Abstract
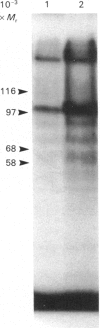
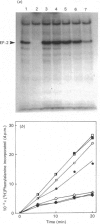
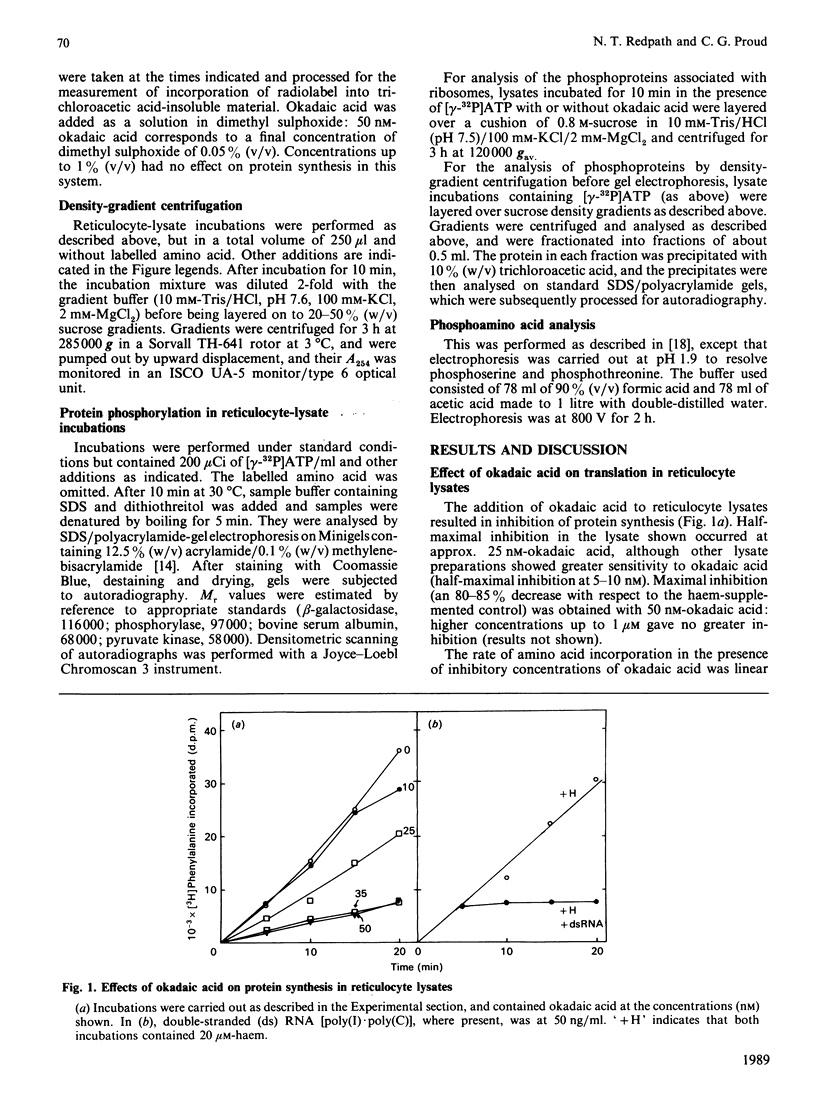
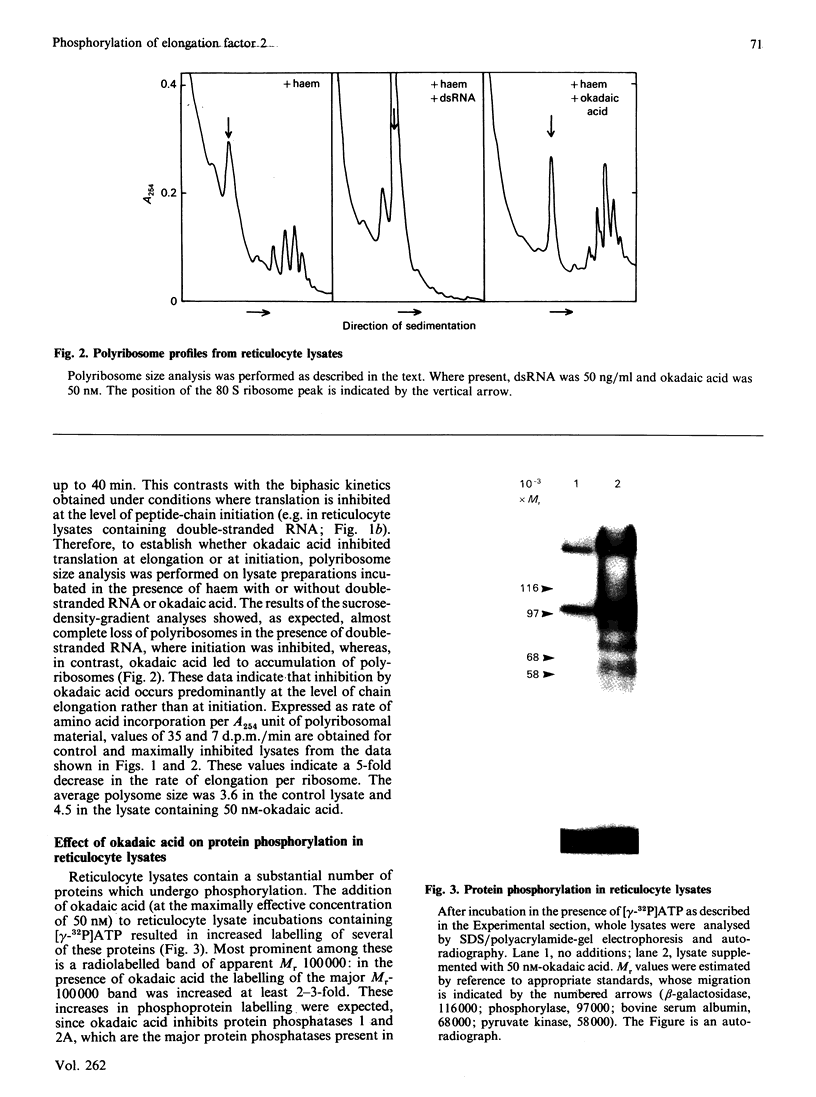
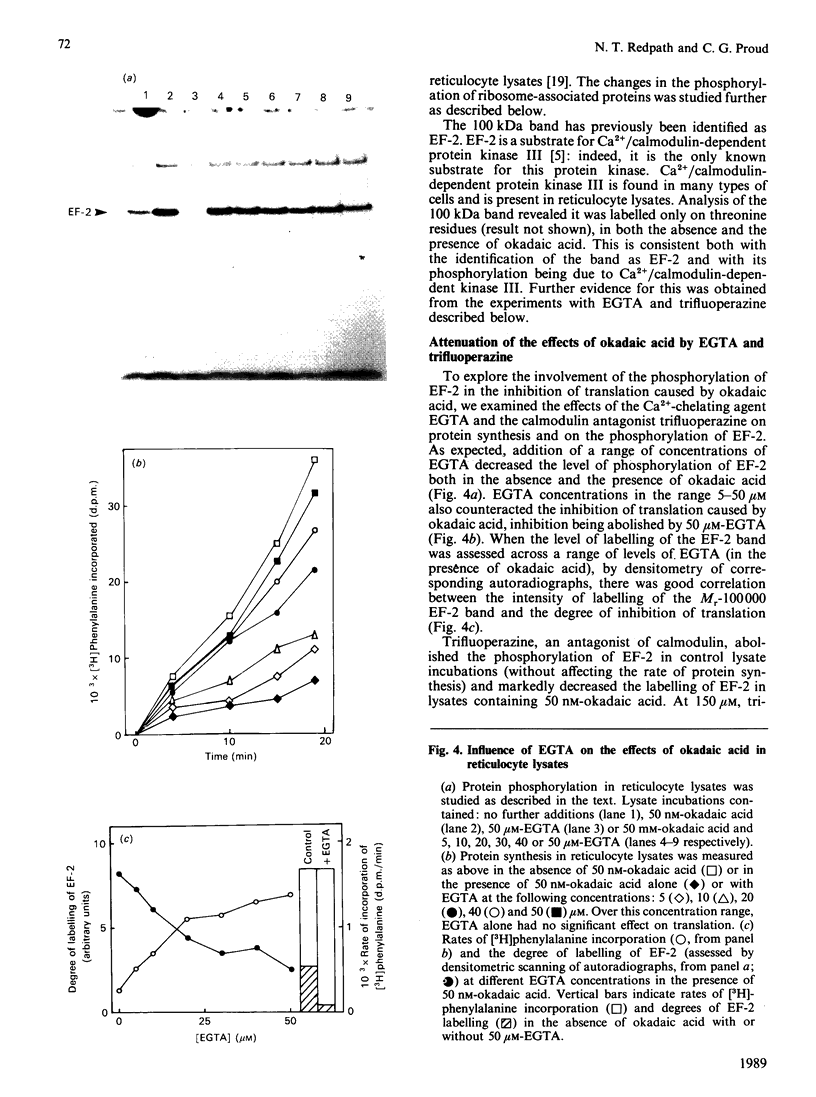
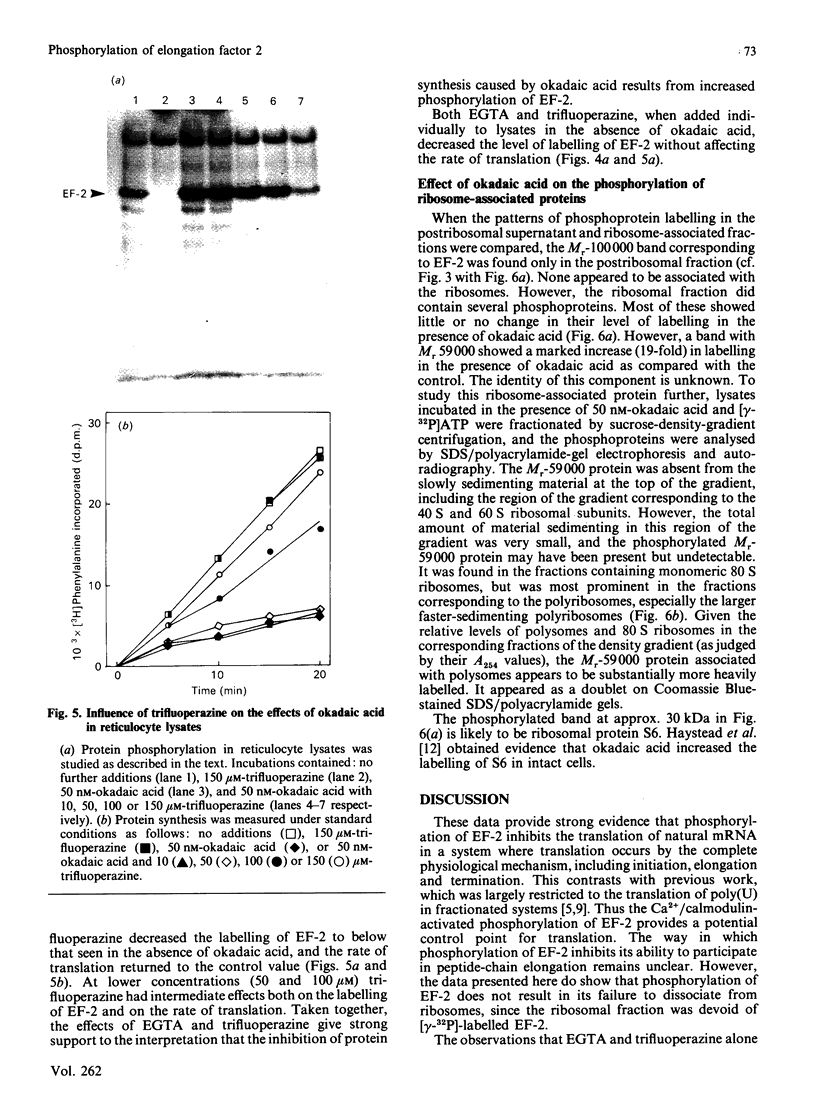
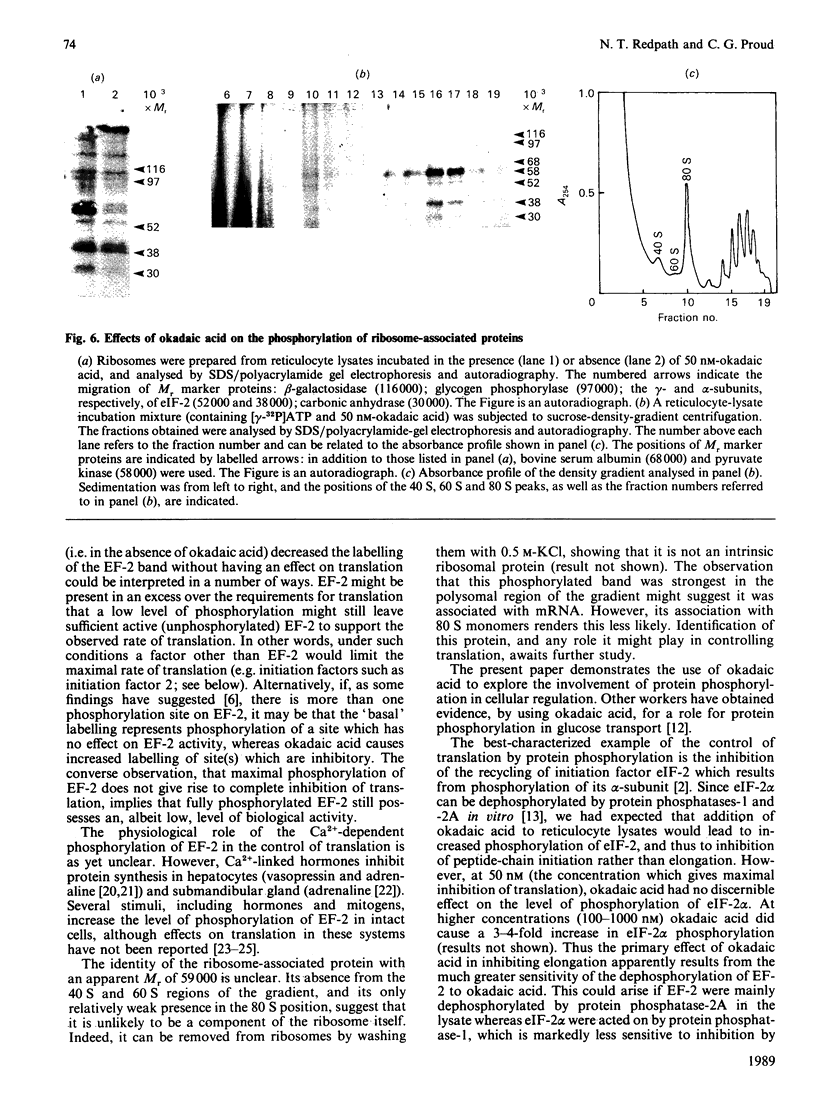
Okadaic acid, a tumour promoter which potently inhibits protein phosphatases, inhibited translation in the reticulocyte-lysate cell-free system. Inhibition was dose-dependent, with half-maximal effects occurring at 20-40 nM-okadaic acid. Inhibition of translation by okadaic acid resulted in the accumulation of polyribosomes, indicating that it was due to a decrease in the rate of elongation relative to initiation. Okadaic acid (at concentrations which inhibited translation) caused increased phosphorylation of a number of proteins in the lysate. Prominent among these was a protein of Mr 100,000, which has previously been identified as elongation factor 2 (EF-2). EF-2 is a specific substrate for a Ca2+/calmodulin-dependent protein kinase, which phosphorylates EF-2 on threonine residues. The Mr-100,000 band was phosphorylated exclusively on threonine residues, and its degree of 32P labelling was decreased by the Ca2+ chelator EGTA and by the calmodulin antagonist trifluoperazine. These agents