Abstract
Aims
Hospital readmissions due to recurrent fluid overload in diabetes and diabetic kidney disease can be avoided with evidence-based interventions. We aimed to identify at-risk patients who can benefit from these interventions by developing risk prediction models for readmissions for fluid overload in people living with diabetes and diabetic kidney disease.
Methods
This was a single-center retrospective cohort study of 1,531 adults with diabetes and diabetic kidney disease hospitalized for fluid overload, congestive heart failure, pulmonary edema, and generalized edema between 2015 and 2017. The multivariable regression models for 30-day and 90-day readmission for fluid overload were compared with the LACE score for discrimination, calibration, sensitivity, specificity, and net reclassification index (NRI).
Results
Readmissions for fluid overload within 30 days and 90 days occurred in 8.6% and 17.2% of patients with diabetes, and 8.2% and 18.3% of patients with diabetic kidney disease, respectively. After adjusting for demographics, comorbidities, clinical parameters, and medications, a history of alcoholism (HR 3.85, 95% CI: 1.41–10.55) and prior hospitalization for fluid overload (HR 2.50, 95% CI: 1.26–4.96) were independently associated with 30-day readmission in patients with diabetic kidney disease, as well as in individuals with diabetes. Additionally, current smoking, absence of hypertension, and high-dose intravenous furosemide were also associated with 30-day readmission in individuals with diabetes. Prior hospitalization for fluid overload (HR 2.43, 95% CI: 1.50–3.94), cardiovascular disease (HR 1.44, 95% CI: 1.03–2.02), eGFR ≤45 mL/min/1.73 m2 (HR 1.39, 95% CI: 1.003–1.93) was independently associated with 90-day readmissions in individuals with diabetic kidney disease. Additionally, thiazide prescription at discharge reduced 90-day readmission in diabetic kidney disease, while the need for high-dose intravenous furosemide predicted 90-day readmission in diabetes. The clinical and clinico-psychological models for 90-day readmission in individuals with diabetes and diabetic kidney disease had better discrimination and calibration than the LACE score. The NRI for the clinico-psychosocial models to predict 30- and 90-day readmissions in diabetes was 22.4% and 28.9%, respectively. The NRI for the clinico-psychosocial models to predict 30- and 90-day readmissions in diabetic kidney disease was 5.6% and 38.9%, respectively.
Conclusion
The risk models can potentially be used to identify patients at risk of readmission for fluid overload for evidence-based interventions, such as patient education or transitional care programs to reduce preventable hospitalizations.
Keywords: Hospitalization, Readmission, Fluid overload, Heart failure, Diabetes
Introduction
Diabetes mellitus is a global health issue affecting approximately 529 million adults in 2021 and is likely to affect 1.3 billion by 2050 [1]. Health expenditure for the treatment of diabetes and its complications was largely driven by hospitalizations and unplanned readmissions that burden healthcare resources [2]. An authoritative call to action by the Lancet Commission on diabetes in 2020 was emphasized using data to improve diabetes care via better recognition and understanding of populations at risk of adverse health outcomes [2]. It is thus important to characterize potentially avoidable hospitalizations and readmissions among people with diabetes. During the country-wide National Diabetes Collaborative in the nation’s “War on Diabetes” [3], administrative data identified fluid overload as one of the most frequent diagnoses for hospitalizations and 30-day readmissions in our healthcare institutions. Fluid overload is a common complication of cardiovascular and kidney diseases that are frequent in people living with diabetes [4]. It reflects altered extracellular fluid homeostasis with accumulation of total body sodium and water and is associated with increased morbidity and mortality in heart failure [5], chronic kidney disease [6], and acute kidney injury [7]. However, early identification of fluid overload symptoms and timely institution of effective pharmacological and lifestyle modifications may avert hospitalizations for severe symptomatic fluid overload. The multidisciplinary integrated care and community support programs required to achieve this are likely to be resource-intensive [2, 8–10], so there is a need to stratify risk and identify patients who will benefit most from them.
There is currently a lack of validated tools to identify people with diabetes who are at risk of hospital readmissions for fluid overload. Studies restricted to heart failure used a limited range of International Classification of Diseases and Related Health Problems 10th Revision (ICD-10) codes [11, 12]. Hence, the spectrum of patients with fluid overload in kidney disease or who had unrecognized heart failure with preserved ejection fraction is often neglected. The studies typically evaluated all-cause readmissions [11, 13–16], rather than specifically examining readmissions for recurrent fluid overload. However, domain segmentation by disease-specific prediction is recommended due to the complexities of hospital readmissions [17]. Predicting readmission for fluid overload is relevant to select patients for targeted programs that aim to reduce recurrent severe symptomatic fluid overload. We hypothesized that risk models can predict readmissions for fluid overload. Hence, this study aimed to (1) evaluate the factors associated with hospital readmissions for recurrent fluid overload among individuals with diabetes and diabetic kidney disease and (2) develop and assess clinically relevant and easily applied risk prediction models for 30- and 90-day readmissions for fluid overload.
Methods
This was a retrospective cohort study of a convenience sampling of all adults with diabetes hospitalized for fluid overload at the Singapore General Hospital, an academic medical center and tertiary care hospital, between January 1, 2015, and December 31, 2017. The index hospitalization was defined as the first hospitalization for fluid overload during the study period. Fluid overload was identified from ICD-10 discharge codes E877 (fluid overload), I500 (congestive heart failure), J81 (pulmonary edema), and R601 (generalized edema) shown in online supplementary Table S1 (for all online suppl. material, see https://doi.org/10.1159/000538036). These were based on the diagnoses used in prior studies related to fluid overload in kidney disease [6, 18]. Patients were included if they had diabetes mellitus identified by the SingHealth Diabetes Registry according to diagnosis codes, laboratory results (fasting plasma glucose ≥7 mmol/L, oral glucose tolerance test plasma glucose ≥11.1 mmol/L at 2 h, HbA1c ≥ 7%), and/or the use of glucose-lowering medication [19]. Diabetic kidney disease was defined if there was reduced kidney function with estimated glomerular filtration rate (eGFR) ≤60 mL/min/1.73 m2. Patients were excluded if (1) they did not have any subsequent laboratory tests and prescriptions (up to 12 months post-discharge) or hospital visits (until December 30, 2018) since these patients were considered lost to follow-up or (2) they had kidney failure, defined as eGFR < 15 mL/min/1.73 m2.
