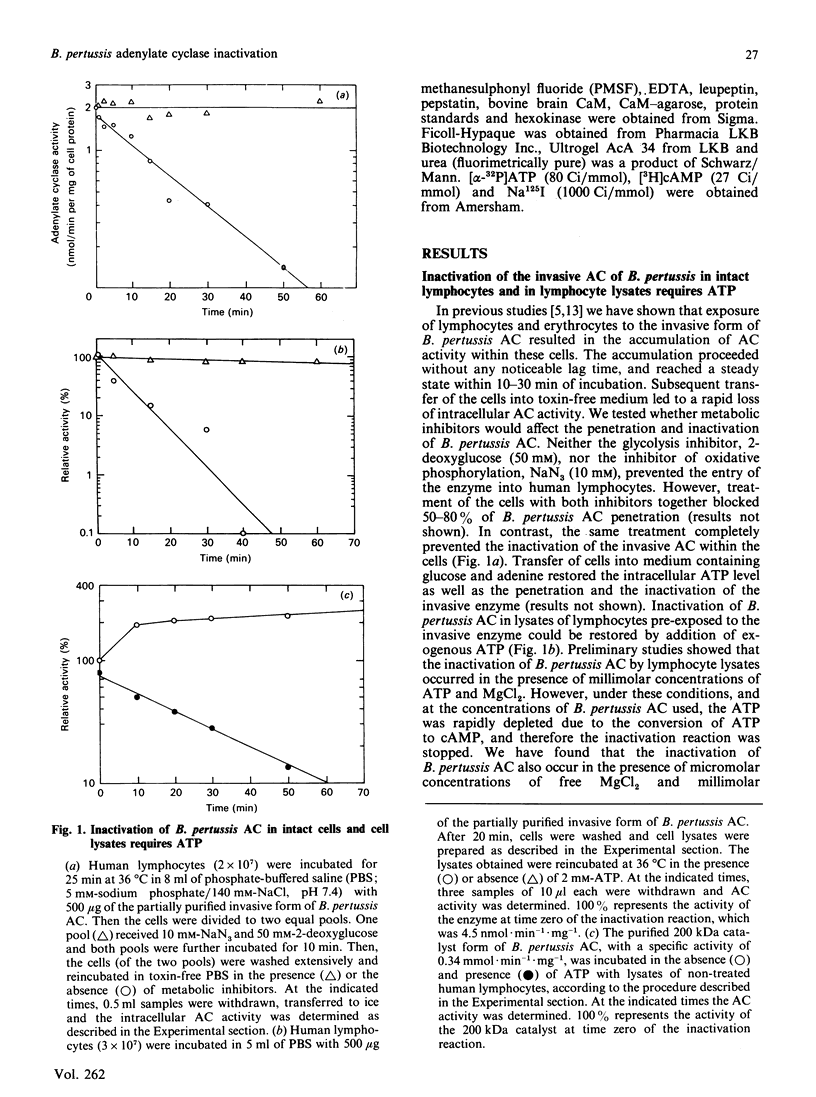
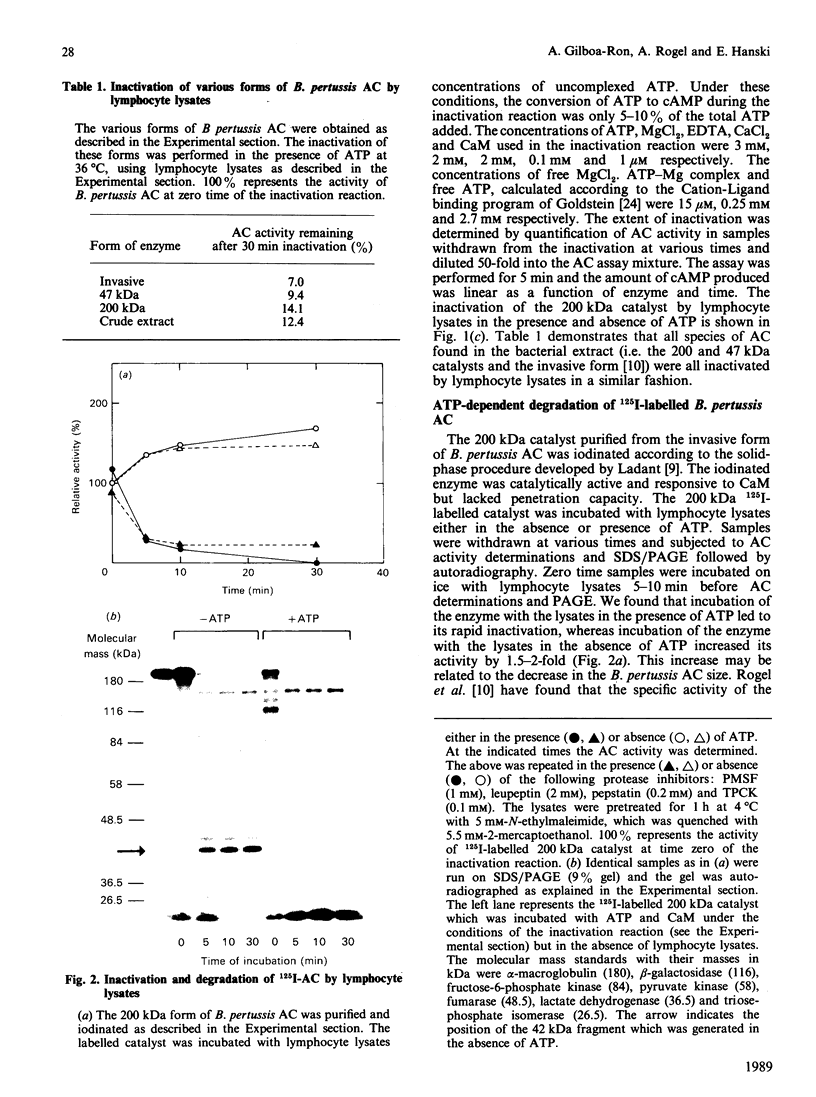
Bordetella pertussis produces a calmodulin-dependent adenylate cyclase (AC) which acts as a toxin capable of penetrating eukaryotic cells and generating high levels of intracellular cyclic AMP. Transfer of target cells into B. pertussis AC-free medium leads to a rapid decay in the intracellular AC activity, implying that the invasive enzyme is unstable in the host cytoplasm. We report here that treatment of human lymphocytes with a glycolysis inhibitor and an uncoupler of oxidative phosphorylation completely blocked the intracellular inactivation of B. pertussis AC. Lymphocyte lysates inactivated all forms of B. pertussis AC in the presence of exogenous ATP. This inactivation was associated with degradation of an 125I-labelled 200 kDa form of B. pertussis AC. It appears that ATP is required for the proteolytic pathway, but not as an energy source, since non-hydrolysable ATP analogues supported inactivation and complete degradation of the enzyme. The possibility that binding of ATP to B. pertussis AC renders it susceptible to degradation by the host cell protease is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Confer D. L., Eaton J. W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982 Sep 3;217(4563):948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- Dice J. F. Molecular determinants of protein half-lives in eukaryotic cells. FASEB J. 1987 Nov;1(5):349–357. doi: 10.1096/fasebj.1.5.2824267. [DOI] [PubMed] [Google Scholar]
- Farfel Z., Friedman E., Hanski E. The invasive adenylate cyclase of Bordetella pertussis. Intracellular localization and kinetics of penetration into various cells. Biochem J. 1987 Apr 1;243(1):153–158. doi: 10.1042/bj2430153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E., Farfel Z., Hanski E. The invasive adenylate cyclase of Bordetella pertussis. Properties and penetration kinetics. Biochem J. 1987 Apr 1;243(1):145–151. doi: 10.1042/bj2430145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Ladant D., Sezer O., Pichot F., Ullmann A., Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):19–30. [PubMed] [Google Scholar]
