SUMMARY
Functional genomics is the use of systematic gene perturbation approaches to determine the contributions of genes under conditions of interest. Although functional genomic strategies have been used in bacteria for decades, recent studies have taken advantage of CRISPR (clustered regularly interspaced short palindromic repeats) technologies, such as CRISPRi (CRISPR interference), that are capable of precisely modulating expression of all genes in the genome. Here, we discuss and review the use of CRISPRi and related technologies for bacterial functional genomics. We discuss the strengths and weaknesses of CRISPRi as well as design considerations for CRISPRi genetic screens. We also review examples of how CRISPRi screens have defined relevant genetic targets for medical and industrial applications. Finally, we outline a few of the many possible directions that could be pursued using CRISPR-based functional genomics in bacteria. Our view is that the most exciting screens and discoveries are yet to come.
KEYWORDS: CRISPR-Cas9, genetic screens, ESKAPE pathogens, antibiotics, biofuels, bioproducts
INTRODUCTION
Functional genomics overview and classic approaches
Functional genomics is the use of systematic genetic approaches to uncover gene phenotypes (1). Functional genomics attempts to measure phenotypes of all genes being queried/perturbed under a given condition, in contrast to forward genetic or experimental evolution screens that attempt to identify a small number of outlier genes. Systematic phenotyping has several advantages. Because most phenotypes lie on a continuum (e.g., growth rates), rather than existing as a binary, functional genomics can capture the full distribution of phenotypic diversity (2). Furthermore, systematic approaches can catalog both direct and indirect effects of a given condition on cell physiology; this can be valuable in situations where indirect players have a major role in a biological outcome. For example, chemical-gene interaction screens (i.e., chemical genomics) have been used to investigate the direct and indirect contribution of various genes to antibiotic sensitivity and resistance phenotypes in bacteria (3). Finally, similar patterns of gene phenotypes across conditions provide stronger support for a functional relationship between genes than any single phenotype (4).
“Classic” functional genomics approaches in bacteria have used insertion/deletion mutagenesis to systematically disrupt non-essential genes. Gene deletion libraries, in which coding sequences are precisely replaced with antibiotic resistance markers using various recombination strategies, are gold-standard genetic resources for phenotyping non-essential genes (5–9). Mutants from these libraries are typically stored as individual isolates (arrayed), enabling rapid follow-up examination of phenotypes found in screens and detection of cell autonomous phenotypes. However, creating deletion libraries is labor and resource intensive, and as such, these libraries are only available for a handful of model organisms. An additional downside is that copying/expanding deletion libraries requires multiple passages that allows deletion strains to pick up secondary mutations that mitigate deletion phenotypes or are involved in acclimation to the laboratory environment (10). Gene deletion libraries contain complete loss-of-function mutants and, thus, do not contain essential genes by definition. One often unanticipated complication of using gene deletion libraries is that phenotypes can occasionally arise from overexpression of genes downstream of the deletion due to read through transcription from the promoter of the antibiotic resistance marker (7).
Transposon sequencing (Tn-seq) and its predecessor, transposon site hybridization (11, 12), use random transposon insertions for genome-wide mutagenesis, providing a global view of gene phenotypes without the high costs or planning requirements associated with other approaches. Since its introduction in 2009 (11, 12), Tn-seq has been used in dozens of organisms and has become the backbone of bacterial functional genomics (13, 14). Tn-seq is effective at identifying essential and conditionally essential genes, which appear as genes lacking or with greatly reduced transposon insertions in the essential condition (15–17). Creative investigators have used Tn-seq to explore antibiotic function (18–22), measure the rate of depletion of essential genes in naturally competent bacteria, and identify synthetic lethal gene interactions between redundant or parallel pathways (14, 23, 24). However, Tn-seq is not without limitations. Smaller genes may not be phenotyped correctly by Tn-seq libraries with sparse insertion coverage (i.e., low insertion density); this could result in false positives in screens for essentiality. If Tn-seq libraries are made larger to better assess the essentiality of short genes, they might become less manageable due to size, complicating their use in high-throughput phenotypic assays. Non-random insertion biases of some transposons can prevent uniform insertion coverage and complicate analysis (25, 26). As with deletion libraries, Tn-seq can be used to predict essential genes, but not characterize their phenotypes [with some exceptions (23, 24)]. Finally, Tn-seq approaches often utilize transposons carrying a strong promoter to retain expression of downstream genes; however, such promoters can result in overexpression of downstream genes. Although this feature has been used as a deliberate strategy to identify overexpression phenotypes (25), it necessitates validation of Tn-seq findings since they may result from either interruption of an upstream gene or overexpression of a downstream gene.
Deletion libraries and Tn-seq offer insights into gene function by abolishment of function, thus limiting their scope to a binary nature of gene disruption. Systems that use conditional proteolysis of the gene product, such as PROSPECT (27), offer a nuanced approach. By fusing essential genes to C-terminal tags targeted for protein degradation in hypomorph strains, target levels of protein depletion can be achieved. Once PROSPECT strains are constructed, they can be barcoded and combined together to assess phenotypic analysis in a pooled competition context. However, some proteins may not be suitable for this approach if the tag alone renders the gene product inviable.
Introduction to CRISPRi
In recent years, functional genomics studies have taken advantage of CRISPR (clustered regularly interspaced short palindromic repeats) technologies. There are many reviews covering CRISPR types, mechanisms, and applications in gene editing (28–32); therefore, we only provide a brief background here. Multiple varieties of CRISPR systems exist; class I systems depend on a multi-protein effector complex, while class II systems utilize a single effector protein. Generally, class II systems are more widely employed in research for their simplicity. DNA editing in both eukaryotes and bacteria can be achieved when CRISPR-induced double-stranded breaks (DSBs) are repaired through homology-directed repair (HDR) using a provided DNA repair template with homology to the target locus. In eukaryotes, CRISPR can also be used for genome-scale screens due to efficient non-homologous end joining (NHEJ) that can repair DSBs in the absence of a target-specific HDR repair template, an error-prone process which usually disrupts gene function (30). Since NHEJ is often absent or inefficient in bacteria (33), CRISPR-induced DSBs are fatal without an HDR template. In part, for this reason, functional genomics screens in the bacterial domain have commonly employed CRISPRi technology.
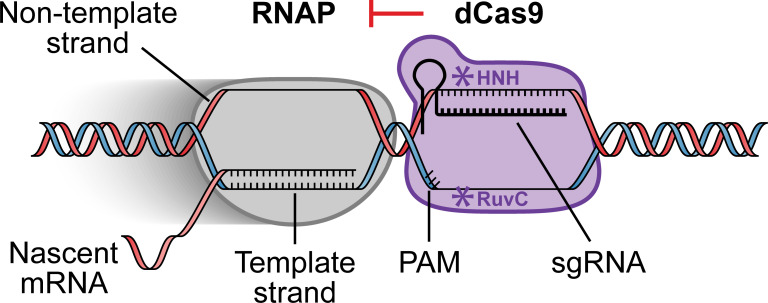
Among other advantages, CRISPRi powerfully interrogates gene function without permanent modification of the DNA target. First developed in 2013, CRISPRi is a programmable gene knockdown technology that utilizes a modified, catalytically inactive variant of the CRISPR-associated (Cas) nuclease to bind to the target DNA and block progression of RNA polymerase (RNAP) (34). CRISPRi can be finely tuned for partial knockdowns and scaled to target all genes in the genome (35–38). The vast majority of CRISPRi systems use a catalytically inactive variant of the Cas9 nuclease (dCas9) and a fusion of cr- and tracr-RNAs into a single-guide RNA (sgRNA) to silence gene expression (Fig. 1) (34), though derivatives from other CRISPR types exist (39). The first 20 nucleotides of the sgRNA, known as the spacer, direct dCas9 to a complementary sequence in target genes (protospacer) with an adjacent PAM (protospacer adjacent motif) where it physically blocks either RNAP association with promoter DNA or RNAP elongation throughout the gene body (34, 40), depending on the protospacer location. Unlike eukaryotic CRISPRi systems that rely on fused silencing factors (41), dCas9-sgRNA binding to DNA is sufficient for robust gene repression in bacteria (34, 42).
Fig 1.
Mechanism of gene knockdown by CRISPRi. An sgRNA binds to dead dCas9 and directs the complex to a complementary DNA target sequence (protospacer) with an adjacent PAM where the complex physically blocks elongation by RNAP. Asterisks in dCas9 mark point mutations which inactivate nucleolytic activity. sgRNA designs that target the non-template strand are generally more effective for CRISPRi knockdowns.
There are numerous benefits to using CRISPRi including simplicity, ability to control knockdown levels, targeting of essential genes, and multiplexing. The sgRNA spacer sequence can be readily changed through simple cloning procedures (43), enabling facile reprogramming of the CRISPRi system to recognize genomic targets of interest. CRISPRi knockdown levels can be titrated in multiple ways, which is advantageous for targeting essential genes where full knockdown would result in lethality. For example, controlling expression of the CRISPRi machinery under inducible promoters enables either complete repression or partial knockdown using saturating or sub-saturating inducer concentrations, respectively (30, 31). Titrating inducer concentrations is perhaps the most straightforward approach to achieving partial knockdowns, though this strategy may not be ideal for single-cell studies since it has been shown to yield noisy knockdown in single Escherichia coli cells (34, 40, 44). Alternatively, modifying the sgRNA using truncations (44) or mismatches (45, 46) can partially reduce knockdown levels. By combining perfect and mismatched sgRNAs into a single pooled or arrayed library, both non-essential and essential genes can be phenotyped simultaneously using a saturating inducer concentration. CRISPRi can also be used with constitutive promoters of varying strengths to achieve knockdown gradients in contexts where varying inducer concentrations may be challenging. For instance, one study utilized a constitutively expressed CRISPRi system in Pseudomonas aeruginosa to explore the contribution of conditionally essential genes on virulence using a murine pneumonia model (47). Multiplexing with multiple sgRNAs or through the use of CRISPR arrays to explore genetic interactions is also quite feasible with CRISPRi—phenotypes of strains with up to 10 knockdowns have been reported (48).
Nonetheless, CRISPRi has some drawbacks that limit its utility in specific situations. Because CRISPRi represses transcription, downstream genes in a transcription unit (TU) will also be knocked down [a phenomenon known as polarity (34)]. Depending on the organism or CRISPRi system employed, targeting downstream genes in a TU can also cause knockdown of upstream genes, a phenomenon we call “reverse polarity.” Reverse polarity ranges in severity from near complete knockdown of upstream genes [e.g., Bacillus subtilis (40)] to more modest effects on upstream expression [e.g., Escherichia coli (49) and Zymomonas mobilis (50)] to no detectable effects [e.g., Mycobacterium smegmatis (51)]. The mechanistic basis for reverse polarity is currently unknown. CRISPRi systems may also interact with native machinery which can produce off-target effects. This can be addressed by deleting or inactivating the native CRISPR (52, 53). CRISPRi components can also be toxic when expressed at high levels, either individually [dCas9 alone (54–56)] or through toxic sgRNAs seen in the “bad seed” effect (49, 57). Although CRISPRi is more straightforward than other strategies used to phenotype essential genes, suppressors can emerge that inactivate CRISPRi when essentials are targeted and fitness is greatly reduced; mutant strains with inactivated CRISPRi systems behave like wild type and outgrow the knockdown strain in the same culture (58). This problem can be mitigated, if necessary, by expressing a second copy of dCas9 as most CRISPRi inactivating mutations occur in the dcas9 gene (58) or by including additional barcodes near the sgRNA that can detect CRISPRi “escaper” strains that show divergent fitness (46).
Strategies to deliver CRISPRi systems to diverse bacteria typically rely on either replicative plasmids or genome integration. Utilizing CRISPRi via plasmids offers flexibility, as new plasmids can be easily introduced by transformation or conjugation in many bacteria (34, 59). However, the persistent need for selective pressure to retain these plasmids can be resource-intensive, potentially limiting in prolonged studies, and can introduce variables that might affect chemical-gene interactions. Furthermore, expression of dCas9 from plasmids must be tightly controlled as excessive dCas9 expression can be toxic on its own or contribute to bad seed effects (49, 54).