Data were retrieved from electronic medical records (EMRs) and included demographic data (age, gender, ethnicity), comorbidities (cardiovascular disease, atrial fibrillation, history of alcoholism, smoking status, systolic and diastolic blood pressure [BP] on admission), biochemistry, and hospitalization dates and discharge diagnoses from 6 months prior to index hospitalization until December 30, 2018. Hypertension was defined as systolic BP >140 mm Hg, diastolic BP >90 mm Hg (at index hospitalization), or the use of BP-lowering medications. eGFR was calculated by the Chronic Kidney Disease Epidemiology Collaboration 2009 equation with the first serum creatinine value on admission for the index hospitalization [20]. Cardiovascular disease was defined as the presence of ischemic heart disease, congestive cardiac failure, acute myocardial infarct, stroke, and peripheral vascular disease within 6 months prior to the index hospitalization. The Charlson Comorbidity Index (CCI) was obtained using the algorithm by Quan et al. [21]. Assuming that acute kidney disease due to cardiorenal syndrome should resolve by discharge, we adapted the Kidney Disease: Improving Global Outcomes definition so that acute kidney disease was present if serum creatinine on admission was 1.5 times or more compared to discharge [22]. Prescriptions for renin-angiotensin system blockers such as angiotensin-converting enzyme inhibitor and angiotensin receptor blocker, diuretics, mineralocorticoid receptor blockers (MRBs), statins, and antidepressants (online suppl. Table S2) within 6 months prior to the index hospitalization and at discharge were retrieved from electronic prescription records. In addition to clinical factors related to disease severity and healthcare utilization, this study also included a history of alcoholism, use of antidepressants, smoking status, and subsidized ward admission as proxies for psycho-socioeconomic determinants of health in recognition of their potential impact on readmissions [23, 24].
The primary outcomes of interest were hospital readmissions for fluid overload within 30 days and 90 days of discharge from the index hospitalization. The non-readmission group was defined as those who did not have readmission for fluid overload within the period of interest. A 30-day readmission was the most frequent evaluated outcome in several systematic reviews [25, 26], since this was frequently used as a quality indicator for value-driven care practices and financial reimbursements [27]. Although there are several prediction models for 30-day hospital readmissions [25], the LACE index was one of the most frequently evaluated models in patients with and without heart disease [26, 28], possibly because of its discriminatory ability and simplicity for easy clinical application [23]. The LACE index assigns scores according to length of stay, acuity of admission, the CCI, and number of emergency department visits in the past 6 months to identify those at high risk for repeat hospitalizations [29]. Hence, the LACE index was chosen as the comparator for the developed models for 30-day readmission for fluid overload. Few studies have evaluated prediction models for risk of 90-day readmissions [26]. Yet, the 90-day interval is clinically relevant to identify hospitalized patients who may benefit from post-discharge transitional care programs that typically last several weeks to months [8, 10]. The LACE score was used in a handful of studies for unplanned emergency department visits and readmissions within 90 days after discharge for heart failure [13] and other conditions [30]. In contrast, other models included variables that were not readily available in the medical records or not relevant to our healthcare setting (such as “discharge during winter”) [16], or meant only for specific age groups [14, 15]. In the absence of generalizable and validated risk prediction models for fluid overload or all-cause hospitalization readmission at 90 days, the LACE score was chosen as the comparator for the developed models for 90-day readmission for fluid overload.
This study abided by the Declaration of Helsinki. Our institution’s Centralized Institutional Review Board (2020/3061) determined that the study did not require ethical deliberation for the use of de-identified data generated during routine clinical care. The study was reported according to the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) checklist (online suppl. Table S3).
Statistical Analyses
Statistical analysis was performed using IBM SPSS Statistics 26 (IBM Corp., Armonk, NY, USA), while model development and performance were evaluated using R 4.3.0 (R Core Team, 2023). Categorical variables were presented as proportions and continuous variables summarized as medians with interquartile ranges (25th percentile, 75th percentile) as appropriate. Baseline characteristics were compared according to the primary outcome using Pearson χ2 test or Fisher’s exact test for categorical variables and Mann-Whitney U test for continuous variables. To make the risk prediction model more interpretable and transparent for clinicians [17], optimal cut-off values for continuous variables (systolic BP, number of emergency visits and CCI, rounded to nearest whole number, and eGFR, rounded to the nearest 5 mL/min/1.73 m2) were identified using the Youden index, transforming them into categorical variables in the models.
Cox proportional hazards regression was performed to examine the association of demographic and clinical characteristics with hazards of readmission for fluid overload within 30 and 90 days. Missing data were excluded in the complete-case analyses. Data were censored at the last follow-up or at 30 or 90 days. For the clinical and clinico-psychosocial models, factors were chosen if p was ≤0.10 in univariable analysis. Multicollinearity was checked by examining the correlation matrix for coefficient values ≥0.80. Admission eGFR and discharge eGFR were highly correlated; hence, only discharge eGFR was included in the models. Additionally, we created parsimonious models where the factors were chosen if p was <0.05 in multivariable analysis. The Cox regression models were compared with the LACE index to predict risk of 30- and 90-day readmission for fluid overload. The concordance index (c index), the equivalent of the area under curve of the receiver operating curve (ROC), was computed to evaluate the ability of the models to discriminate risk. A c index with a value of 0.5 suggests poor discrimination, while a value of 1.0 suggests perfect ability to discriminate risk. Akaike information criterion was used to evaluate the goodness of fit while accounting for model complexity, with a difference in Akaike information criterion >10 between the models considered as significant [25]. The optimum cut-off for each model was determined by the Youden index of the model that corresponds to the point nearest to the upper left corner on the ROC. Sensitivity (proportion of readmissions correctly identified by the model), specificity (proportion of those without readmissions correctly identified by the model), positive predictive value (PPV, ability of a positive result by the model to identify those with readmissions), and negative predictive value (NPV, ability of a negative result by the model to identify those without readmission) were evaluated at the optimal cutoffs. The change in risk prediction by the Cox regression models, compared to LACE, was assessed by net reclassification index (NRI). Risk groups (low, intermediate, and high) were determined by the risk tertiles. The NRIe is the net proportion of events assigned to a higher risk category, while the NRIne is the net proportion of nonevents assigned a lower risk category. All tests were two-tailed, and statistical significance was defined as p < 0.05 unless otherwise stated.
Results
Individuals with Diabetes and Diabetic Kidney Disease Admitted for Fluid Overload
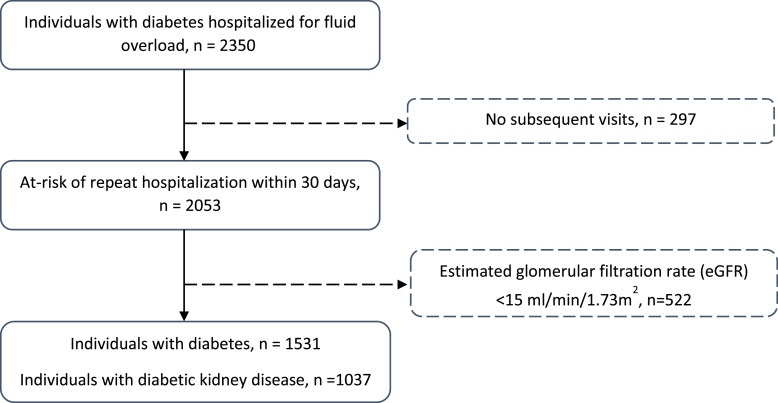
Figure 1 shows the study flow diagram for inclusion in this study. Among 1,531 patients with diabetes and hospitalized for fluid overload, 1,037 (67.7%) had diabetic kidney disease. Table 1 shows that comorbidities such as older age, hypertension, and cardiovascular disease were frequent among both the diabetes and diabetic kidney disease groups. The median eGFR on admission was 45.6 (29.0, 67.1) mL/min/1.73 m2 in the group with diabetes and 34.5 (23.9, 46.5) mL/min/1.73 m2 in the group with diabetic kidney disease. Intravenous (IV) furosemide was prescribed to the majority during the hospitalization, while high-dose frusemide ≥80 mg per day was required in 21.8% and 24.7% of individuals with diabetes and diabetic kidney disease, respectively; almost all patients received at least one oral diuretic at discharge. Most had LACE scores of 10 or more (traditionally considered “high risk” for hospital readmission).