- Goldstein D. A. Calculation of the concentrations of free cations and cation-ligand complexes in solutions containing multiple divalent cations and ligands. Biophys J. 1979 May;26(2):235–242. doi: 10.1016/S0006-3495(79)85247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon V. M., Leppla S. H., Hewlett E. L. Inhibitors of receptor-mediated endocytosis block the entry of Bacillus anthracis adenylate cyclase toxin but not that of Bordetella pertussis adenylate cyclase toxin. Infect Immun. 1988 May;56(5):1066–1069. doi: 10.1128/iai.56.5.1066-1069.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E., Farfel Z. Bordetella pertussis invasive adenylate cyclase. Partial resolution and properties of its cellular penetration. J Biol Chem. 1985 May 10;260(9):5526–5532. [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. doi: 10.1146/annurev.bi.51.070182.002003. [DOI] [PubMed] [Google Scholar]
- Hewlett E. L., Urban M. A., Manclark C. R., Wolff J. Extracytoplasmic adenylate cyclase of Bordetella pertussis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1926–1930. doi: 10.1073/pnas.73.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang B. J., Woo K. M., Goldberg A. L., Chung C. H. Protease Ti, a new ATP-dependent protease in Escherichia coli, contains protein-activated ATPase and proteolytic functions in distinct subunits. J Biol Chem. 1988 Jun 25;263(18):8727–8734. [PubMed] [Google Scholar]
- Ladant D., Brezin C., Alonso J. M., Crenon I., Guiso N. Bordetella pertussis adenylate cyclase. Purification, characterization, and radioimmunoassay. J Biol Chem. 1986 Dec 5;261(34):16264–16269. [PubMed] [Google Scholar]
- Ladant D. Interaction of Bordetella pertussis adenylate cyclase with calmodulin. Identification of two separated calmodulin-binding domains. J Biol Chem. 1988 Feb 25;263(6):2612–2618. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leppla S. H. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M. C., Salomon Y., Rendell M., Rodbell M. The hepatic adenylate cyclase system. II. Substrate binding and utilization and the effects of magnesium ion and pH. J Biol Chem. 1975 Jun 10;250(11):4246–4252. [PubMed] [Google Scholar]