We previously developed Mobile-CRISPRi, a suite of integrating vectors capable of delivering CRISPRi systems to diverse bacteria (43, 60). Mobile-CRISPRi for Gram-negative bacteria uses conjugation from E. coli donors and integrates into recipient genomes using the broad host range Tn7 transposase. The Gram-positive version of the system uses conjugation from B. subtilis donors and transfers CRISPRi via the ICEBs1 conjugatable element. ICEBs1 transfers CRISPRi at high efficiency to some organisms (e.g., Listeria monocytogenes) but is limited in host range due to an attachment site that is only found in close relatives of B. subtilis. Mobile-CRISPRi insertion into the genome offers consistency in experimental designs and eliminates the need for antibiotic selection to maintain CRISPRi. However, we and others have found that Tn7 insertion downstream of the essential glmS gene, while typically benign, is sometimes associated with organism- and condition-specific defects [e.g, in Agrobacterium tumefaciens (61)].
CRISPRi FUNCTIONAL GENOMICS
Design considerations
One major distinction between functional genomics experiments using CRISPRi or Tn-seq is that CRISPRi libraries require more upfront design considerations (Table 1). Because researchers can define the set of genes under investigation in a CRISPRi experiment by including sgRNAs targeting only those genes, CRISPRi allows for substantial flexibility to define the scope (which genes are being targeted) and resolution (how many times those genes are targeted) of functional genomics (62). Some technical or biological parameters of a screen may impose limits on the size of a CRISPRi library. For instance, high-throughput screens for antibiotic activity often occur in small volumes of growth medium (e.g., in 384-well plates) in which a tradeoff exists between the size and diversity of the initial culture inoculum and the number of doublings required to detect a drug-gene phenotype (63). Such limits could be mitigated by including only essential genes in the CRISPRi library, although conditionally essential resistance mechanisms may be missed. Functional genomic screens for genes involved in host-microbe interactions often encounter population bottlenecks imposed by host biology (37). Designing more compact CRISPRi libraries may decrease the impact of bottlenecks on strain fitness measurements while ensuring coverage of all genes. In terms of resolution, including more sgRNAs per gene allows for detection of subtle phenotypes while requiring that the initial inoculum must be large enough to accommodate additional sgRNAs and increasing library construction costs.
TABLE 1.
Cautions and potential pitfalls in CRISPRi library design
| Genomic feature | gRNA design challenge | Pitfall explanation | Reference |
|---|---|---|---|
| Genes of unknown function and unannotated regions | Absence of functional annotation hampers gRNA design. | Interference with unknown function genes may yield uninterpretable or misleading phenotypic outcomes. | (35) |
| Origin of replication | Targeting oriC arrests replication and regulatory protein binding. | Disruption can interfere with the DNA replication, complicating interpretation of the rest of the library. | (64) |
| GC content | Regions with extreme GC content can reduce uniqueness and alter binding affinity. | A non-representative GC content can skew binding efficiency, leading to variable knockdown effectiveness or off-target interactions. | (65) |
| Regulatory elements (e.g., promoters and terminators) | Regulatory elements can share nucleotide sequences, making them challenging to uniquely target. | Non-specific guide binding or drastic alterations of cellular expression profiles can result in data that are difficult to interpret. | (66) |
| Transcription unit | Targeting operons risks polarity (multi-gene perturbation) due to polycistronic mRNA structures. | gRNAs may affect adjacent genes within an operon, making it challenging to ascribe observed effects to the knockdown of a single gene. | (40) |
| Overlapping genes | Segments of DNA shared between multiple genes cannot be targeted individually. | Attributing phenotyptes to a single gene may not be possible if guides target a common genomic region containing multiple genes. | (67) |
| Essential genes | Reduction of expression may be lethal to cells. Sub-maximal knockdown in expression is likely required. (e.g., mismatched guides) | Identifying interactions between gene knockdown and chemical perturbations may not be possible with extreme phenotypic consequences. | (45) |
Importantly, a well-annotated genome is required to plan and construct CRISPRi libraries. Identifying specific genome characteristics is a fundamental aspect for sgRNA design to ensure interpretation of results. When considering the design of a CRISPRi library, the presence of annotated features within a genome contributes to precision and influences the library’s overall scope. Although genes containing repetitive elements are amenable to targeting, their outcomes can be complex to interpret. Genome features that can complicate mapping phenotypes to spacer sequences, such as large variations in GC content/skew (68), mobile genetic elements (69), duplicated genes, or consistent motifs in multi-domain proteins (70, 71), may make it necessary to forgo targeting any of these regions.
The requirement of PAM sequences must also be considered as part of CRISPRi library design. The most extensively characterized dCas9 protein is derived from Streptococcus pyogenes Cas9 which requires recognition of a PAM with the sequence “NGG” prior to spacer-protospacer base-pairing. In a uniformly distributed genome, such recognition allows for the generation of a spacer at approximately 16-nucleotide intervals (72). However, genomic regions with deviations in GC content can disrupt the frequency of viable target sites for spacer design aimed at essential genes or features. Banta (43) and Gestel (73) mitigate such issues by providing frameworks for constructing Mobile-CRISPRi libraries based on NGG PAMs.
Seed sequences adjacent to the PAM introduce an additional layer of complexity beyond PAM specificity in spacer design. Research from Bikard’s group reveals that certain seed sequences may lead to unanticipated cellular toxicity via non-specific off-target binding to essential genes (49). Seeds with nine-nucleotide complementarity generally induce a loss in cellular fitness, whereas four-nucleotide seeds sporadically cause significant fitness loss despite their prevalence genome-wide. Both types of adverse effects are mitigated through reduced dCas9 expression.
To further enhance the validity of conclusions drawn from CRISPRi experiments, utilizing multiple sgRNAs to target each gene or TU is recommended (57). Once sgRNA spacers are designed, they can be ordered individually as annealed oligos (for individual knockdown strain construction or arrayed libraries), or as large oligo pools that are amplified by PCR and cloned into delivery vectors (Fig. 2).
Fig 2.
Graphic overview of a CRISPRi functional genomics screen and analysis for a library of k strains in n competition assays. Oligo libraries can be amplified and cloned into delivery vectors to generate knockdown strain libraries. Phenotyping typically consists of growth in a pooled, competitive environment in which some strains increase in frequency (increased relative fitness) while others are depleted from the pool (decreased relative fitness). Amplicons of sgRNA spacers act as barcodes to identify and count each knockdown strain. Once counted, information about the fitness of knockdown strains can be analyzed and modeled.
In CRISPRi experimental design, considerations of controls, selective pressure, and population diversity can improve screening outcomes. Inclusion of non-targeting control sgRNAs creates a set of strains with no expected phenotypes that can facilitate downstream normalization and allow for quantification of population diversity (74). Weaker selective pressures allow quantification of gene-condition interactions for entire libraries, while stronger selective pressures enrich for outlier gene knockdowns with greater fitness than non-targeting controls. Because CRISPRi targets gene transcription, protein function is lost over time due to dilution and turnover that occurs during growth. Accordingly, knockdown phenotypes are more detectable with increasing doublings post CRISPRi induction. Non-targeting controls may also function as measures of population diversity (75). In scenarios where strong selective pressure or bottlenecks arise (e.g., CRISPRi screens in a pathogenesis context), non-targeting controls may act in an analogous way to barcoded population approaches, such as sequence tag-based analysis of microbial populations (STAMP) experiments (76). Thus, inclusion of non-targeting controls allows within experiment measures of selective pressure and strain diversity.
One notable exception to CRISPRi library design requirements is an approach from Tavazoie and colleagues that leverages the natural CRISPR spacer acquisition machinery to generate high-complexity sgRNA libraries (77). This “adaptation-mediated library manufacturing” produces diverse libraries and more closely resembles Tn-seq in terms of quasi-random perturbation of gene function. The high resolution of such libraries enables detection of subtle phenotypes at the cost of added complexity. Such acquisition approaches could help democratize the use of CRISPRi libraries by reducing upfront library construction costs; however, it is important to note that the costs of both library construction and sequencing should be taken into account when planning CRISPRi functional genomics screens, since larger libraries require deeper sequencing.
Essential genes underpin cell survival; thus, altering their expression can lead to substantial phenotypes. To phenotype essentials, researchers must take care to precisely modulate knockdown, as greatly reduced expression will result in lethality and insufficient knockdown may not produce observable changes (78). To address this issue, researchers have developed multiple strategies to modulate knockdown including inducible/titratable expression of CRISPRi components, sgRNA truncation, introducing mismatches between sgRNA spacers and protospacers, and use of “alternative” PAMs that lead to reduced knockdown (2). In particular, use of mismatched sgRNAs is promising as mismatched sgRNA efficacy is predictable by machine learning approaches, shows demonstrated applicability in several bacterial hosts, and simplifies experimental design by not requiring multiple concentrations of CRISPRi inducer (45). Mismatch-CRISPRi and related approaches have underpinned quantitative modeling efforts that characterize the relationship between essential gene function and expression (2, 45, 46, 74).
Analysis of CRISPRi functional genomics data
There are numerous acceptable analytical practices for analyzing CRISPRi screen data. For a summary of available tools for designing and analyzing CRISPRi screens, see Table 2. Generally, CRISPRi-seq experiments include a measure of initial strain diversity (Tinitial) compared to diversity after growth in a condition of interest (Tfinal) (Fig. 2). For instance, to identify essential genes, researchers may compare Tinitial to Tfinal of knockdown mutants grown in rich medium with CRISPRi induced. Alternatively, for conditions such as drug or inhibitor treatment, one could compare Tfinal untreated to Tfinal treated to identify genes involved in drug/inhibitor targeting or resistance. In addition, timecourse experiments after CRISPRi induction can provide additional information for quantitative models of knockdown-fitness relationships (46, 79). Short-read aligners (e.g., Bowtie) and K-mer-based algorithms (e.g., Seal.sh) and other approaches [2FAST2Q (80), FeatureCounts (81), MAGeCK (82)] have been used to count sgRNA spacers—a proxy for knockdown strain abundance—from Illumina-sequenced amplicons. As with most sequencing approaches, sufficient depth is required for confident abundance measurements.
TABLE 2.
Tools for designing and analyzing CRISPRi screens
| Stage of process | Tool | Description | Website | Reference(s) |
|---|---|---|---|---|
| Library design | CCTop | Web-based tool to identify targets for sequences, identifies off-targets and aids in primer design. | https://cctop.cos.uni-heidelberg.de/ | (83) |
| CRISPOR | Predicts sgRNA efficiency and identifies off-targets for multiple types of CRISPR experiments. | http://crispor.tefor.net/ | (84) | |
| CHOPCHOP and CHOPCHOP v2 | Provides sgRNA design with predictions for off-targets with multiple species built-in. | https://chopchop.cbu.uib.no/ | (85, 86) | |
| Cas-OFFinder | Identifies off-target in genomic data by examining mismatches and genomic topology. | http://www.rgenome.net/cas-offinder/ | (87) | |
| CRISPR-ERA | Web-based tool for sgRNA design that includes functionality for predicting off-targets. | http://crispr-era.stanford.edu/ | (88) | |
| sgRNA Design | Provides all possible guides to uniquely target a genome, while minimizing off-targets for CRISPRi. | https://github.com/ryandward/sgrna_design | (43) | |
| Sequence analysis and visualization | SnapGene | Visualizes prokaryotic annotations, also useful for plasmid mapping and simulating experiments. | https://www.snapgene.com/ | N/A |
| Geneious | Comprehensive suite for sequence analysis including alignment, assembly, and annotation of genomic data. | https://www.geneious.com/ | N/A | |
| High-throughput screening | MAGeCK | Broad suite of tools for comprehensive analysis CRISPR/Cas9 and CRISPRi screen data, includes visualizations. | https://github.com/liulab-dfci/MAGeCK | (82) |
| CRISPRAnalyzeR | Interactive suite for analyzing CRISPR screens, with a focus on modularity and visualization. | https://github.com/boutroslab/CRISPRAnalyzeR | (89) | |
| Custom data analysis | DESeq2 | R packages that track composition changes in library competition assays. Originally designed for RNA-seq, but adapted for CRISPR genome-scale experiments. | https://bioconductor.org/packages/release/bioc/html/DESeq2.html | (90) |
| edgeR | https://bioconductor.org/packages/release/bioc/html/edgeR.html | (91) | ||
| limma | https://bioconductor.org/packages/release/bioc/html/limma.html | (92) | ||
| Alignment, mapping, and guide counting | Bowtie | Aligns sgRNA sequences to reference genomes, identifying targeted regions and off-targets. | https://bowtie-bio.sourceforge.net/index.shtml | (93) |
| BBMap & seal.sh (BBTools) | A suite of tools including an aligner for short reads to reference genomes (BBMap) and counting sgRNA occurrences from raw read data (seal.sh). | https://jgi.doe.gov/data-and-tools/software-tools/bbtools/ | N/A | |
| BLAST | Searches nucleotide or protein databases to identify sequence matches, aiding in validation of targets and finding orthologs. | https://blast.ncbi.nlm.nih.gov/Blast.cgi | (94) | |
| Functional analysis | OrthoFinder | Identifies orthologous groups across species, useful for comparative genomic analyses and sgRNA design for understudied species. | https://github.com/davidemms/OrthoFinder | (95) |
| STRING-db | Provides known and predicted protein-protein interactions, permitting broad characterization of pathways and protein complexes. | https://string-db.org/ | (96) |
Several tools have been utilized to quantify changes in relative knockdown fitness. For instance, edgeR (91) and DESeq2 (90), originally designed for RNA-seq analysis, have been adapted for CRISPRI-seq and integrated with pooled statistical approaches such as Stouffer’s method to calculate false discovery rates for individual genes from groups of sgRNAs that target that gene (97). More specialized tools [e.g., MAGeCK (82)] were designed specifically for CRISPR screening and offer functionalities such as counting, data quality control, hit identification, and result visualization. Considering the behavior of all sgRNAs targeting a gene or TU is advisable, as, on rare occasions, guides can fail to function or have off-target effects (98)—some analysis tools, such as MAGeCK, take this into account by default (82).