attenuated the effects of okadaic acid on EF-2 phosphorylation and translation. When ranges of concentrations of each agent were tested, their effects on EF-2 labelling correlated well with their ability to reverse the okadaic acid-induced inhibition of translation. These findings demonstrate that increased phosphorylation of EF-2 results in an impairment of peptide-chain elongation when natural mRNA is used. The possible physiological role of EF-2 phosphorylation in the control of translation is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou L. M., Siegmann M., Thomas G. S6 kinase in quiescent Swiss mouse 3T3 cells is activated by phosphorylation in response to serum treatment. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7154–7158. doi: 10.1073/pnas.85.19.7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brostrom C. O., Bocckino S. B., Brostrom M. A., Galuska E. M. Regulation of protein synthesis in isolated hepatocytes by calcium-mobilizing hormones. Mol Pharmacol. 1986 Jan;29(1):104–111. [PubMed] [Google Scholar]
- Clark S. J., Colthurst D. R., Proud C. G. Structure and phosphorylation of eukaryotic initiation factor 2. Casein kinase 2 and protein kinase C phosphorylate distinct but adjacent sites in the beta-subunit. Biochim Biophys Acta. 1988 Feb 22;968(2):211–219. doi: 10.1016/0167-4889(88)90010-9. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Henshaw E. C., Rahamimoff H., London I. M. Met-tRNAfMet binding to 40S ribosomal subunits: a site for the regulation of initiation of protein synthesis by hemin. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2946–2950. doi: 10.1073/pnas.71.8.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colthurst D. R., Proud C. G. Eukaryotic initiation factor 2 from rat liver: no apparent function for the beta-subunit in the formation of initiation complexes. Biochim Biophys Acta. 1986 Oct 16;868(1):77–86. doi: 10.1016/0167-4781(86)90089-8. [DOI] [PubMed] [Google Scholar]
- Ernst V., Levin D. H., Foulkes J. G., London I. M. Effects of skeletal muscle protein phosphatase inhibitor-2 on protein synthesis and protein phosphorylation in rabbit reticulocyte lysates. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7092–7096. doi: 10.1073/pnas.79.23.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes J. G., Ernst V., Levin D. H. Separation and identification of type 1 and type 2 protein phosphatases from rabbit reticulocyte lysates. J Biol Chem. 1983 Feb 10;258(3):1439–1443. [PubMed] [Google Scholar]