- Masure H. R., Oldenburg D. J., Donovan M. G., Shattuck R. L., Storm D. R. The interaction of Ca2+ with the calmodulin-sensitive adenylate cyclase from Bordetella pertussis. J Biol Chem. 1988 May 15;263(14):6933–6940. [PubMed] [Google Scholar]
- Minocherhomjee M., Selfe S., Flowers N. J., Storm D. R. Direct interaction between the catalytic subunit of the calmodulin-sensitive adenylate cyclase from bovine brain with 125I-labeled wheat germ agglutinin and 125I-labeled calmodulin. Biochemistry. 1987 Jul 14;26(14):4444–4448. doi: 10.1021/bi00388a038. [DOI] [PubMed] [Google Scholar]
- Ristroph J. D., Ivins B. E. Elaboration of Bacillus anthracis antigens in a new, defined culture medium. Infect Immun. 1983 Jan;39(1):483–486. doi: 10.1128/iai.39.1.483-486.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogel A., Farfel Z., Goldschmidt S., Shiloach J., Hanski E. Bordetella pertussis adenylate cyclase. Identification of multiple forms of the enzyme by antibodies. J Biol Chem. 1988 Sep 15;263(26):13310–13316. [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Maguire M. E., Sturgill T. W., Biltonen R. L., Gilman A. G. Relationship between the beta-adrenergic receptor and adenylate cyclase. J Biol Chem. 1977 Aug 25;252(16):5761–5775. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Waxman L., Fagan J. M., Tanaka K., Goldberg A. L. A soluble ATP-dependent system for protein degradation from murine erythroleukemia cells. Evidence for a protease which requires ATP hydrolysis but not ubiquitin. J Biol Chem. 1985 Oct 5;260(22):11994–12000. [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis. 1984 Aug;150(2):219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- Wolff J., Cook G. H., Goldhammer A. R., Berkowitz S. A. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haën C. Adenylate cyclase. A new kinetic analysis of the effects of hormones and fluoride ion. J Biol Chem. 1974 May 10;249(9):2756–2762. [PubMed] [Google Scholar]