In CRISPRi studies, gene knockdowns can be conceptualized as reductions in gene “dose.” Analyzing the sigmoidal relationship between gene dose and the resulting cellular fitness demands rigorous statistical methodologies, with an emphasis on the need to minimize overfitting. A broad spectrum of mismatch knockdowns proves crucial for establishing a robust statistical relationship. Investigations by Rock and colleagues in Mycobacterium tuberculosis (Mtb) and M. smegmatis explored the correlation between PAM sequences, spacer lengths, and GC content with gene knockdown efficiency. A critical threshold, termed “vulnerability,” emerges when significant gene expression reductions lead to fitness loss, bearing similarities to the enzymological concept of the effective concentration required to cause a 50% change in response (2, 99). Otto et al. employed continuous epistasis combined with Mismatch-CRISPRi for gene expression titration in double CRISPRi knockdown for 20 genes in E. coli (79). Their methodology relied on quantitative reverse transcription PCR (RT-qPCR) to measure knockdown, setting parameters for subsequent curve fitting. They also found similar dose-response relationships between knockdown and fitness. Hawkins et al. utilized green fluorescent protein (GFP) as a species-neutral indicator to ascertain gene expression reductions by constructing 30,000 mismatch sgRNAs targeting GFP in B. subtilis and E. coli. Their approach involved fluorescence-activated cell sorting (FACS) and binning to identify sequences with reduced fluorescence and thus efficacy (45).
We previously extended the application of dose-response modeling to an antibiotic context in Acinetobacter baumannii (74). Building upon mismatch sgRNA efficacy predictions developed by Hawkins et al. (45), we adapted the Brain-Cousens model [a sigmoidal model traditionally used to model herbicide response (100)] to interactions between essential gene knockdowns and antibiotics. This modeling revealed that multiple “phases” of knockdown-antibiotic interactions were possible; for instance, at low levels of knockdown, the tRNA synthetase gene, glnS, showed resistance to the carbapenem antibiotic, imipenem, but sensitivity at higher levels of knockdown. The extent to which this phenomenon occurs across genes, antibiotics (or other environmental conditions), and genetic backgrounds remains an open question.
MEDICAL APPLICATIONS OF CRISPRi
CRISPRi can be used to identify and validate the targets of antibacterial compounds or discover unique pathogen vulnerabilities that could be used to develop new antibiotics (Fig. 3). This application is critical as the rate of multi-drug-resistant bacterial infections continues to climb. Antimicrobial resistant (AMR) organisms cause ~3 million infections that result in 50,000 deaths in the USA per year, and the World Health Organization estimates that worldwide deaths could climb to 10 million if no action is taken (101). This health crisis was exacerbated by the COVID-19 global pandemic that resulted in at least a 15% increase in AMR infections and deaths in the first year alone (102). Identifying novel small molecule antibiotics to treat AMR infections has proven challenging as no new antibiotic classes were introduced between 1962 and 2000 (103).
Fig 3.
Schematic depicting medical or industrial and environmental applications for chemical genomics using CRISPRi. In this representation, “target” is the gene product or pathway that is directly inhibited by the antimicrobial or toxin. Once identified, targets may be leveraged in further basic and/or applied research.
Antibiotics target essential cellular processes to either kill bacteria or inhibit their replication. However, understanding the function and roles of essential genes in these antibiotic-resistant bacteria has been a major bottleneck for antibiotic drug discovery. Uncharacterized essential genes or pathways are a promising new source of potential antibiotic targets. As approximately one-third of bacterial coding genes have unknown functions, CRISPRi screens could be particularly valuable in defining cellular roles for these genes.
Elucidating mechanisms of antibiotic activity
FDA-approved antibiotics target essential processes in bacteria, but characterizing antibiotic action is difficult because of the limited tools that can be used to study essential gene function (104). CRISPRi can genetically validate suspected targets of new chemical agents or identify biologic targets for chemicals with unknown function (Fig. 3). For instance, we previously identified the direct target of the largely uncharacterized antimicrobial compound MAC-0170636 using a Bacillus subtilis essential gene knockdown library (40). Knockdown of the uppS gene, encoding the undecaprenyl pyrophosphate synthase (UppS), was the most sensitized knockdown strain in the CRISPRi library, and follow-up genetic and biochemical experiments confirmed that UppS was the target (40). CRISPRi has also been used to genetically distinguish the activity profiles of chemicals thought to behave similarly. In a study investigating the activity profiles of ceragenins and cationic antimicrobial peptides in an E. coli knockdown library, it was found that although there was overlapping activity between these compounds, ceragenins also displayed unique activity against phosphate transport genes (105). This level of sensitivity is made possible by analyzing the fitness effect across all gene-chemical conditions. In a similar fashion, other groups have used CRISPRi knockdown libraries to validate the activity of new antibiotics with more than one biologic target, which have very low frequencies of resistance compared to single-target drugs. For example, Martin et al. found that the dual-acting chemical, SCH-79797, had activity against Gram-negative and Gram-positive pathogens and displayed extremely low rates of antibiotic resistance development (106). Thus, a major advantage of creating pooled CRISPRi libraries treated with antibiotics is the ability to simultaneously detect multiple genetic hypersensitivities.
CRISPRi studies that determine the cellular impacts of antibiotics are not limited to model bacteria; these approaches have also been extended to clinically relevant pathogens. In one study utilizing machine learning algorithms to identify compounds that inhibited growth against the hospital-associated pathogen, Acinetobacter baumannii, the authors discovered a chemical that inhibited lipoprotein trafficking to effectively clear a wound infection in a mouse model (107). We recently screened an A. baumannii essential gene knockdown library against therapeutically relevant antibiotics, finding antibiotic-gene interactions that potentiate or mitigate antibiotic efficacy (108). These chemical-genomics approaches give broader insight into potential combinations of antibiotics that could be used to treat infections caused by multi-drug-resistant pathogens.
Of equal importance to its ability to characterize antibiotic function, CRISPRi can also identify genetic vulnerabilities in pathogens that could serve as targets for the next generation of antimicrobials. Species-specific vulnerabilities could be exploited to create more targeted and effective antibiotics for infections caused by different organisms (Fig. 4A). For example, one group utilized Tn-seq to determine gene essentiality in an opportunistic pathogen, Streptococcus pneumoniae. The researchers were then able to limit the scope of their subsequent CRISPRi screen to these putative essential genes with the goal of identifying functions of uncharacterized essentials. They uncovered two genes, renamed tarP and tarQ, responsible for teichoic acid biosynthesis that had substantial growth defects when targeted by their CRISPRi system (109). In a similar fashion, other groups have utilized CRISPRi libraries in Acinetobacter baumannii and Vibrio cholerae to demonstrate the essentiality of a novel cell division protein, AdvA, and targeting lipoprotein transport systems lead to cell death, respectively (110, 111). Uncovering the function and vulnerability of these essential cellular processes paves the way for antibiotic discovery in bacteria that are notoriously difficult to treat (110).
Fig 4.
Examples of gene targets with (A) medical or (B) industrial and environmental relevance which may be identified and/or characterized with CRISPRi. Blunt arrows depict gene knockdown by CRISPRi (red) or chemical-mediated inhibition (black). Pointed arrows depict specific outcomes (black).
Slow-growing pathogenic bacteria with limited treatment options such as Mtb present great challenges for interrogating the function of unknown genes using genetic tools. Mtb takes approximately 1–3 weeks to form colonies on agar plates, and its hydrophobic cell wall makes it difficult to transfer genetic material (112). As a result, genetic manipulations in Mtb are slow and time-consuming, which necessitates the development of high-throughput genetic tools. Attempts to develop an S. pyogenes dCas9-based CRISPRi system in Mtb showed limited promise (113), necessitating a search for alternative CRISPRi systems with greater efficacy. After screening 11 diverse Cas9 variants to increase the effectiveness of CRISPRi, the investigators achieved 20- to 100-fold knockdown of gene expression with little toxicity using dCas9 from Streptococcus thermophilus (51). This optimization unlocked the ability to screen thousands of gene knockdown mutants simultaneously to explore their biology and discover new vulnerable cellular pathways to treat with antibiotics. A few years later, a full-genome CRISPRi library was used for this exact purpose to study the vulnerability and lethality of genes from diverse pathways (2). This paper and others found cell wall and protein folding targeting genes to be highly sensitive to gene repression effects (2, 114). This Mtb CRISPRi library was further challenged with an array of different antibiotics in a chemical genomics screen. These experiments revealed hundreds of genes associated with intrinsic antibiotic resistance that can be used to guide antibiotic treatment of multi-drug-resistant Mtb (99).
Investigating pathogenesis
Bacterial genes responsible for pathogenesis, the ability of an organism to cause disease, are an area of major interest for clinicians and basic scientists. Gaining information about the mechanisms bacteria use to infect and survive in their host provides insight into creating solutions to disrupt these gene products. For instance, the intracellular pathogen, Legionella pneumophila, secretes up to 300 different effector proteins through a Dot/Icm type IV secretion system to infect its host (115–118). One major challenge in understanding the role of these proteins in the context of pathogenesis is the high level of redundancy in their functions; individually knocking out effector proteins rarely provides phenotypes (119). To combat this issue, the Machner lab created a multiplex CRISPRi system with the ability to transcriptionally repress 10 genes at once. In addition, they demonstrated that they could study these knockdowns during pathogenesis in a macrophage model. This multi-gene suppression platform has great potential to discover novel functions of gene products and their role in causing disease (48). Multiplexed gene silencing has also been demonstrated in Enterococcus faecalis. In this case, a nisin-inducible CRISPRi system was used to study the function of genes in biofilm development over time. They found that disrupting pili formation after 24 hours of normal growth with inducible CRISPRi resulted in less biofilm development, showing a role for pili in biofilm maintenance in addition to their established role in attachment (120). Additionally, CRISPRi has been used to study the function of essential and conditionally essential genes in animal models. We previously investigated the knockdown of conditionally essential genes for Pseudomonas aeruginosa in a murine pneumonia infection model (47). In this proof of principle experiment, we used a constitutively active Mobile-CRISPRi system to knock down the expression of exsA, an activator of the type III secretion system required for pathogenesis. Our results confirmed the essential role of exsA in pathogenesis and highlight the benefit of using this highly modular system to probe the function of essential genes in a variety of pathogenic bacteria (47). Perhaps the most comprehensive CRISPRi screen in pathogenic conditions to date is that of Veening and colleagues who screened a library targeting all known operons in S. pneumoniae in a murine pneumonia model. Although strong bottleneck effects precluded genome-scale fitness measurements with S. pneumoniae alone, superinfection with influenza virus bypassed these bottlenecks, enabling discovery of pathogenesis-related genes (75). Overall, these studies highlight the potential for CRISPRi to identify key virulence factors critical for pathogenesis and new biologic targets that can be exploited as future antibiotics.