Fig. 1.
Study cohort of 1,531 adults with diabetes hospitalized for fluid overload, among whom 1,037 had diabetic kidney disease. Patients were excluded if they were lost to follow-up, had kidney failure, or had missing values for the covariates included in the multivariable analyses.
Table 1.
Characteristics of study cohort of individuals with diabetes and diabetic kidney disease hospitalized for fluid overload
| Diabetes (N = 1,531) | Diabetic kidney disease (N = 1,037) | |
|---|---|---|
| Before hospitalization | ||
| Age, years | 73 (64, 81) | 75 (66, 82) |
| Older age ≥65 years, n (%) | 1,116 (72.9) | 811 (78.2) |
| Male gender, n (%) | 775 (50.6) | 496 (47.8) |
| Ethnicity | ||
| Chinese, n (%) | 1,005 (65.6) | 701 (67.6) |
| Malay, n (%) | 212 (13.8) | 145 (14.0) |
| Indian, n (%) | 243 (15.9) | 145 (14.0) |
| Other, n (%) | 71 (4.6) | 46 (4.4) |
| Hospitalization for fluid overload in past 6 months, n (%) | 62 (4.0) | 48 (4.6) |
| Emergency department visits in past 6 months, n (%) | 1 (1, 2) | 1 (1, 2) |
| Two or more ED visits in 6 months, n (%) | 704 (46.0) | 470 (45.3) |
| Cardiovascular disease, n (%) | 885 (57.8) | 611 (58.9) |
| Atrial fibrillation, n (%) | 281 (18.4) | 198 (19.1) |
| HbA1c#, % | 7.2 (6.4, 8.4) | 7.2 (6.4, 8.4) |
| Hypertension, n (%) | 1,239 (80.9) | 882 (85.1) |
| CCI | 6 (5, 9) | 7 (5, 9) |
| History of alcoholism, n (%) | 28 (1.8) | 15 (1.4) |
| Antidepressant use, n (%) | 118 (7.7) | 82 (7.9) |
| Current smoker, n (%) | 92 (6.0) | 57 (5.5) |
| During index hospitalization | ||
| Admission via emergency department, n (%) | 1,387 (90.6) | 956 (92.2) |
| Subsidized ward#, n (%) | 484 (93.3) | 387 (93.5%) |
| eGFR at admission, mL/min/1.73 m2 | 45.6 (29.0, 67.1) | 34.5 (23.9, 46.5) |
| AKD, n (%) | 104 (6.8) | 85 (8.2) |
| IV furosemide, n (%) | 1,255 (80.0) | 836 (80.6) |
| IV furosemide peak dose >80 mg/day, n (%) | 334 (21.8) | 256 (24.7) |
| Length of stay, days | 4 (2, 8) | 4 (2, 8) |
| LACE score | 13 (11, 14) | 13 (11, 15) |
| LACE score ≥10, n (%) | 1,411 (92.2) | 978 (94.3) |
| At discharge | ||
| Systolic BP, mm Hg | 129 (115, 141) | 130 (118, 144) |
| Systolic BP at discharge <125 mm Hg#, n (%) | 652 (43.0) | 401 (39.0) |
| Diastolic BP, mm Hg | 66 (60, 72) | 66 (60, 72) |
| eGFR, mL/min/1.73 m2 | 48.5 (30.6, 75.0) | 36.4 (25.4, 50.1) |
| eGFR at discharge ≤45 mL/min/1.73 m2, n (%) | 693 (45.3) | 685 (66.1) |
| Diuretic, n (%) | 1,474 (96.3) | 1,000 (96.4) |
| Loop diuretic, n (%) | 1,466 (95.8) | 996 (96.0) |
| Thiazide | 89 (5.8) | 59 (5.7) |
| MRB, n (%) | 299 (19.5) | 168 (16.2) |
| RAS blocker, n (%) | 1,102 (72.0) | 728 (70.2) |
| Statin, n (%) | 1,325 (86.5) | 915 (88.2) |
| Follow-up, months | 19.6 (9.9, 30.9) | 19.6 (9.5, 30.9) |
BP, blood pressure; eGFR, estimated glomerular filtration rate; IV, intravenous; RAS, renin-angiotensin system; MRB, mineralocorticoid receptor blocker; AKD, acute kidney disease.
#Missing data for HbA1c (n = 779), ward class (n = 1,003), and systolic BP at discharge (n = 13).
Hospital Readmissions for Fluid Overload
The median follow-up was 19.6 (9.9, 30.9) months. Hospital readmission for fluid overload occurred within 30 and 90 days in 8.6% and 17.2%, respectively, among individuals with diabetes; and 8.2% and 18.3% among individuals with diabetic kidney disease. Online supplementary Tables S4 and S5 compare the characteristics of patients with diabetes and diabetic kidney disease, respectively, according to the occurrence of readmission at 30 days and 90 days.
For the group with diabetes (online suppl. Table S4), the factors associated with both 30- or 90-day readmissions were prior hospitalization for fluid overload and more frequent emergency department visits within 6 months before the index hospitalization, current smoker, cardiovascular disease, absence of hypertension, and need for high-dose IV furosemide during the index hospitalization. A history of alcoholism was associated with 30-day but not 90-day readmission. Instead, the presence of atrial fibrillation, higher CCI, higher LACE score, lower eGFR at admission and discharge, and MRB on discharge were associated with 90-day readmission for fluid overload.
For the group with diabetic kidney disease (online suppl. Table S5), the factors associated with both 30- or 90-day readmissions were prior hospitalization for fluid overload and a history of alcoholism. Those with 90-day readmission were younger, had more frequent emergency department visits before hospitalization, and were more likely to have cardiovascular disease, atrial fibrillation, CCI ≥7, high-dose IV furosemide, eGFR ≤45 mL/min/1.73 m2, and MRB, but less likely to have thiazide prescibed at discharge, than those without readmission.