- Haystead T. A., Sim A. T., Carling D., Honnor R. C., Tsukitani Y., Cohen P., Hardie D. G. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989 Jan 5;337(6202):78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Mieskes G., Rüegg J. C., Takai A., Trautwein W. Effects of a protein phosphatase inhibitor, okadaic acid, on membrane currents of isolated guinea-pig cardiac myocytes. Pflugers Arch. 1988 Aug;412(3):248–252. doi: 10.1007/BF00582504. [DOI] [PubMed] [Google Scholar]
- Ingebritsen T. S., Cohen P. The protein phosphatases involved in cellular regulation. 1. Classification and substrate specificities. Eur J Biochem. 1983 May 2;132(2):255–261. doi: 10.1111/j.1432-1033.1983.tb07357.x. [DOI] [PubMed] [Google Scholar]
- Mackie K. P., Nairn A. C., Hampel G., Lam G., Jaffe E. A. Thrombin and histamine stimulate the phosphorylation of elongation factor 2 in human umbilical vein endothelial cells. J Biol Chem. 1989 Jan 25;264(3):1748–1753. [PubMed] [Google Scholar]
- Mandl J., Garzo T., Antoni F. Epinephrine inhibits protein synthesis in isolated mouse hepatocytes through alpha adrenergic receptors in a calcium dependent way. Biochem Pharmacol. 1982 Apr 15;31(8):1656–1658. doi: 10.1016/0006-2952(82)90399-9. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Bhagat B., Palfrey H. C. Identification of calmodulin-dependent protein kinase III and its major Mr 100,000 substrate in mammalian tissues. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7939–7943. doi: 10.1073/pnas.82.23.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn A. C., Nichols R. A., Brady M. J., Palfrey H. C. Nerve growth factor treatment or cAMP elevation reduces Ca2+/calmodulin-dependent protein kinase III activity in PC12 cells. J Biol Chem. 1987 Oct 15;262(29):14265–14272. [PubMed] [Google Scholar]
- Nairn A. C., Palfrey H. C. Identification of the major Mr 100,000 substrate for calmodulin-dependent protein kinase III in mammalian cells as elongation factor-2. J Biol Chem. 1987 Dec 25;262(36):17299–17303. [PubMed] [Google Scholar]
- Pain V. M. Initiation of protein synthesis in mammalian cells. Biochem J. 1986 May 1;235(3):625–637. doi: 10.1042/bj2350625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfrey H. C., Nairn A. C., Muldoon L. L., Villereal M. L. Rapid activation of calmodulin-dependent protein kinase III in mitogen-stimulated human fibroblasts. Correlation with intracellular Ca2+ transients. J Biol Chem. 1987 Jul 15;262(20):9785–9792. [PubMed] [Google Scholar]
- Proud C. G., Clemens M. J., Pain V. M. Regulation of binding of initiator tRNA to eukaryotic initiation factor eIF-2. Effects of the haem-controlled repressor on the kinetics of ternary complex formation. FEBS Lett. 1982 Nov 8;148(2):214–220. doi: 10.1016/0014-5793(82)80810-7. [DOI] [PubMed] [Google Scholar]