Remaining challenges in medical applications
Although CRISPRi has been broadly used to explore the function of essential and non-essential genes across diverse, pathogenic bacteria, there are some challenges that remain. As mentioned above, suppressors arising from excessive knockdown (58) or bottleneck effects (121) hinder CRISPRi studies—especially in the context of pathogenesis. Additionally, the efficacy of CRISPRi varies among bacterial species for unknown reasons. This variability can lead to dCas9 toxicity from high levels of expression or insufficient levels of knockdown for genes of interest (51, 58). Efforts have been made to mitigate these issues by substituting dCas9 variants for GC-rich organisms to improve knockdown efficiencies (122).
INDUSTRIAL AND ENVIRONMENTAL APPLICATIONS OF CRISPRi
Evading chemical inhibition
Just as chemical-gene interactions can be used to decipher the function of antimicrobial therapeutics, they can also inform the mechanism of growth impediment by inhibitory compounds encountered during industrial fermentations or environmental exposure (Fig. 3). Illuminating these chemical-gene relationships is an important first step toward engineering robust production strains and understanding the biological impact of bacterial inhibitors in the environment. Studies using transposon libraries have identified genes that mitigate stresses, such as those found during biofuel formation (123, 124), which may be used as genetic engineering targets to increase stress tolerance and enhance yields. However, the role of essential genes in such settings remains obscured. Thus, the use of CRISPRi to interrogate the scope of the full genome, including essential genes, will prove fruitful for rational engineering of bacteria (104). For example, in E. coli, laboratory evolution revealed that a point mutation in the essential gene cydC conferred tolerance to stress from exposure to ionic liquids used to break down plant material into biofuel fermentation feedstock. However, no biological explanation for the cydC phenotype could be gleaned. The authors speculated that CRISPRi could address this gap by enabling investigation of essential genes (125). Indeed, a separate study using CRISPRi partial knockdown in Z. mobilis implicated an essential cell envelope gene in conferring tolerance to the valuable bioenergy precursor isobutanol (126), possibly tying isobutanol inhibition to cell envelope disruption or related pathways.
For poorly understood inhibitory conditions, genome-scale CRISPRi screens can inform engineering strategies to overcome inhibition, even if the mechanism of inhibition is unclear. For example, Synechocystis sp. PCC 6803 can be used for L-lactate production; however, accumulation of L-lactate inhibits growth. Using a whole-genome CRISPRi screen in the presence of L-lactate, Yao et al. uncovered genes that confer L-lactate tolerance. These genes could be modified as engineering targets to improve L-lactate tolerance in production strains, though the mechanism of tolerance remains unknown. Thus, these novel engineering targets would have been difficult to predict without a large-scale phenotypic screen (127).
Decoding microbial biology
CRISPRi has been implemented toward understanding basic biology for a variety of important organisms with applications in bioremediation (122), food and health (128), global nutrient cycling (129), and bioproduction (122, 128, 130–134).
Cell division and cell cycle genes are popular choices for interrogation, especially in newly described CRISPRi systems, due to their essential roles in bacterial propagation and visible phenotypes when perturbed. Such genes have been investigated in Pseudomonas spp. (122, 130), Caulobacter crescentus (131), and Lactobacillus plantarum (128). Metabolic genes are similarly popular because of their key roles in nutrient cycling and industrial bioproduction and because perturbations lead to changes in metabolite profiles that can be directly measured, such as has been done for Hungateiclostridium thermocellum DSM 1313 (132) and Pseudoclostridium thermosuccinogenes (135).
In the important methane cycling and industrial bioconversion microbe, Methylotuvimicrobium buryatense 5GB1C, a Cre/lox-mediated chromosome-integrating CRISPRi system was developed. This was a prominent advance in high-throughput genetics for M. buryatense, given that Tn5 insertion efficiency was insufficient to perform Tn-seq. The authors generated a whole-genome CRISPRi library and used it to identify key genes for methane utilization. They postulated that their Cre/lox-mediated method can also be used for mutant generation by insertion of error-prone PCR products into the chromosome (129). This novel Cre/lox approach for generating sgRNA libraries could prove valuable for other difficult-to-transform species.
Moreover, CRISPRi can identify and characterize novel engineering targets (Fig. 4B). A major advantage of CRISPRi in biotechnology research is enhanced speed of hypothesis generation and testing, since CRISPRi provides a straightforward way to screen many genes for phenotypes before embarking on more time-consuming engineering (e.g., knockouts or deletions). For example, multiple groups have used CRISPRi to test for mutations that increase bioproduction of desired compounds, including whole-genome screening for enhanced L-lactate production by Synechocystis sp. PCC 6803 (127), de-repressing biosynthetic gene clusters to activate antibiotic production by Streptomyces (136), and screening non-coding RNAs for enhanced α-amylase yield in B. subtilis (137). Alternatively, CRISPRi has also been used to downregulate genes involved in unwanted byproduct formation or energy-intensive processes (e.g., flagellar genes) with the goal of shifting resources to desired products (138–142). Another strategy to push nutrients to desired products is to increase the speed of substrate uptake. For example, Kim et al. were able to decrease the lag time of Pseudomonas putida during growth in glycerol by knocking down the glycerol-uptake repressor, glpR. A final approach to increase yields is to facilitate collection of the desired product. For instance, to improve extracellular L-proline yields by Corynebacterium glutamicum, Liu et al. used CRISPRi to screen ~400 putative transporters and identified a novel L-proline exporter which improved yields when overexpressed (143). A similar strategy could be used to find efflux pumps that export growth inhibitors. Overexpression of such efflux pumps could yield robust, stress-tolerant production strains. Conversely to exported products, intracellular products are often collected first by cell sedimentation to harvest biomass. Luo et al. set out to ease downstream processing of valuable intracellular wax esters in Acinetobacter baylyi ADP1 by combining CRISPRi knockdown of the cell division gene ftsZ with targeted gene knockouts to produce enlarged cells. It was hypothesized that enlarged cells may sediment more quickly than their wild-type counterparts, though this strategy may only be viable in cases where the desired intracellular product has equal or greater density to the cells being harvested (144).
Remaining challenges in industrial and environmental applications
While CRISPRi is exceptionally useful for screening large numbers of genes for relevant phenotypes, it is currently a less viable option for engineering final production strains. Maintaining CRISPRi plasmids with antibiotics and inducing CRISPRi with synthetic molecules is prohibitively expensive at the industrial scale. A few advances have made CRISPRi more viable for industrial applications, including integration of the CRISPRi machinery onto the chromosome so that selection with antibiotics is no longer required (60, 126, 129), and designing systems that are inducible with molecules that are already present in the feedstock (e.g., xylose) (131) or are constitutively active. Still, the propensity for genetic suppressors of the CRISPRi system remains a major concern. Even so, there continues to be interest in engineering genetic circuits using CRISPR-assisted technologies to titrate flux and optimize the balance between bioproduction and growth (42, 127, 130, 136, 138, 139, 145–149).
CONCLUSIONS AND OUTLOOK
Initial studies have shown the power of CRISPR-based functional genomics to comprehensively identify and characterize relevant physiological targets for medical and industrial applications. Novel, emerging techniques and applications to new, diverse fields point to a bright future for CRISPR-based gene phenotyping. Below, we highlight some of the most compelling new areas where CRISPRi-seq and related approaches can be applied. CRISPRi-seq has only been minimally utilized to study host-microbe interactions from the bacterial perspective (37, 47, 109, 150); however, the ability to precisely target subsets of genes, perturb essential processes with partial knockdowns, and assess bottlenecks using non-targeting sgRNAs provide an excellent fit for studies of pathogenesis and commensalism. Microbiomes, including those found in the mammalian gut, are highly relevant for human health and disease, but have been underexplored with respect to genome-scale genetic approaches. Proof-of-principle experiments using Tn-seq show the power of functional genomics to identify niche-specific metabolisms (151–154), but genome-scale CRISPRi has yet to be applied to this field of study to our knowledge. Genetic tools for delivering and expressing CRISPRi and related gene perturbation technology will need to be optimized to generate comprehensive libraries in the diverse and understudied bacteria; such improvements are already underway (155–157).
Large-scale genetic interaction studies are ideal for discovering novel connections between pathways that may provide synergistic antibiotic targets or ways of controlling metabolic flux. These interactions manifest as synthetic lethal interactions whereby two independent non-essential gene knockdowns result in loss of viability when combined. Elucidating genetic interactions in this way can characterize gene functions that are otherwise obscured by genetic redundancy. Although relatively few large interaction screens have been carried out in bacterial systems (158, 159), extensive work in the yeast (160) and mammalian (161) fields have highlighted the ability of such screens to uncover pathway connections on a massive scale. Here, CRISPRi advantages in scaling and multiplexing will provide a potent new tool for systematically characterizing gene interactions in bacteria. We and others have already shown the power of multiplex CRISPRi to uncover higher-order genetic interactions [e.g., a triple interaction among cell wall genes (40)] or dissect incredible biological complexity [L. pneumophila effectors (48)], but more open-ended screens have yet to emerge. Based on work in mammalian systems (161, 162), large-scale, multiplexed, CRISPRi screens should be achievable. Combining CRISPRi with classic approaches, such as Tn-seq, is also likely to be fruitful in identifying new interactions. Recent work from the van Opijnen group has shown that CRISPRi partial knockdowns can be combined with Tn-seq to systematically detect interactions between essential and non-essential genes (23). We anticipate many more permutations of these approaches in diverse systems.
Most CRISPRi screens performed to date have focused solely on growth phenotypes, leaving massive areas of phenotypic space untouched. We are particularly excited about the possibility of using large-scale CRISPRi experiments to study gene regulation, as has been demonstrated in yeast and mammalian systems. For instance, yeast geneticists have combined CRISPRi knockdowns with readouts of promoter activity via barcoded reporter RNAs to examine the effects of genome-scale gene perturbations on regulation [CiBER-seq (163) or ReporterSeq (164)]. The bacterial field is beginning to see similar examples of CRISPRi screens coupled with transcriptional reporters to understand gene regulation (165). A recent revolution in bacterial single-cell sequencing (scRNAseq) approaches has increased interest in RNA sequencing of CRISPRi knockdown strains in complex pools [Perturb-seq (166)] to examine regulation at scale. However, this single-cell sequencing approach is cost prohibitive at the genome scale and has been limited by the total number of cells that can be studied and the limited quantity of mRNA extracted from bacterial cells (167–170). Morphological phenotypes of essential gene knockdowns have been characterized in low (171) to medium (40, 172, 173) throughput in bacterial systems, but advances in automated microscopy approaches could increase throughput and allow for measurement of microscopy phenotypes under additional conditions (e.g., antibiotic treatment) (167). Improved techniques for arraying complex strain libraries into wells with individual genotypes will greatly facilitate this process (174, 175).
Other CRISPR technologies with incredible untapped potential have yet to be applied at the genome scale. Targeted, CRISPR-mediated transposition (CAST) systems have been used to programmably disrupt bacterial genes (176–178), but not at the library or genome scale. CAST systems have the potential to address one of the key weaknesses of Tn-seq: that random transposon insertion leads to inadequate coverage of short genes unless library sizes become impractically large. CAST systems could also enable new specificity in gene perturbation, such as domain-specific targeting of gene function. CRISPR activation (CRISPRa) systems have been steadily improving in efficacy and utility (147–149), but have not been applied at the genome scale due to the “hit or miss” nature of upregulating bacterial promoters so far. Such approaches may need further optimization or additional empirical measurement of activity as inputs for machine learning to reach their full potential. However, we are optimistic, as steady improvement has defined the field of CRISPR technology over the last decade—a trend we expect to continue unabated.