Table 2 shows that 30-day readmission for fluid overload was associated with a history of alcoholism and prior hospitalization for fluid overload in both diabetes and diabetic kidney disease. Additionally, current smokers, use of high-dose frusemide, and absence of hypertension also predicted 30-day readmission in diabetes. The magnitude of the associations in the clinical models was not materially altered by the addition of psychosocial factors. In bootstrap sampling with 10,000 replications (online suppl. Table S6), the direction of the associations remained consistent but the associations were less precise. Among individuals with diabetes, absence of hypertension remained significantly associated with 30-day readmission. Among individuals with diabetic kidney disease, prior hospitalization for fluid overload remained significantly associated with 30-day readmission.
Table 2.
Multivariable analysis for factors associated with hospital readmissions for fluid overload in individuals with diabetes and diabetic kidney disease
| Clinical model | Clinical + psychosocial | Clinical model | Clinical + psychosocial | |||||
|---|---|---|---|---|---|---|---|---|
| Adjusted HR (95% CI) | p value | Adjusted HR (95% CI) | p value | Adjusted HR (95% CI) | p value | Adjusted HR (95% CI) | p value | |
| For 30-day hospital readmission for fluid overload | ||||||||
| Diabetes | Diabetic kidney disease | |||||||
| Study cohort (N = 1,531), event, n = 131 (8.6%) | Study cohort (N = 1,037), event, n = 85 (8.2%) | |||||||
| Hospitalization for fluid overload within 6 months before index hospitalization, yes | 2.16 (1.20, 3.88) | 0.01 | 2.02 (1.12, 3.64) | 0.02 | 2.45 (1.24, 4.85) | 0.01 | 2.50 (1.26, 4.96) | 0.009 |
| Two or more emergency visits within 6 months before index hospitalization, yes | 1.38 (0.96, 2.00) | 0.08 | 1.37 (0.95, 1.98) | 0.09 | – | – | – | – |
| Cardiovascular disease, yes | 1.38 (0.94, 2.04) | 0.10 | 1.39 (0.94, 2.06) | 0.10 | 1.32 (0.82, 2.11) | 0.26 | 1.34 (0.83, 2.14) | 0.23 |
| Hypertension, yes | 0.64 (0.43, 0.94) | 0.02 | 0.63 (0.43, 0.93) | 0.02 | 0.66 (0.39, 1.11) | 0.12 | 0.65 (0.39, 1.10) | 0.11 |
| IV furosemide peak daily dose >80 mg/day, yes | 1.50 (1.03, 2.19) | 0.03 | 1.47 (1.01, 2.15) | 0.04 | 1.46 (0.92, 2.30) | 0.10 | 1.44 (0.91, 2.27) | 0.12 |
| History of alcoholism, yes | – | – | 2.61 (1.14, 5.98) | 0.02 | – | – | 3.85 (1.41, 10.55) | 0.009 |
| Current smoker, yes | – | – | 1.83 (1.06, 3.15) | 0.03 | – | – | – | – |
| For 90-day hospital readmission for fluid overload | ||||||||
|---|---|---|---|---|---|---|---|---|
| Diabetes | Diabetic kidney disease | |||||||
| Study cohort (N = 1,531), event n = 264 (17.2%) | Study cohort (N = 1,037), event, n = 190 (18.3%) | |||||||
| Hospitalization for fluid overload within 6 months before index hospitalization, yes | 2.14 (1.39, 3.27) | 0.0005 | 2.04 (1.33, 3.14) | 0.001 | 2.38 (1.47, 3.85) | 0.0004 | 2.43 (1.50, 3.94) | 0.0003 |
| Two or more emergency visits within 6 months before index hospitalization, yes | 1.18 (0.90, 1.54) | 0.23 | 1.20 (0.92, 1.57) | 0.18 | 1.04 (0.76, 1.42) | 0.80 | 1.03 (0.75, 1.41) | 0.85 |
| Cardiovascular disease, yes | 1.44 (1.08, 1.92) | 0.01 | 1.43 (1.08, 1.90) | 0.01 | 1.42 (1.01, 1.99) | 0.04 | 1.44 (1.03, 2.02) | 0.03 |
| Hypertension, yes | 0.76 (0.57, 1.02) | 0.07 | 0.76 (0.56, 1.02) | 0.07 | – | – | – | – |
| Atrial fibrillation, yes | 1.26 (0.95, 1.68) | 0.11 | 1.28 (0.96, 1.71) | 0.09 | 1.21 (0.86, 1.70) | 0.27 | 1.21 (0.86, 1.69) | 0.28 |
| CCI ≥7, yes | 1.26 (0.97, 1.62) | 0.08 | 1.24 (0.96, 1.60) | 0.10 | 1.25 (0.92, 1.69) | 0.16 | 1.22 (0.90, 1.66) | 0.20 |
| Emergency admission, yes | 1.25 (0.75, 2.07) | 0.39 | 1.24 (0.75, 2.06) | 0.40 | – | – | – | – |
| IV furosemide peak daily dose >80 mg/day, yes | 1.35 (1.02, 1.77) | 0.03 | 1.35 (1.02, 1.77) | 0.03 | 1.34 (0.98, 1.83) | 0.06 | 1.33 (0.98, 1.82) | 0.07 |
| eGFR at discharge ≤45 mL/min/1.73 m2, yes | 1.34 (1.03, 1.73) | 0.03 | 1.34 (1.03, 1.73) | 0.03 | 1.39 (1.00, 1.93) | 0.05 | 1.39 (1.003, 1.93) | 0.048 |
| Thiazide at discharge, yes | 0.70 (0.36, 1.38) | 0.30 | 0.71 (0.36, 1.39) | 0.32 | 0.30 (0.10, 0.94) | 0.04 | 0.30 (0.10, 0.95) | 0.04 |
| MRB at discharge, yes | 1.24 (0.92, 1.66) | 0.16 | 1.23 (0.92, 1.66) | 0.17 | 1.38 (0.97, 1.97) | 0.07 | 1.34 (0.93, 1.91) | 0.11 |
| Male, yes | 1.12 (0.87, 1.44) | 0.40 | 1.08 (0.84, 1.40) | 0.55 | 1.20 (0.89, 1.61) | 0.23 | 1.17 (0.87, 1.58) | 0.29 |
| History of alcoholism, yes | – | – | – | – | – | – | 2.14 (0.93, 4.94) | 0.08 |
| Current smoker, yes | – | – | 1.44 (0.93, 2.23) | 0.10 | – | – | – | – |
CI, confidence interval; IV, intravenous; BP, blood pressure; eGFR, estimated glomerular filtration rate; MRB, mineralocorticoid receptor blocker.
Readmission for fluid overload within 90 days was associated with prior hospitalization for fluid overload, cardiovascular disease, and reduced eGFR ≤45 mL/min/1.73 m2 at discharge in both diabetes and diabetic kidney disease (Table 2). Additionally, the need for high-dose frusemide predicted 90-day readmission in diabetes. The associations between the clinical factors and the outcome were not affected by the addition of psychosocial factors. Online supplementary Table S6 showed that all these predictors remained significantly associated with the outcome in bootstrapped regression models.