- Ryazanov A. G. Ca2+/calmodulin-dependent phosphorylation of elongation factor 2. FEBS Lett. 1987 Apr 20;214(2):331–334. doi: 10.1016/0014-5793(87)80081-9. [DOI] [PubMed] [Google Scholar]
- Ryazanov A. G., Natapov P. G., Shestakova E. A., Severin F. F., Spirin A. S. Phosphorylation of the elongation factor 2: the fifth Ca2+/calmodulin-dependent system of protein phosphorylation. Biochimie. 1988 May;70(5):619–626. doi: 10.1016/0300-9084(88)90245-3. [DOI] [PubMed] [Google Scholar]
- Ryazanov A. G., Shestakova E. A., Natapov P. G. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature. 1988 Jul 14;334(6178):170–173. doi: 10.1038/334170a0. [DOI] [PubMed] [Google Scholar]
- Takuma T., Kuyatt B. L., Baum B. J. Alpha 1-adrenergic inhibition of protein synthesis in rat submandibular cells. Am J Physiol. 1984 Sep;247(3 Pt 1):G284–G289. doi: 10.1152/ajpgi.1984.247.3.G284. [DOI] [PubMed] [Google Scholar]
- Tuhácková Z., Ullrichová J., Hradec J. Regulation of the activity of eukaryotic peptide elongation factor 1 by autocatalytic phosphorylation. Eur J Biochem. 1985 Jan 2;146(1):161–166. doi: 10.1111/j.1432-1033.1985.tb08633.x. [DOI] [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]