ACKNOWLEDGMENTS
A.L.E. was supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1747503. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. A.L.E. and W.J.H. were supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number T32GM135066. W.J.H. was supported by the National Institutes of Health Loan Repayment Program under Award Number LRP0000028821. R.D.W. was supported by the Predoctoral Training Program in Genetics (NIH 5T32GM007133). This material is based upon work supported in part by the U.S. Department of Energy, Office of Science and Office of Biological and Environmental Research, Great Lakes Bioenergy Research Center, under award number DE-SC0018409. This work was also supported by the National Institutes of Health under award number 1R35GM150487-01 to J.M.P.
Biographies

Amy L. Enright holds a BS degree in microbiology from the University of Wisconsin-La Crosse. She received postbaccalaureate training in cell biology and genetics at the University of Wisconsin-Madison under the mentorship of Dr. Judith Kimble. She is currently a doctoral student in microbiology at the University of Wisconsin-Madison in the laboratory of Dr. Jason Peters. She currently performs CRISPRi-based functional genomics for improved industrial phenotypes in the biofuel producing bacterium Zymomonas mobilis.

William J. Heelan graduated from Aurora University with a BS in Health Science and minors in chemistry, physiology, and psychology. He then completed his Doctor of Pharmacy (PharmD) at the University of Wisconsin-Madison School of Pharmacy. He is currently a Pharmaceutical Sciences PhD student in the laboratories of Dr. Jason Peters and Dr. Warren Rose at the University of Wisconsin-Madison, where he studies biofilm formation in the pathogenic bacterium, Pseudomonas aeruginosa. William is currently using an essential gene knockdown library to discover novel connections between core cellular processes and biofilm formation.

Ryan D. Ward, originally from New Mexico, holds a bachelor’s degree in Genetics and Biotechnology, as well as both a bachelor’s and master’s degree in Economics. His diverse professional background includes foodborne risk prediction, endangered species conservation, and drought impact analysis. Currently, Ryan is a PhD student in Genetics at the University of Wisconsin-Madison, under the advisement of Dr. Jason Peters. His research focuses on the differential response to antibiotics in pathogenic bacteria across phylogenetic space. Ryan has developed innovative high-throughput screening techniques and analytical methods, contributing to research papers on pathogens as well as bioenergy production.

Jason M. Peters received BS degrees in Biology and Education from Southeast Missouri State University, a Ph.D. in Genetics from the University of Wisconsin-Madison under the mentorship of Robert Landick, and postdoctoral training in Systems Biology from the University of California San Francisco under the mentorship of Carol Gross. He is currently an Assistant Professor of Pharmaceutical Sciences at the University of Wisconsin-Madison and has been in the field of bacterial functional genomics since 2012.
Contributor Information
Jason M. Peters, Email: jason.peters@wisc.edu.
Kumaran S. Ramamurthi, National Cancer Institute, Bethesda, Maryland, USA
REFERENCES
- 1. Przybyla L, Gilbert LA. 2022. A new era in functional genomics screens. Nat Rev Genet 23:89–103. doi: 10.1038/s41576-021-00409-w [DOI] [PubMed] [Google Scholar]
- 2. Bosch B, DeJesus MA, Poulton NC, Zhang W, Engelhart CA, Zaveri A, Lavalette S, Ruecker N, Trujillo C, Wallach JB, Li S, Ehrt S, Chait BT, Schnappinger D, Rock JM. 2021. Genome-wide gene expression tuning reveals diverse vulnerabilities of M. tuberculosis. Cell 184:4579–4592. doi: 10.1016/j.cell.2021.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown ED, Wright GD. 2016. Antibacterial drug discovery in the resistance era. Nature 529:336–343. doi: 10.1038/nature17042 [DOI] [PubMed] [Google Scholar]
- 4. Cacace E, Kritikos G, Typas A. 2017. Chemical genetics in drug discovery. Curr Opin Syst Biol 4:35–42. doi: 10.1016/j.coisb.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, et al. 1999. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285:901–906. doi: 10.1126/science.285.5429.901 [DOI] [PubMed] [Google Scholar]
- 6. Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006. doi: 10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koo B-M, Kritikos G, Farelli JD, Todor H, Tong K, Kimsey H, Wapinski I, Galardini M, Cabal A, Peters JM, Hachmann A-B, Rudner DZ, Allen KN, Typas A, Gross CA. 2017. Construction and analysis of two genome-scale deletion libraries for Bacillus subtilis. Cell Syst 4:291–305. doi: 10.1016/j.cels.2016.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Porwollik S, Santiviago CA, Cheng P, Long F, Desai P, Fredlund J, Srikumar S, Silva CA, Chu W, Chen X, Canals R, Reynolds MM, Bogomolnaya L, Shields C, Cui P, Guo J, Zheng Y, Endicott-Yazdani T, Yang H-J, Maple A, Ragoza Y, Blondel CJ, Valenzuela C, Andrews-Polymenis H, McClelland M. 2014. Defined single-gene and multi-gene deletion mutant collections in Salmonella enterica sv Typhimurium. PLoS One 9:e99820. doi: 10.1371/journal.pone.0099820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Berardinis V, Vallenet D, Castelli V, Besnard M, Pinet A, Cruaud C, Samair S, Lechaplais C, Gyapay G, Richez C, Durot M, Kreimeyer A, Le Fèvre F, Schächter V, Pezo V, Döring V, Scarpelli C, Médigue C, Cohen GN, Marlière P, Salanoubat M, Weissenbach J. 2008. A complete collection of single-gene deletion mutants of Acinetobacter baylyi ADP1. Mol SYST Biol 4:174. doi: 10.1038/msb.2008.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Leeuwen J, Pons C, Tan G, Wang JZ, Hou J, Weile J, Gebbia M, Liang W, Shuteriqi E, Li Z, Lopes M, Ušaj M, Dos Santos Lopes A, van Lieshout N, Myers CL, Roth FP, Aloy P, Andrews BJ, Boone C. 2020. Systematic analysis of bypass suppression of essential genes. Mol Syst Biol 16:e9828. doi: 10.15252/msb.20209828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sassetti CM, Boyd DH, Rubin EJ. 2001. Comprehensive identification of conditionally essential genes in mycobacteria. Proc Natl Acad Sci U S A 98:12712–12717. doi: 10.1073/pnas.231275498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murry JP, Sassetti CM, Lane JM, Xie Z, Rubin EJ. 2008. Transposon site hybridization in Mycobacterium tuberculosis. Methods Mol Biol 416:45–59. doi: 10.1007/978-1-59745-321-9_4 [DOI] [PubMed] [Google Scholar]
- 13. Goodman AL, McNulty NP, Zhao Y, Leip D, Mitra RD, Lozupone CA, Knight R, Gordon JI. 2009. Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe 6:279–289. doi: 10.1016/j.chom.2009.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Opijnen T, Bodi KL, Camilli A. 2009. Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat Methods 6:767–772. doi: 10.1038/nmeth.1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cain AK, Barquist L, Goodman AL, Paulsen IT, Parkhill J, van Opijnen T. 2020. A decade of advances in transposon-insertion sequencing. Nat Rev Genet 21:526–540. doi: 10.1038/s41576-020-0244-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poulsen BE, Yang R, Clatworthy AE, White T, Osmulski SJ, Li L, Penaranda C, Lander ES, Shoresh N, Hung DT. 2019. Defining the core essential genome of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 116:10072–10080. doi: 10.1073/pnas.1900570116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosconi F, Rudmann E, Li J, Surujon D, Anthony J, Frank M, Jones DS, Rock C, Rosch JW, Johnston CD, van Opijnen T. 2022. A bacterial pan-genome makes gene essentiality strain-dependent and evolvable. Nat Microbiol 7:1580–1592. doi: 10.1038/s41564-022-01208-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coe KA, Lee W, Stone MC, Komazin-Meredith G, Meredith TC, Grad YH, Walker S. 2019. Multi-strain Tn-Seq reveals common daptomycin resistance determinants in Staphylococcus aureus. PLoS Pathog 15:e1007862. doi: 10.1371/journal.ppat.1007862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vitale A, Pessi G, Urfer M, Locher HH, Zerbe K, Obrecht D, Robinson JA, Eberl L. 2020. Identification of genes required for resistance to peptidomimetic antibiotics by transposon sequencing. Front Microbiol 11:1681. doi: 10.3389/fmicb.2020.01681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leshchiner D, Rosconi F, Sundaresh B, Rudmann E, Ramirez LMN, Nishimoto AT, Wood SJ, Jana B, Buján N, Li K, Gao J, Frank M, Reeve SM, Lee RE, Rock CO, Rosch JW, van Opijnen T. 2022. A genome-wide atlas of antibiotic susceptibility targets and pathways to tolerance. Nat Commun 13:3165. doi: 10.1038/s41467-022-30967-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gallagher LA, Shendure J, Manoil C. 2011. Genome-scale identification of resistance functions in Pseudomonas aeruginosa using Tn-seq. mBio 2:e00315-10. doi: 10.1128/mBio.00315-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bailey J, Gallagher L, Barker WT, Hubble VB, Gasper J, Melander C, Manoil C. 2022. Genetic dissection of antibiotic adjuvant activity. mBio 13:e0308421. doi: 10.1128/mbio.03084-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jana B, Liu X, Dénéréaz J, Park H, Leshchiner D, Liu B, Gallay C, Veening J-W, van Opijnen T. 2023. CRISPRi-TnSeq: a genome-wide high-throughput tool for bacterial essential-nonessential genetic interaction mapping. bioRxiv:2023.05.31.543074. doi: 10.1101/2023.05.31.543074 [DOI]
- 24. Truong TT, Vettiger A, Bernhardt TG. 2020. Cell division is antagonized by the activity of peptidoglycan endopeptidases that promote cell elongation. Mol Microbiol 114:966–978. doi: 10.1111/mmi.14587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DeJesus MA, Gerrick ER, Xu W, Park SW, Long JE, Boutte CC, Rubin EJ, Schnappinger D, Ehrt S, Fortune SM, Sassetti CM, Ioerger TR. 2017. Comprehensive essentiality analysis of the Mycobacterium tuberculosis genome via saturating transposon mutagenesis. mBio 8:e02133-16. doi: 10.1128/mBio.02133-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Green B, Bouchier C, Fairhead C, Craig NL, Cormack BP. 2012. Insertion site preference of Mu, Tn5, and Tn7 transposons. Mob DNA 3:3. doi: 10.1186/1759-8753-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson EO, LaVerriere E, Office E, Stanley M, Meyer E, Kawate T, Gomez JE, Audette RE, Bandyopadhyay N, Betancourt N, et al. 2019. Large-scale chemical–genetics yields new M. tuberculosis inhibitor classes. Nature 571:72–78. doi: 10.1038/s41586-019-1315-z [DOI] [PubMed] [Google Scholar]
- 28. Doudna JA, Charpentier E. 2014. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346:1258096. doi: 10.1126/science.1258096 [DOI] [PubMed] [Google Scholar]
- 29. van der Oost J, Westra ER, Jackson RN, Wiedenheft B. 2014. Unravelling the structural and mechanistic basis of CRISPR-Cas systems. Nat Rev Microbiol 12:479–492. doi: 10.1038/nrmicro3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shalem O, Sanjana NE, Zhang F. 2015. High-throughput functional genomics using CRISPR-Cas9. Nat Rev Genet 16:299–311. doi: 10.1038/nrg3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barrangou R. 2015. The roles of CRISPR–Cas systems in adaptive immunity and beyond. Curr Opin Immunol 32:36–41. doi: 10.1016/j.coi.2014.12.008 [DOI] [PubMed] [Google Scholar]
- 32. Hille F, Richter H, Wong SP, Bratovič M, Ressel S, Charpentier E. 2018. The biology of CRISPR-Cas: backward and forward. Cell 172:1239–1259. doi: 10.1016/j.cell.2017.11.032 [DOI] [PubMed] [Google Scholar]