Risk Prediction Models for 30- and 90-Day Readmission for Fluid Overload
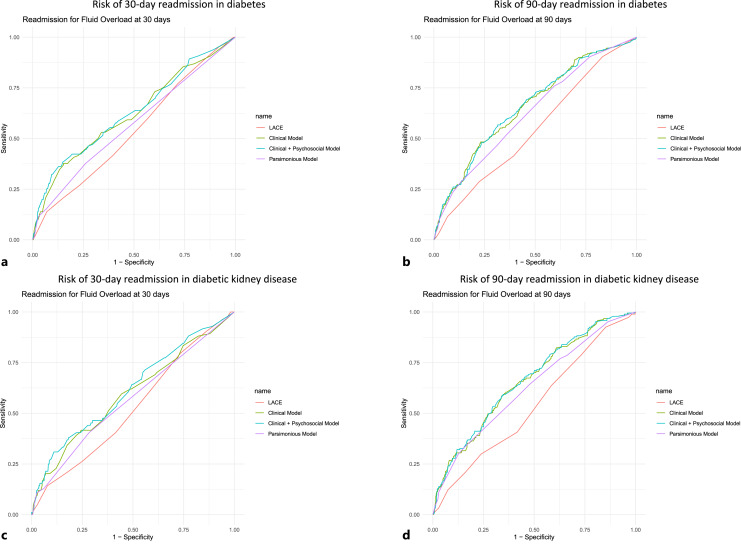
The performance of the clinical, clinico-psychosocial, and parsimonious Cox regression models was compared with LACE index in Table 3, with the corresponding ROCs in Figure 2. For predicting 30-day readmission for fluid overload in individuals with diabetes, the clinical, clinico-psychological models, as well as the parsimonious model, had better discrimination (c indices ranging 0.600–0.618) and goodness of fit compared to the LACE index. At the optimal cutoffs, the clinical, clinico-psychosocial, and parsimonious models had higher sensitivity, PPV, and NPV, but slightly lower specificity, than the LACE index. In diabetic kidney disease, the clinical and clinico-psychological models, but not the parsimonious model, had better discrimination compared to the LACE index. However, the c indices (ranging 0.527–0.594) were modest for all models. The goodness of fit was not significantly different between the models. At the optimal cutoffs, the clinical, clinico-psychosocial, and parsimonious models had lower sensitivity, higher specificity, and PPV, but similar NPV, compared with the LACE index.
Table 3.
Performance of the risk prediction models for 30- and 90-day readmission for fluid overload among individuals with diabetes and diabetic kidney disease
| For 30-day hospital readmission for fluid overload in diabetes | ||||
|---|---|---|---|---|
| Clinical model | Clinical + psychosocial | Parsimonious model | LACE index | |
| C index | 0.609 | 0.618 | 0.600 | 0.538 |
| AIC | 1,887.7 | 1,883.3 | 1,886.1 | 1,904.7 |
| Optimum cut-off | 0.119 | 0.118 | 0.128 | 0.109 |
| Sensitivity | 0.382 | 0.420 | 0.267 | 0.137 |
| Specificity | 0.847 | 0.824 | 0.906 | 0.931 |
| PPV | 0.189 | 0.182 | 0.210 | 0.157 |
| NPV | 0.936 | 0.938 | 0.930 | 0.920 |
| For 90-day hospital readmission for fluid overload in diabetes | ||||
|---|---|---|---|---|
| Clinical model | Clinical + psychosocial | Parsimonious model | LACE index | |
| C index | 0.660 | 0.659 | 0.629 | 0.546 |
| AIC | 3,755.1 | 3,754.7 | 3,759.1 | 3,795.9 |
| Optimum cut-off | 0.211 | 0.209 | 0.154 | 0.153 |
| Sensitivity | 0.477 | 0.473 | 0.761 | 0.905 |
| Specificity | 0.775 | 0.768 | 0.405 | 0.170 |
| PPV | 0.307 | 0.298 | 0.210 | 0.185 |
| NPV | 0.877 | 0.875 | 0.891 | 0.896 |
| For 30-day hospital readmission for fluid overload in diabetic kidney disease | ||||
|---|---|---|---|---|
| Clinical model | Clinical + psychosocial | Parsimonious model | LACE index | |
| C index | 0.586 | 0.594 | 0.551 | 0.527 |
| AIC | 1,165.3 | 1,162.6 | 1,162.7 | 1,171.7 |
| Optimum cut-off | 0.093 | 0.089 | 0.140 | 0.076 |
| Sensitivity | 0.412 | 0.435 | 0.153 | 0.800 |
| Specificity | 0.750 | 0.739 | 0.949 | 0.266 |
| PPV | 0.128 | 0.130 | 0.210 | 0.089 |
| NPV | 0.935 | 0.936 | 0.926 | 0.937 |
| For 90-day hospital readmission for fluid overload in diabetic kidney disease | ||||
|---|---|---|---|---|
| Clinical model | Clinical + psychosocial | Parsimonious model | LACE index | |
| C index | 0.659 | 0.665 | 0.630 | 0.540 |
| AIC | 2,551.8 | 2,551.2 | 2,552.8 | 2,583.6 |
| Optimum cut-off | 0.160 | 0.162 | 0.207 | 0.164 |
| Sensitivity | 0.789 | 0.784 | 0.547 | 0.926 |
| Specificity | 0.439 | 0.458 | 0.630 | 0.150 |
| PPV | 0.240 | 0.245 | 0.249 | 0.196 |
| NPV | 0.903 | 0.904 | 0.861 | 0.901 |
Clinical model: hospitalization for fluid overload within 6 months before index hospitalization, two or more emergency visits within 6 months before index hospitalization, cardiovascular disease, hypertension, IV furosemide peak daily dose >80 mg/day.
Clinical + psychosocial model: hospitalization for fluid overload within 6 months before index hospitalization, two or more emergency visits within 6 months before index hospitalization, cardiovascular disease, hypertension, IV furosemide peak daily dose >80 mg/day, history of alcoholism, current smoker.
Parsimonious model: hospitalization for fluid overload within 6 months before index hospitalization, hypertension, IV furosemide peak daily dose >80 mg/day during hospitalization, history of alcoholism, current smoker.
LACE index: length of stay, acuity of admission, CCI, number of emergency department visits in 6 months before index hospitalization.
Clinical model: hospitalization for fluid overload within 6 months before index hospitalization, two or more emergency visits within 6 months before index hospitalization, cardiovascular disease, hypertension, atrial fibrillation, CCI ≥7, emergency admission, IV furosemide peak daily dose >80 mg/day, eGFR at discharge ≤45 mL/min/1.73 m2, thiazide at discharge, MRB at discharge, male sex.
Clinical + psychosocial model: hospitalization for fluid overload within 6 months before index hospitalization, two or more emergency visits within 6 months before index hospitalization, cardiovascular disease, hypertension, atrial fibrillation, CCI ≥7, emergency admission, IV furosemide peak daily dose >80 mg/day, eGFR at discharge ≤45 mL/min/1.73 m2, thiazide at discharge, MRB at discharge, male sex, current smoker.