- 33. Sharda M, Badrinarayanan A, Seshasayee ASN. 2020. Evolutionary and comparative analysis of bacterial nonhomologous end joining repair. Genome Biol Evol 12:2450–2466. doi: 10.1093/gbe/evaa223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. 2013. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152:1173–1183. doi: 10.1016/j.cell.2013.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Todor H, Silvis MR, Osadnik H, Gross CA. 2021. Bacterial CRISPR screens for gene function. Curr Opin Microbiol 59:102–109. doi: 10.1016/j.mib.2020.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rousset F, Bikard D. 2020. CRISPR screens in the era of microbiomes. Curr Opin Microbiol 57:70–77. doi: 10.1016/j.mib.2020.07.009 [DOI] [PubMed] [Google Scholar]
- 37. de Bakker V, Liu X, Bravo AM, Veening J-W. 2022. CRISPRi-seq for genome-wide fitness quantification in bacteria. Nat Protoc 17:252–281. doi: 10.1038/s41596-021-00639-6 [DOI] [PubMed] [Google Scholar]
- 38. Wong AI, Rock JM. 2021. CRISPR interference (CRISPRi) for targeted gene silencing in mycobacteria. Methods Mol Biol 2314:343–364. doi: 10.1007/978-1-0716-1460-0_16 [DOI] [PubMed] [Google Scholar]
- 39. Liu Z, Dong H, Cui Y, Cong L, Zhang D. 2020. Application of different types of CRISPR/Cas-based systems in bacteria. Microb Cell Fact 19:172. doi: 10.1186/s12934-020-01431-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peters JM, Colavin A, Shi H, Czarny TL, Larson MH, Wong S, Hawkins JS, Lu CHS, Koo B-M, Marta E, Shiver AL, Whitehead EH, Weissman JS, Brown ED, Qi LS, Huang KC, Gross CA. 2016. A comprehensive, CRISPR-based functional analysis of essential genes in bacteria. Cell 165:1493–1506. doi: 10.1016/j.cell.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, Lim WA, Weissman JS, Qi LS. 2013. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotesotes. Cell 154:442–451. doi: 10.1016/j.cell.2013.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bikard D, Jiang W, Samai P, Hochschild A, Zhang F, Marraffini LA. 2013. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res 41:7429–7437. doi: 10.1093/nar/gkt520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Banta AB, Ward RD, Tran JS, Bacon EE, Peters JM. 2020. Programmable gene knockdown in diverse bacteria using mobile-CRISPRi. Curr Protoc Microbiol 59:e130. doi: 10.1002/cpmc.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vigouroux A, Oldewurtel E, Cui L, Bikard D, van Teeffelen S. 2018. Tuning dCas9’s ability to block transcription enables robust, noiseless knockdown of bacterial genes. Mol Syst Biol 14:e7899. doi: 10.15252/msb.20177899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hawkins JS, Silvis MR, Koo B-M, Peters JM, Osadnik H, Jost M, Hearne CC, Weissman JS, Todor H, Gross CA. 2020. Mismatch-CRISPRi reveals the co-varying expression-fitness relationships of essential genes in Escherichia coli and Bacillus subtilis. Cell Syst 11:523–535. doi: 10.1016/j.cels.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mathis AD, Otto RM, Reynolds KA. 2021. A simplified strategy for titrating gene expression reveals new relationships between genotype, environment, and bacterial growth. Nucleic Acids Res 49:e6. doi: 10.1093/nar/gkaa1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Qu J, Prasad NK, Yu MA, Chen S, Lyden A, Herrera N, Silvis MR, Crawford E, Looney MR, Peters JM, Rosenberg OS. 2019. Modulating pathogenesis with Mobile-CRISPRi. J Bacteriol 201:e00304-19. doi: 10.1128/JB.00304-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ellis NA, Kim B, Tung J, Machner MP. 2021. A multiplex CRISPR interference tool for virulence gene interrogation in Legionella pneumophila. Commun Biol 4:157. doi: 10.1038/s42003-021-01672-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cui L, Vigouroux A, Rousset F, Varet H, Khanna V, Bikard D. 2018. A CRISPRi screen in E. coli reveals sequence-specific toxicity of dCas9. Nat Commun 9:1912. doi: 10.1038/s41467-018-04209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Enright AL, Banta AB, Ward RD, Rivera Vazquez J, Felczak MM, Wolfe MB, TerAvest MA, Amador-Noguez D, Peters JM. 2023. The genetics of aerotolerant growth in an alphaproteobacterium with a naturally reduced genome. mBio 14:e0148723. doi: 10.1128/mbio.01487-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rock JM, Hopkins FF, Chavez A, Diallo M, Chase MR, Gerrick ER, Pritchard JR, Church GM, Rubin EJ, Sassetti CM, Schnappinger D, Fortune SM. 2017. Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nat Microbiol 2:16274. doi: 10.1038/nmicrobiol.2016.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zheng Y, Han J, Wang B, Hu X, Li R, Shen W, Ma X, Ma L, Yi L, Yang S, Peng W. 2019. Characterization and repurposing of the endogenous Type I-F CRISPR–Cas system of Zymomonas mobilis for genome engineering. Nucleic Acids Res 47:11461–11475. doi: 10.1093/nar/gkz940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shields RC, Walker AR, Maricic N, Chakraborty B, Underhill SAM, Burne RA. 2020. Repurposing the Streptococcus mutans CRISPR-Cas9 system to understand essential gene function. PLoS Pathog 16:e1008344. doi: 10.1371/journal.ppat.1008344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cho S, Choe D, Lee E, Kim SC, Palsson B, Cho B-K. 2018. High-level dCas9 expression induces abnormal cell morphology in Escherichia coli. ACS Synth Biol 7:1085–1094. doi: 10.1021/acssynbio.7b00462 [DOI] [PubMed] [Google Scholar]
- 55. Lee YJ, Hoynes-O’Connor A, Leong MC, Moon TS. 2016. Programmable control of bacterial gene expression with the combined CRISPR and antisense RNA system. Nucleic Acids Res 44:2462–2473. doi: 10.1093/nar/gkw056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang S, Voigt CA. 2018. Engineered dCas9 with reduced toxicity in bacteria: implications for genetic circuit design. Nucleic Acids Res 46:11115–11125. doi: 10.1093/nar/gky884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rostain W, Grebert T, Vyhovskyi D, Pizarro PT, Tshinsele-Van Bellingen G, Cui L, Bikard D. 2023. Cas9 off-target binding to the promoter of bacterial genes leads to silencing and toxicity. Nucleic Acids Res 51:3485–3496. doi: 10.1093/nar/gkad170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhao H, Sun Y, Peters JM, Gross CA, Garner EC, Helmann JD. 2016. Depletion of undecaprenyl pyrophosphate phosphatases disrupts cell envelope biogenesis in Bacillus subtilis. J Bacteriol 198:2925–2935. doi: 10.1128/JB.00507-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Depardieu F, Bikard D. 2020. Gene silencing with CRISPRi in bacteria and optimization of dCas9 expression levels. Methods 172:61–75. doi: 10.1016/j.ymeth.2019.07.024 [DOI] [PubMed] [Google Scholar]
- 60. Peters JM, Koo B-M, Patino R, Heussler GE, Hearne CC, Qu J, Inclan YF, Hawkins JS, Lu CHS, Silvis MR, Harden MM, Osadnik H, Peters JE, Engel JN, Dutton RJ, Grossman AD, Gross CA, Rosenberg OS. 2019. Enabling genetic analysis of diverse bacteria with Mobile-CRISPRi. Nat Microbiol 4:244–250. doi: 10.1038/s41564-018-0327-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Figueroa-Cuilan W, Daniel JJ, Howell M, Sulaiman A, Brown PJB. 2016. Mini-Tn7 insertion in an artificial AttTn7 site enables depletion of the essential master regulator CtrA in the phytopathogen Agrobacterium tumefaciens. Appl Environ Microbiol 82:5015–5025. doi: 10.1128/AEM.01392-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang T, Guan C, Guo J, Liu B, Wu Y, Xie Z, Zhang C, Xing X-H. 2018. Pooled CRISPR interference screening enables genome-scale functional genomics study in bacteria with superior performance. Nat Commun 9:2475. doi: 10.1038/s41467-018-04899-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang R, Xu W, Shao S, Wang Q. 2021. Gene silencing through CRISPR interference in bacteria: current advances and future prospects. Front Microbiol 12:635227. doi: 10.3389/fmicb.2021.635227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wiktor J, Lesterlin C, Sherratt DJ, Dekker C. 2016. CRISPR-mediated control of the bacterial initiation of replication. Nucleic Acids Res 44:3801–3810. doi: 10.1093/nar/gkw214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Javaid N, Choi S. 2021. CRISPR/Cas system and factors affecting its precision and efficiency. Front Cell Dev Biol 9:761709. doi: 10.3389/fcell.2021.761709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Akinci E, Hamilton MC, Khowpinitchai B, Sherwood RI. 2021. Using CRISPR to understand and manipulate gene regulation. Development 148:dev182667. doi: 10.1242/dev.182667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fonseca MM, Harris DJ, Posada D. 2014. Origin and length distribution of unidirectional prokaryotic overlapping genes. G3 (Bethesda) 4:19–27. doi: 10.1534/g3.113.005652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Spoto M, Riera Puma JP, Fleming E, Guan C, Ondouah Nzutchi Y, Kim D, Oh J. 2022. Large-scale CRISPRi and transcriptomics of Staphylococcus epidermidis identify genetic factors implicated in lifestyle versatility. mBio 13:e0263222. doi: 10.1128/mbio.02632-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Treangen TJ, Salzberg SL. 2011. Repetitive DNA and next-generation sequencing: computational challenges and solutions. Nat Rev Genet 13:36–46. doi: 10.1038/nrg3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Larson MH, Gilbert LA, Wang X, Lim WA, Weissman JS, Qi LS. 2013. CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat Protoc 8:2180–2196. doi: 10.1038/nprot.2013.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jost M, Santos DA, Saunders RA, Horlbeck MA, Hawkins JS, Scaria SM, Norman TM, Hussmann JA, Liem CR, Gross CA, Weissman JS. 2020. Titrating gene expression using libraries of systematically attenuated CRISPR guide RNAs. Nat Biotechnol 38:355–364. doi: 10.1038/s41587-019-0387-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Medley JC, Hebbar S, Sydzyik JT, Zinovyeva AY. 2022. Single nucleotide substitutions effectively block Cas9 and allow for scarless genome editing in Caenorhabditis elegans. Genetics 220:iyab199. doi: 10.1093/genetics/iyab199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. van Gestel J, Hawkins JS, Todor H, Gross CA. 2021. Computational pipeline for designing guide RNAs for mismatch-CRISPRi. STAR Protoc 2:100521. doi: 10.1016/j.xpro.2021.100521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ward RD, Tran JS, Banta AB, Bacon EE, Rose WE, Peters JM. 2023. Essential gene knockdowns reveal genetic vulnerabilities and antibiotic sensitivities in Acinetobacter baumannii. bioRxiv mBio 15:e02051–23. doi: 10.1101/2023.08.02.551708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu X, Kimmey JM, Matarazzo L, de Bakker V, Van Maele L, Sirard J-C, Nizet V, Veening J-W. 2021. Exploration of bacterial bottlenecks and Streptococcus pneumoniae pathogenesis by CRISPRi-seq. Cell Host Microbe 29:107–120. doi: 10.1016/j.chom.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Abel S, Abel zur Wiesch P, Chang H-H, Davis BM, Lipsitch M, Waldor MK. 2015. Sequence tag-based analysis of microbial population dynamics. Nat Methods 12:223–226. doi: 10.1038/nmeth.3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jiang W, Oikonomou P, Tavazoie S. 2020. Comprehensive genome-wide perturbations via CRISPR adaptation reveal complex genetics of antibiotic sensitivity. Cell 180:1002–1017. doi: 10.1016/j.cell.2020.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Byun G, Yang J, Seo SW. 2023. CRISPRi-mediated tunable control of gene expression level with engineered single-guide RNA in Escherichia coli. Nucleic Acids Res 51:4650–4659. doi: 10.1093/nar/gkad234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Otto RM, Turska-Nowak A, Brown PM, Reynolds KA. 2023. A continuous epistasis model for predicting growth rate given combinatorial variation in gene expression and environment. Syst Biol. doi: 10.1101/2022.08.19.504444 [DOI] [PMC free article] [PubMed]