Parsimonious model: hospitalization for fluid overload within 6 months before index hospitalization, cardiovascular disease, need for IV furosemide peak daily dose >80 mg/day during hospitalization, eGFR at discharge ≤45 mL/min/1.73 m2.
LACE index: length of stay, acuity of admission, CCI, number of emergency department visits in 6 months before index hospitalization.
Clinical model: hospitalization for fluid overload within 6 months before index hospitalization, cardiovascular disease, hypertension, IV furosemide peak daily dose >80 mg/day.
Clinical + psychosocial model: hospitalization for fluid overload within 6 months before index hospitalization, cardiovascular disease, hypertension, IV furosemide peak daily dose >80 mg/day, history of alcoholism.
Parsimonious model: hospitalization for fluid overload within 6 months before index hospitalization, history of alcoholism.
LACE index: length of stay, acuity of admission, CCI, number of emergency department visits in 6 months before index hospitalization.
Clinical model: hospitalization for fluid overload within 6 months before index hospitalization, two or more emergency visits within 6 months before index hospitalization, cardiovascular disease, atrial fibrillation, CCI ≥7, IV furosemide peak daily dose >80 mg/day, eGFR at discharge ≤45 mL/min/1.73 m2, thiazide at discharge, MRB at discharge, male sex.
Clinical + psychosocial model: hospitalization for fluid overload within 6 months before index hospitalization, two or more emergency visits within 6 months before index hospitalization, cardiovascular disease, atrial fibrillation, CCI ≥7, IV furosemide peak daily dose >80 mg/day, eGFR at discharge ≤45 mL/min/1.73 m2, thiazide at discharge, MRB at discharge, male sex, history of alcoholism.
Parsimonious model: hospitalization for fluid overload within 6 months before index hospitalization, cardiovascular disease, eGFR at discharge ≤45 mL/min/1.73 m2, thiazide at discharge.
LACE index: length of stay, acuity of admission, CCI, number of emergency department visits in 6 months before index hospitalization. eGFR, estimated glomerular filtration rate; IV, intravenous; MRB, mineralocorticoid receptor blocker; AIC, Akaike information criterion.
Fig. 2.
ROC for the models to predict risk of readmission for fluid overload within 30 days (a) and 90 days (b) in diabetes and 30 days (c) and 90 days (d) in diabetic kidney disease.
For predicting 90-day readmission for fluid overload in individuals with diabetes and diabetic kidney disease, the clinical, clinico-psychological, and the parsimonious models had better discrimination and goodness of fit compared to the LACE index. At the optimal cutoffs, the clinical, clinico-psychosocial, and parsimonious models for both diabetes and diabetic kidney disease had lower sensitivity, higher specificity, and PPV, and similar NPV compared to the LACE index. In diabetes, the parsimonious model had higher sensitivity, lower specificity, and PPV, and similar NPV compared to the clinical and clinico-psychosocial models. In diabetic kidney disease, the parsimonious model had lower sensitivity, higher specificity, similar PPV, and lower NPV compared to the clinical and clinico-psychosocial models.
Supplementary Table S7 showed that as the risk threshold for 30-day readmission increased from 5% to 10%, the sensitivity decreased, specificity increased, PPV increased, and NPV was generally unchanged in both the clinical and clinico-psychological models for diabetes and diabetic kidney disease. As the risk threshold for 90-day readmission increased from 15% to 25%, the sensitivity decreased, specificity increased, PPV increased, and NPV decreased slightly in both the clinical and clinico-psychological models for diabetes and diabetic kidney disease.
The NRI of the clinical and clinic-psychological models in predicting risk of 30-day readmission for fluid overload was 22.1% and 22.4%, respectively, in diabetes (online suppl. Table S8); and 9.5% and 5.6%, respectively, in diabetic kidney disease (online suppl. Table S9). Compared to the LACE index, the models improved reclassification of individuals with readmissions to a higher risk category in both diabetes and diabetic kidney disease, while in individuals without readmission, the models improved classification to a lower risk category in diabetes but not in diabetic kidney disease.
The NRI of the clinical and clinic-psychological models in predicting risk of 90-day readmission for fluid overload was 27.9% and 28.9%, respectively, in diabetes (online suppl. Table S10); and 37.5% and 38.9%, respectively, in diabetic kidney disease (online suppl. Table S11). Compared to the LACE index, the models improved reclassification of individuals with readmissions to a higher risk category in both diabetes and diabetic kidney disease. In individuals without readmission, the models improved classification to a lower risk category.
Discussion
We identified clinical and psychosocial predictors for 30- and 90-day hospital readmissions for recurrent fluid overload among individuals with diabetes and developed highly specific multivariable models to identify at-risk patients. This study found that psychosocial factors such as a history of alcoholism and current smoking were associated with readmissions for fluid overload. This suggests that unhealthy lifestyles may influence or reflect poor chronic disease self-management [2, 4], including lack of sodium and fluid restriction and diuretic adherence in the case of recurrent fluid overload. Other studies have noted that socioeconomic factors (or their proxies) impact readmission risk [27]. Thus, our findings also support the call by the Lancet Commission for social or wider community support to address suboptimal health literacy in diabetes [2]. Clinical factors such as prior hospitalization for fluid overload, cardiovascular disease, need for high-dose IV furosemide, and reduced kidney function likely indicate the severity of fluid overload and its contributory diseases. A recent systematic review of 23 cohort and case-control studies of 30-day readmissions among 748,700 adults with diabetes noted that most studies were from North America [31]. Few studies specifically addressed the issues of recurrent fluid overload, heart failure, or severe edema. Pooled analysis from the systematic review and subsequent publications similarly found that heart failure, kidney disease, and alcohol abuse were predictors of readmissions [31, 32]. It was interesting that in individuals with diabetic kidney disease, thiazide use appeared to have a protective effect in reducing hospital readmission at 90 days. Since almost all patients received loop diuretics on discharge, this observation suggests that sequential blockade was effective in reducing recurrent fluid overload in reduced kidney function. This improvement in healthcare service utilization adds to current clinical trial and observational data on the improved diuresis when thiazides were added to loop diuretics [33–35], and may be a potential intervention to reduce readmissions for fluid overload in diabetic kidney disease.
The Lancet Commission emphasized using data to identify at-risk populations and drive integrated care [2]. While there were 23 studies published between 1998 and 2018 that used both EMR and administrative healthcare claim data to examine the occurrence and factors associated with hospital readmission among individuals with diabetes [31], only 4 studies published between 2015 and 2019 had utilized large EMR databases to develop or validate risk prediction models for 30-day hospital readmission in diabetes [27]. Our multivariable predictive models were comprehensively evaluated for accuracy and clinical usefulness [27], so that they can potentially be utilized in quality improvement or implementation projects to identify patients for interventions or transitional care in the community to reduce readmissions. The models performed better than the commonly used LACE score in both discrimination and calibration. The regression models have high NPV, which indicates that the models can correctly identify low-risk individuals. Thus, the models can be used to exclude lower risk individuals from resource-intensive measures, such as close monitoring via home visits or telephone calls, or multidisciplinary care with a holistic approach to clinical and psychosocial circumstances [2, 8, 9]. Additionally, implementation teams may select a higher risk threshold that corresponds with a higher PPV to better identify patients who will benefit most from the interventions.
Although our regression-based readmission risk models performed better than the clinical rule-based LACE index [17], the c indices for predicting 30-day readmission in diabetic kidney disease were modest, given that the c index for a typical predictive model is between 0.6 and 0.85 [36]. Improving discrimination by identifying strong predictors will be ideal but likely to face data and model challenges [17]. We had used EMR data because readmission risk prediction models using EMR data had better performance than those using administrative data [27], but predictors such as complex psychosocial issues may not be readily assessed or obtained during routine clinical care and thus not available in the EMR [2, 27]. Our models for predicting 30-day readmissions for fluid overload in diabetes and diabetic kidney disease had low PPV; hence, the models may identify “false positives,” i.e., individuals at low risk for readmission. This may be influenced by the low prevalence of 30-day readmissions in the study cohorts and mitigated by applying a higher risk threshold, as shown in online supplementary Table S7. Prognostic models developed in the secondary or tertiary care setting may not perform comparably in the primary care setting [37]. This is an important consideration if the prediction models are to be adopted upstream as our nation aims to improve population healthcare delivery in the “Healthier SG” strategy [38]. Hence, further research will be required to improve the discrimination and perform external validation of the risk models in different healthcare settings. Another study limitation is potential misclassification bias since the use of discharge codes may underestimate the true prevalence of the outcome [39]. However, the study inclusion criteria were comprehensive for fluid overload conditions such as congestive heart failure, pulmonary edema, and generalized edema, rather than “fluid overload” or “heart failure” alone. Echocardiography and New York Heart Association class were not available in the registry. While heart failure severity may be a confounder in fluid overload severity and recurrence risk, heart failure severity is not easily assessed in kidney disease because heart failure with preserved ejection fraction is prevalent and NT-proBNP may be inaccurate to determine cardiac dysfunction [40]. Few patients in our cohort were prescribed sodium/glucose cotransporter-2 inhibitors (SGLT2i) while hospitalized for fluid overload, possibly due to drug costs or because SGLT2i was withheld during acute illness. SGLT2i were similarly not included in older studies of readmission risks but reduced heart failure hospitalizations and readmissions in diabetes and kidney disease in recent trials [12, 41, 42]. Further evaluation of the risk models in the future may include a higher prevalence of SGLT2i use after subsidies became available. However, real-world prescription rates of SGLT2i among people with diabetes ranged 6–10% in Singapore and worldwide in the past 5 years [43–45]; hence, SGLT2i use may not significantly impact fluid overload hospitalizations for now.
Despite these limitations, this study added knowledge in identifying individuals with diabetes at risk of hospitalization for recurrent fluid overload [2]. The risk prediction models for readmissions for fluid overload were comprehensively evaluated, reported following the TRIPOD statement for greater transparency and reproducibility [46], and potentially easily applied in real-world settings since the predictors are commonly available to clinicians. Future studies to validate the models in different populations should consider how pharmacotherapy such as SGLT2i may alter the prediction models and evaluate the application of the prediction models in quality improvement and “real-world” implementation studies to reduce hospitalizations among people living with diabetes and diabetic kidney disease.
Acknowledgment
The authors thank Ms Xin Xiaohui from the Health Service Research Unit, Singapore General Hospital, for her support for the project.
Statement of Ethics
This study abided by the Declaration of Helsinki, and the Centralized Institutional Review Board (2020/3061) determined that the study did not require further ethical deliberation for use of de-identified data.
Conflict of Interest Statement
All authors declare no relevant conflict of interest.
Funding Sources
This study was supported by the SHF-Foundation Research Grant (SHF/HSRHO014/2017).
Author Contributions
Jiashen Cai and Cynthia Lim conceptualized and designed the study; Hanis Abdul Kadir, Jiashen Cai, and Cynthia Lim analyzed and interpreted data; Jiashen Cai and Dorothy Huang drafted the manuscript; Zhihua Huang, Li Choo Ng, Andrew Ang, Ngiap Chuan Tan, Yong Mong Bee, Chieh Suai Tan, and Cynthia Lim provided critical input to the manuscript.
Funding Statement
This study was supported by the SHF-Foundation Research Grant (SHF/HSRHO014/2017).
Data Availability Statement
Data sharing is available and subjected to institutional data sharing policies. Further inquiries can be directed to the corresponding author.
Supplementary Material.
References
- 1. GBD 2021 Diabetes Collaborators, Stafford LK, McLaughlin SA, Boyko EJ, Vollset SE, Smith AE. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):P203–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan JCN, Lim LL, Wareham NJ, Shaw JE, Orchard TJ, Zhang P, et al. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet. 2021;396(10267):2019–82. [DOI] [PubMed] [Google Scholar]
- 3. Bee YM, Tai ES, Wong TY. Singapore's “war on diabetes”. Lancet Diab Endocrinol. 2022;10(6):391–2. [DOI] [PubMed] [Google Scholar]
- 4. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. [DOI] [PubMed] [Google Scholar]
- 5. Miller WL. Fluid volume overload and congestion in heart failure: time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail. 2016;9(8):e002922. [DOI] [PubMed] [Google Scholar]
- 6. Banerjee D, Ma JZ, Collins AJ, Herzog CA. Long-term survival of incident hemodialysis patients who are hospitalized for congestive heart failure, pulmonary edema, or fluid overload. Clin J Am Soc Nephrol. 2007;2(6):1186–90. [DOI] [PubMed] [Google Scholar]
- 7. Bagshaw SM, Brophy PD, Cruz D, Ronco C. Fluid balance as a biomarker: impact of fluid overload on outcome in critically ill patients with acute kidney injury. Crit Care. 2008;12(4):169–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Low LL, Vasanwala FF, Ng LB, Chen C, Lee KH, Tan SY. Effectiveness of a transitional home care program in reducing acute hospital utilization: a quasi-experimental study. BMC Health Serv Res. 2015;15(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takeda A, Martin N, Taylor RS, Taylor SJ. Disease management interventions for heart failure. Cochrane Database Syst Rev. 2019;1(1):CD002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bamforth RJ, Chhibba R, Ferguson TW, Sabourin J, Pieroni D, Askin N, et al. Strategies to prevent hospital readmission and death in patients with chronic heart failure, chronic obstructive pulmonary disease, and chronic kidney disease: a systematic review and meta-analysis. PLoS One. 2021;16(4):e0249542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tan BY, Gu JY, Wei HY, Chen L, Yan SL, Deng N. Electronic medical record-based model to predict the risk of 90-day readmission for patients with heart failure. BMC Med Inform Decis Mak. 2019;19(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park S, Jeong HE, Lee H, You SC, Shin JY. Association of sodium-glucose cotransporter 2 inhibitors with post-discharge outcomes in patients with acute heart failure with type 2 diabetes: a cohort study. Cardiovasc Diabetol. 2023;22(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang H, Robinson RD, Johnson C, Zenarosa NR, Jayswal RD, Keithley J, et al. Using the LACE index to predict hospital readmissions in congestive heart failure patients. BMC Cardiovasc Disord. 2014;14(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Formiga F, Masip J, Chivite D, Corbella X. Applicability of the heart failure Readmission Risk score: a first European study. Int J Cardiol. 2017;236:304–9. [DOI] [PubMed] [Google Scholar]