- 80. Bravo AM, Typas A, Veening J-W. 2022. 2FAST2Q: a general-purpose sequence search and counting program for FASTQ files. PeerJ 10:e14041. doi: 10.7717/peerj.14041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liao Y, Smyth GK, Shi W. 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30:923–930. doi: 10.1093/bioinformatics/btt656 [DOI] [PubMed] [Google Scholar]
- 82. Li W, Xu H, Xiao T, Cong L, Love MI, Zhang F, Irizarry RA, Liu JS, Brown M, Liu XS. 2014. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol 15:554. doi: 10.1186/s13059-014-0554-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Stemmer M, Thumberger T, Del Sol Keyer M, Wittbrodt J, Mateo JL. 2015. CCTop: an intuitive, flexible and reliable CRISPR/Cas9 target prediction tool. PLoS One 10:e0124633. doi: 10.1371/journal.pone.0124633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Concordet J-P, Haeussler M. 2018. CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res 46:W242–W245. doi: 10.1093/nar/gky354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Montague TG, Cruz JM, Gagnon JA, Church GM, Valen E. 2014. CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res 42:W401–W407. doi: 10.1093/nar/gku410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Labun K, Montague TG, Gagnon JA, Thyme SB, Valen E. 2016. CHOPCHOP v2: a web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res 44:W272–W276. doi: 10.1093/nar/gkw398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bae S, Park J, Kim J-S. 2014. Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30:1473–1475. doi: 10.1093/bioinformatics/btu048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liu H, Wei Z, Dominguez A, Li Y, Wang X, Qi LS. 2015. CRISPR-ERA: a comprehensive design tool for CRISPR-mediated gene editing, repression and activation. Bioinformatics 31:3676–3678. doi: 10.1093/bioinformatics/btv423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Winter J, Schwering M, Pelz O, Rauscher B, Zhan T, Heigwer F, Boutros M. 2017. CRISPRAnalyzeR: interactive analysis, annotation and documentation of pooled CRISPR screens. Bioinformatics. doi: 10.1101/109967 [DOI]
- 90. Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. doi: 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. 2015. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43:e47–e47. doi: 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Langmead B, Trapnell C, Pop M, Salzberg SL. 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10:R25. doi: 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 95. Emms DM, Kelly S. 2019. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol 20:238. doi: 10.1186/s13059-019-1832-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Szklarczyk D, Kirsch R, Koutrouli M, Nastou K, Mehryary F, Hachilif R, Gable AL, Fang T, Doncheva NT, Pyysalo S, Bork P, Jensen LJ, von Mering C. 2023. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res 51:D638–D646. doi: 10.1093/nar/gkac1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bodapati S, Daley TP, Lin X, Zou J, Qi LS. 2020. A benchmark of algorithms for the analysis of pooled CRISPR screens. Genome Biol 21:62. doi: 10.1186/s13059-020-01972-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Daley TP, Lin Z, Lin X, Liu Y, Wong WH, Qi LS. 2018. CRISPhieRmix: a hierarchical mixture model for CRISPR pooled screens. Genome Biol 19:159. doi: 10.1186/s13059-018-1538-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Li S, Poulton NC, Chang JS, Azadian ZA, DeJesus MA, Ruecker N, Zimmerman MD, Eckartt KA, Bosch B, Engelhart CA, Sullivan DF, Gengenbacher M, Dartois VA, Schnappinger D, Rock JM. 2022. CRISPRi chemical genetics and comparative genomics identify genes mediating drug potency in Mycobacterium tuberculosis. Nat Microbiol 7:766–779. doi: 10.1038/s41564-022-01130-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Brain P, Cousens R. 1989. An equation to describe dose responses where there is stimulation of growth at low doses. Weed Research 29:93–96. doi: 10.1111/j.1365-3180.1989.tb00845.x [DOI] [Google Scholar]
- 101. CDC . 2019. Antibiotic resistance threatens everyone. Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/drugresistance/index.html. Retrieved 21 Feb 2020. [Google Scholar]
- 102. COVID-19: U.S. impact on antimicrobial resistance, special report 2022. 2022. National Center for Emerging and Zoonotic Infectious Diseases
- 103. Silver LL. 2011. Challenges of antibacterial discovery. Clin Microbiol Rev 24:71–109. doi: 10.1128/CMR.00030-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Juhas M, Eberl L, Church GM. 2012. Essential genes as antimicrobial targets and cornerstones of synthetic biology. Trends Biotechnol 30:601–607. doi: 10.1016/j.tibtech.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 105. Mitchell G, Silvis MR, Talkington KC, Budzik JM, Dodd CE, Paluba JM, Oki EA, Trotta KL, Licht DJ, Jimenez-Morales D, Chou S, Savage PB, Gross CA, Marletta MA, Cox JS. 2022. Ceragenins and antimicrobial peptides kill bacteria through distinct mechanisms. mBio 13:e0272621. doi: 10.1128/mbio.02726-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Martin JK, Sheehan JP, Bratton BP, Moore GM, Mateus A, Li SH-J, Kim H, Rabinowitz JD, Typas A, Savitski MM, Wilson MZ, Gitai Z. 2020. A dual-mechanism antibiotic kills gram-negative bacteria and avoids drug resistance. Cell 181:1518–1532. doi: 10.1016/j.cell.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Liu G, Catacutan DB, Rathod K, Swanson K, Jin W, Mohammed JC, Chiappino-Pepe A, Syed SA, Fragis M, Rachwalski K, Magolan J, Surette MG, Coombes BK, Jaakkola T, Barzilay R, Collins JJ, Stokes JM. 2023. Deep learning-guided discovery of an antibiotic targeting Acinetobacter baumannii. Nat Chem Biol 19:1342–1350. doi: 10.1038/s41589-023-01349-8 [DOI] [PubMed] [Google Scholar]
- 108. Ward RD, Tran JS, Banta AB, Bacon EE, Rose WE, Peters JM. 2023. Essential gene knockdowns reveal genetic vulnerabilities and antibiotic sensitivities in Acinetobacter baumannii. bioRxiv:2023.08.02.551708. doi: 10.1101/2023.08.02.551708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Liu X, Gallay C, Kjos M, Domenech A, Slager J, van Kessel SP, Knoops K, Sorg RA, Zhang J-R, Veening J-W. 2017. High‐throughput CRISPRi phenotyping identifies new essential genes in Streptococcus pneumoniae. Mol Syst Biol 13:931. doi: 10.15252/msb.20167449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bai J, Dai Y, Farinha A, Tang AY, Syal S, Vargas-Cuebas G, van Opijnen T, Isberg RR, Geisinger E. 2021. Essential gene analysis in Acinetobacter baumannii by high-density transposon mutagenesis and CRISPR interference. J Bacteriol 203:e0056520. doi: 10.1128/JB.00565-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Caro F, Place NM, Mekalanos JJ. 2019. Analysis of lipoprotein transport depletion in Vibrio cholerae using CRISPRi. Proc Natl Acad Sci U S A 116:17013–17022. doi: 10.1073/pnas.1906158116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Larsen MH, Biermann K, Tandberg S, Hsu T, Jacobs, WR. 2007. Genetic manipulation of Mycobacterium tuberculosis. CP Microbiology 6. doi: 10.1002/9780471729259.mc10a02s6 [DOI] [PubMed] [Google Scholar]
- 113. Choudhary E, Thakur P, Pareek M, Agarwal N. 2015. Gene silencing by CRISPR interference in mycobacteria. Nat Commun 6:6267. doi: 10.1038/ncomms7267 [DOI] [PubMed] [Google Scholar]
- 114. McNeil MB, Keighley LM, Cook JR, Cheung C-Y, Cook GM. 2021. CRISPR interference identifies vulnerable cellular pathways with bactericidal phenotypes in Mycobacterium tuberculosis. Mol Microbiol 116:1033–1043. doi: 10.1111/mmi.14790 [DOI] [PubMed] [Google Scholar]
- 115. Luo Z-Q, Isberg RR. 2004. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc Natl Acad Sci U S A 101:841–846. doi: 10.1073/pnas.0304916101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Burstein D, Zusman T, Degtyar E, Viner R, Segal G, Pupko T. 2009. Genome-scale identification of Legionella pneumophila effectors using a machine learning approach. PLoS Pathog 5:e1000508. doi: 10.1371/journal.ppat.1000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zhu W, Banga S, Tan Y, Zheng C, Stephenson R, Gately J, Luo Z-Q. 2011. Comprehensive identification of protein substrates of the Dot/Icm type IV transporter of Legionella pneumophila. PLoS One 6:e17638. doi: 10.1371/journal.pone.0017638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lifshitz Z, Burstein D, Peeri M, Zusman T, Schwartz K, Shuman HA, Pupko T, Segal G. 2013. Computational modeling and experimental validation of the Legionella and Coxiella virulence-related type-IVB secretion signal. Proc Natl Acad Sci U S A 110:E707–15. doi: 10.1073/pnas.1215278110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wexler M, Zusman T, Linsky M, Lifshitz Z, Segal G. 2022. The Legionella genus core effectors display functional conservation among orthologs by themselves or combined with an accessory protein. Curr Res Microb Sci 3:100105. doi: 10.1016/j.crmicr.2022.100105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Afonina I, Ong J, Chua J, Lu T, Kline KA. 2020. Multiplex CRISPRi system enables the study of stage-specific biofilm genetic requirements in Enterococcus faecalis. mBio 11:e01101-20. doi: 10.1128/mBio.01101-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Rousset F, Cui L, Siouve E, Becavin C, Depardieu F, Bikard D. 2018. Genome-wide CRISPR-dCas9 screens in E. coli identify essential genes and phage host factors. PLoS Genet 14:e1007749. doi: 10.1371/journal.pgen.1007749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tan SZ, Reisch CR, Prather KLJ. 2018. A robust CRISPR interference gene repression system in Pseudomonas. J Bacteriol 200:e00575-17. doi: 10.1128/JB.00575-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Skerker JM, Leon D, Price MN, Mar JS, Tarjan DR, Wetmore KM, Deutschbauer AM, Baumohl JK, Bauer S, Ibáñez AB, Mitchell VD, Wu CH, Hu P, Hazen T, Arkin AP. 2013. Dissecting a complex chemical stress: chemogenomic profiling of plant hydrolysates. Mol Syst Biol 9:674. doi: 10.1038/msb.2013.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Borchert AJ, Bleem A, Beckham GT. 2023. RB-TnSeq identifies genetic targets for improved tolerance of Pseudomonas putida towards compounds relevant to lignin conversion. Metab Eng 77:208–218. doi: 10.1016/j.ymben.2023.04.007 [DOI] [PubMed] [Google Scholar]