- 15. Kitamura M, Izawa KP, Taniue H, Mimura Y, Imamura K, Nagashima H, et al. Relationship between activities of daily living and readmission within 90 days in hospitalized elderly patients with heart failure. Biomed Res Int. 2017;2017:7420738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huynh Q, Negishi K, De Pasquale CG, Hare JL, Leung D, Stanton T, et al. Validation of predictive score of 30-day hospital readmission or death in patients with heart failure. Am J Cardiol. 2018;121(3):322–9. [DOI] [PubMed] [Google Scholar]
- 17. Wang S, Zhu X. Predictive modeling of hospital readmission: challenges and solutions. IEEE/ACM Trans Comput Biol Bioinform. 2022;19(5):2975–95. [DOI] [PubMed] [Google Scholar]
- 18. Plantinga LC, Masud T, Lea JP, Burkart JM, O'Donnell CM, Jaar BG. Post-hospitalization dialysis facility processes of care and hospital readmissions among hemodialysis patients: a retrospective cohort study. BMC Nephrol. 2018;19(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lim DYZ, Chia SY, Abdul Kadir H, Mohamed Salim NN, Bee YM. Establishment of the SingHealth diabetes Registry. Clin Epidemiol. 2021;13:215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9. [DOI] [PubMed] [Google Scholar]
- 22. Lameire NH, Levin A, Kellum JA, Cheung M, Jadoul M, Winkelmayer WC, et al. Harmonizing acute and chronic kidney disease definition and classification: report of a kidney disease: improving global outcomes (KDIGO) consensus conference. Kidney Int. 2021;100(3):516–26. [DOI] [PubMed] [Google Scholar]
- 23. Low LL, Liu N, Wang S, Thumboo J, Ong ME, Lee KH. Predicting 30-day readmissions in an asian population: building a predictive model by incorporating markers of hospitalization severity. PLoS One. 2016;11(12):e0167413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lim CC, Huang D, Huang Z, Ng LC, Tan NC, Tay WY, et al. Early repeat hospitalization for fluid overload in individuals with cardiovascular disease and risks: a retrospective cohort study. Int Urol Nephrol. 2024;56(3):1083–91. [DOI] [PubMed] [Google Scholar]
- 25. Kansagara D, Englander H, Salanitro A, Kagen D, Theobald C, Freeman M, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Grootven B, Jepma P, Rijpkema C, Verweij L, Leeflang M, Daams J, et al. Prediction models for hospital readmissions in patients with heart disease: a systematic review and meta-analysis. BMJ Open. 2021;11(8):e047576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mahmoudi E, Kamdar N, Kim N, Gonzales G, Singh K, Waljee AK. Use of electronic medical records in development and validation of risk prediction models of hospital readmission: systematic review. BMJ. 2020;369:m958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou H, Della PR, Roberts P, Goh L, Dhaliwal SS. Utility of models to predict 28-day or 30-day unplanned hospital readmissions: an updated systematic review. BMJ Open. 2016;6(6):e011060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Walraven C, Dhalla IA, Bell C, Etchells E, Stiell IG, Zarnke K, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ (Can Med Assoc J). 2010;182(6):551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rajaguru V, Han W, Kim TH, Shin J, Lee SG. LACE index to predict the high risk of 30-day readmission: a systematic review and meta-analysis. J Pers Med. 2022;12(4):545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soh JGS, Wong WP, Mukhopadhyay A, Quek SC, Tai BC. Predictors of 30-day unplanned hospital readmission among adult patients with diabetes mellitus: a systematic review with meta-analysis. BMJ Open Diab Res Care. 2020;8(1):e001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koziol M, Towpik I, Zurek M, Niemczynowicz J, Wasaznik M, Sanchak Y, et al. Predictors of rehospitalization and mortality in diabetes-related hospital admissions. J Clin Med. 2021;10(24):5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56(19):1527–34. [DOI] [PubMed] [Google Scholar]
- 34. de la Espriella R, Cobo M, Núñez J. Thiazides in chronic kidney disease:“back to the future”. Clin Kidney J. 2023;16(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trullas JC, Morales-Rull JL, Casado J, Carrera-Izquierdo M, Sanchez-Marteles M, Conde-Martel A, et al. Combining loop with thiazide diuretics for decompensated heart failure: the CLOROTIC trial. Eur Heart J. 2023;44(5):411–21. [DOI] [PubMed] [Google Scholar]
- 36. Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ. 2009;338:b604. [DOI] [PubMed] [Google Scholar]
- 37. Moons KG, Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ. 2009;338:b606. [DOI] [PubMed] [Google Scholar]
- 38. Abraham M, Lim MJ, Tan WS, Cheah J. Global trends towards population health management and key lessons and initiatives in the Singapore context. Int J Integr Care. 2022;22(3):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of heart failure diagnoses in administrative databases: a systematic review and meta-analysis. PLoS One. 2014;9(8):e104519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Méndez AB, Azancot MA, Olivella A, Soler MJ. New aspects in cardiorenal syndrome and HFpEF. Clin Kidney J. 2022;15(10):1807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jhund PS, Kondo T, Butt JH, Docherty KF, Claggett BL, Desai AS, et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat Med. 2022;28(9):1956–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mark PB, Sarafidis P, Ekart R, Ferro CJ, Balafa O, Fernandez-Fernandez B, et al. SGLT2i for evidence-based cardiorenal protection in diabetic and non-diabetic chronic kidney disease: a comprehensive review by EURECA-m and ERBP working groups of ERA. Nephrol Dial Transplant. 2023;38(11):2444–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goh L, Sun J, Ng K. Changes in prescribing of sodium-glucose co-transporter 2 inhibitors and dipeptidyl peptidase 4 inhibitors in Singapore. Pharmacoepidem Drug Saf. 2020:141–1. [Google Scholar]
- 44. Baek JH, Yang YS, Ko SH, Han KD, Kim JH, Moon MK, et al. Real-world prescription patterns and barriers related to the use of sodium-glucose cotransporter 2 inhibitors among Korean patients with type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab J. 2022;46(5):701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Limonte CP, Hall YN, Trikudanathan S, Tuttle KR, Hirsch IB, de Boer IH, et al. Prevalence of SGLT2i and GLP1RA use among US adults with type 2 diabetes. J Diab Complicat. 2022;36(6):108204. [DOI] [PubMed] [Google Scholar]
- 46. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is available and subjected to institutional data sharing policies. Further inquiries can be directed to the corresponding author.