- 125. Eng T, Demling P, Herbert RA, Chen Y, Benites V, Martin J, Lipzen A, Baidoo EEK, Blank LM, Petzold CJ, Mukhopadhyay A. 2018. Restoration of biofuel production levels and increased tolerance under ionic liquid stress is enabled by a mutation in the essential Escherichia coli gene cydC. Microb Cell Fact 17:159. doi: 10.1186/s12934-018-1006-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Banta AB, Enright AL, Siletti C, Peters JM. 2020. A high-efficacy CRISPR interference system for gene function discovery in Zymomonas mobilis. Appl Environ Microbiol 86:e01621-20. doi: 10.1128/AEM.01621-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yao L, Shabestary K, Björk SM, Asplund-Samuelsson J, Joensson HN, Jahn M, Hudson EP. 2020. Pooled CRISPRi screening of the cyanobacterium Synechocystis sp PCC 6803 for enhanced industrial phenotypes. Nat Commun 11:1666. doi: 10.1038/s41467-020-15491-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Myrbråten IS, Wiull K, Salehian Z, Håvarstein LS, Straume D, Mathiesen G, Kjos M. 2019. CRISPR interference for rapid knockdown of essential cell cycle genes in Lactobacillus plantarum. mSphere 4:e00007-19. doi: 10.1128/mSphere.00007-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cheng M, Pei D, He L, Fei Q, Yan X. 2023. Cre/lox-mediated CRISPRi library reveals core genome of a type I methanotroph Methylotuvimicrobium buryatense 5GB1C. Appl Environ Microbiol 89:e0188322. doi: 10.1128/aem.01883-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Batianis C, Kozaeva E, Damalas SG, Martín-Pascual M, Volke DC, Nikel PI, Martins Dos Santos VAP. 2020. An expanded CRISPRi toolbox for tunable control of gene expression in Pseudomonas putida. Microb Biotechnol 13:368–385. doi: 10.1111/1751-7915.13533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Guzzo M, Castro LK, Reisch CR, Guo MS, Laub MT. 2020. A CRISPR interference system for efficient and rapid gene knockdown in Caulobacter crescentus. mBio 11:e02415-19. doi: 10.1128/mBio.02415-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ganguly J, Martin-Pascual M, van Kranenburg R. 2020. CRISPR interference (CRISPRi) as transcriptional repression tool for Hungateiclostridium thermocellum DSM 1313. Microb Biotechnol 13:339–349. doi: 10.1111/1751-7915.13516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Göttl VL, Schmitt I, Braun K, Peters-Wendisch P, Wendisch VF, Henke NA. 2021. CRISPRi-library-guided target identification for engineering carotenoid production by Corynebacterium glutamicum. Microorganisms 9:670. doi: 10.3390/microorganisms9040670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Yin L, Xi D, Shen Y, Ding N, Shao Q, Qian Y, Fang Y. 2024. Rewiring metabolic flux in Corynebacterium glutamicum using a CRISPR/dCpf1-based bifunctional regulation system. J Agric Food Chem 72:3077–3087. doi: 10.1021/acs.jafc.3c08529 [DOI] [PubMed] [Google Scholar]
- 135. Ganguly J, Martin-Pascual M, Montiel González D, Bulut A, Vermeulen B, Tjalma I, Vidaki A, van Kranenburg R. 2022. Breaking the restriction barriers and applying CRISPRi as a gene silencing tool in Pseudoclostridium thermosuccinogenes. Microorganisms 10:698. doi: 10.3390/microorganisms10040698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ameruoso A, Villegas Kcam MC, Cohen KP, Chappell J. 2022. Activating natural product synthesis using CRISPR interference and activation systems in Streptomyces. Nucleic Acids Res 50:7751–7760. doi: 10.1093/nar/gkac556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Geissler AS, Fehler AO, Poulsen LD, González-Tortuero E, Kallehauge TB, Alkan F, Anthon C, Seemann SE, Rasmussen MD, Breüner A, Hjort C, Vinther J, Gorodkin J. 2023. CRISPRi screen for enhancing heterologous α-amylase yield in Bacillus subtilis. J Ind Microbiol Biotechnol 50:kuac028. doi: 10.1093/jimb/kuac028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Huang C-H, Shen CR, Li H, Sung L-Y, Wu M-Y, Hu Y-C. 2016. CRISPR interference (CRISPRi) for gene regulation and succinate production in cyanobacterium S. elongatus PCC 7942. Microb Cell Fact 15:196. doi: 10.1186/s12934-016-0595-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gordon GC, Korosh TC, Cameron JC, Markley AL, Begemann MB, Pfleger BF. 2016. CRISPR interference as a titratable, trans-acting regulatory tool for metabolic engineering in the cyanobacterium Synechococcus sp. strain PCC 7002. Metab Eng 38:170–179. doi: 10.1016/j.ymben.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wang C, Cao Y, Wang Y, Sun L, Song H. 2019. Enhancing surfactin production by using systematic CRISPRi repression to screen amino acid biosynthesis genes in Bacillus subtilis. Microb Cell Fact 18:90. doi: 10.1186/s12934-019-1139-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Fehler AO, Kallehauge TB, Geissler AS, González-Tortuero E, Seemann SE, Gorodkin J, Vinther J. 2022. Flagella disruption in Bacillus subtilis increases amylase production yield. Microb Cell Fact 21:131. doi: 10.1186/s12934-022-01861-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Fackler N, Heffernan J, Juminaga A, Doser D, Nagaraju S, Gonzalez-Garcia RA, Simpson SD, Marcellin E, Köpke M. 2021. Transcriptional control of Clostridium autoethanogenum using CRISPRi. Synth Biol (Oxf) 6:ysab008. doi: 10.1093/synbio/ysab008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Liu J, Liu M, Shi T, Sun G, Gao N, Zhao X, Guo X, Ni X, Yuan Q, Feng J, Liu Z, Guo Y, Chen J, Wang Y, Zheng P, Sun J. 2022. CRISPR-assisted rational flux-tuning and arrayed CRISPRi screening of an L-proline exporter for L-proline hyperproduction. Nat Commun 13:891. doi: 10.1038/s41467-022-28501-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Luo J, Efimova E, Volke DC, Santala V, Santala S. 2022. Engineering cell morphology by CRISPR interference in Acinetobacter baylyi ADP1. Microb Biotechnol 15:2800–2818. doi: 10.1111/1751-7915.14133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Peng R, Wang Y, Feng W-W, Yue X-J, Chen J-H, Hu X-Z, Li Z-F, Sheng D-H, Zhang Y-M, Li Y-Z. 2018. CRISPR/dCas9-mediated transcriptional improvement of the biosynthetic gene cluster for the epothilone production in Myxococcus xanthus. Microb Cell Fact 17:15. doi: 10.1186/s12934-018-0867-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Villegas Kcam MC, Tsong AJ, Chappell J. 2022. Uncovering the distinct properties of a bacterial type I-E CRISPR activation system. ACS Synth Biol 11:1000–1003. doi: 10.1021/acssynbio.1c00496 [DOI] [PubMed] [Google Scholar]
- 147. Dong C, Fontana J, Patel A, Carothers JM, Zalatan JG. 2018. Synthetic CRISPR-Cas gene activators for transcriptional reprogramming in bacteria. Nat Commun 9:2489. doi: 10.1038/s41467-018-04901-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kiattisewee C, Karanjia AV, Legut M, Daniloski Z, Koplik SE, Nelson J, Kleinstiver BP, Sanjana NE, Carothers JM, Zalatan JG. 2022. Expanding the scope of bacterial CRISPR activation with PAM-flexible dCas9 variants. ACS Synth Biol 11:4103–4112. doi: 10.1021/acssynbio.2c00405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ho H-I, Fang JR, Cheung J, Wang HH. 2020. Programmable CRISPR-Cas transcriptional activation in bacteria. Mol Syst Biol 16:e9427. doi: 10.15252/msb.20199427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Lai Y, Babunovic GH, Cui L, Dedon PC, Doench JG, Fortune SM, Lu TK. 2020. Illuminating host-mycobacterial interactions with genome-wide CRISPR knockout and CRISPRi screens. Cell Syst 11:239–251. doi: 10.1016/j.cels.2020.08.010 [DOI] [PubMed] [Google Scholar]
- 151. Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. 2019. Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science 363:eaat9931. doi: 10.1126/science.aat9931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Liu H, Shiver AL, Price MN, Carlson HK, Trotter VV, Chen Y, Escalante V, Ray J, Hern KE, Petzold CJ, Turnbaugh PJ, Huang KC, Arkin AP, Deutschbauer AM. 2021. Functional genetics of human gut commensal Bacteroides thetaiotaomicron reveals metabolic requirements for growth across environments. Cell Rep 34:108789. doi: 10.1016/j.celrep.2021.108789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wu M, McNulty NP, Rodionov DA, Khoroshkin MS, Griffin NW, Cheng J, Latreille P, Kerstetter RA, Terrapon N, Henrissat B, Osterman AL, Gordon JI. 2015. Genetic determinants of in vivo fitness and diet responsiveness in multiple human gut Bacteroides. Science 350:aac5992. doi: 10.1126/science.aac5992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Shiver AL, Sun J, Culver R, Violette A, Wynter C, Nieckarz M, Mattiello SP, Sekhon PK, Friess L, Carlson HK, et al. 2023. A mutant fitness compendium in Bifidobacteria reveals molecular determinants of colonization and host-microbe interactions. bioRxiv:2023.08.29.555234. doi: 10.1101/2023.08.29.555234 [DOI] [Google Scholar]
- 155. Rubin BE, Diamond S, Cress BF, Crits-Christoph A, Lou YC, Borges AL, Shivram H, He C, Xu M, Zhou Z, Smith SJ, Rovinsky R, Smock DCJ, Tang K, Owens TK, Krishnappa N, Sachdeva R, Barrangou R, Deutschbauer AM, Banfield JF, Doudna JA. 2022. Species- and site-specific genome editing in complex bacterial communities. Nat Microbiol 7:34–47. doi: 10.1038/s41564-021-01014-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Dorado-Morales P, Lambérioux M, Mazel D. 2023. Unlocking the potential of microbiome editing: a review of conjugation-based delivery. Mol Microbiol. doi: 10.1111/mmi.15147 [DOI] [PubMed] [Google Scholar]
- 157. Huang P-H, Chen S, Shiver AL, Culver RN, Huang KC, Buie CR. 2022. M-TUBE enables large-volume bacterial gene delivery using a high-throughput microfluidic electroporation platform. PLoS Biol 20:e3001727. doi: 10.1371/journal.pbio.3001727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Typas A, Nichols RJ, Siegele DA, Shales M, Collins SR, Lim B, Braberg H, Yamamoto N, Takeuchi R, Wanner BL, Mori H, Weissman JS, Krogan NJ, Gross CA. 2008. High-throughput, quantitative analyses of genetic interactions in E. coli. Nat Methods 5:781–787. doi: 10.1038/nmeth.1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Babu M, Arnold R, Bundalovic-Torma C, Gagarinova A, Wong KS, Kumar A, Stewart G, Samanfar B, Aoki H, Wagih O, Vlasblom J, Phanse S, Lad K, Yeou Hsiung Yu A, Graham C, Jin K, Brown E, Golshani A, Kim P, Moreno-Hagelsieb G, Greenblatt J, Houry WA, Parkinson J, Emili A. 2014. Quantitative genome-wide genetic interaction screens reveal global epistatic relationships of protein complexes in Escherichia coli. PLoS Genet 10:e1004120. doi: 10.1371/journal.pgen.1004120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kuzmin E, VanderSluis B, Wang W, Tan G, Deshpande R, Chen Y, Usaj M, Balint A, Mattiazzi Usaj M, van Leeuwen J, et al. 2018. Systematic analysis of complex genetic interactions. Science 360:eaao1729. doi: 10.1126/science.aao1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Horlbeck MA, Xu A, Wang M, Bennett NK, Park CY, Bogdanoff D, Adamson B, Chow ED, Kampmann M, Peterson TR, Nakamura K, Fischbach MA, Weissman JS, Gilbert LA. 2018. Mapping the genetic landscape of human cells. Cell 174:953–967. doi: 10.1016/j.cell.2018.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Du D, Roguev A, Gordon DE, Chen M, Chen S-H, Shales M, Shen JP, Ideker T, Mali P, Qi LS, Krogan NJ. 2017. Genetic interaction mapping in mammalian cells using CRISPR interference. Nat Methods 14:577–580. doi: 10.1038/nmeth.4286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Muller R, Meacham ZA, Ferguson L, Ingolia NT. 2020. CiBER-seq dissects genetic networks by quantitative CRISPRi profiling of expression phenotypes. Science 370:eabb9662. doi: 10.1126/science.abb9662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Alford BD, Tassoni-Tsuchida E, Khan D, Work JJ, Valiant G, Brandman O. 2021. ReporterSeq reveals genome-wide dynamic modulators of the heat shock response across diverse stressors. Elife 10:e57376. doi: 10.7554/eLife.57376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Poulton NC, DeJesus MA, Munsamy-Govender V, Kanai M, Roberts CG, Azadian ZA, Bosch B, Lin KM, Li S, Rock JM. 2024. Beyond antibiotic resistance: the whiB7 transcription factor coordinates an adaptive response to alanine starvation in mycobacteria. Cell Chem Biol 31:669–682. doi: 10.1016/j.chembiol.2023.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Adamson B, Norman TM, Jost M, Cho MY, Nuñez JK, Chen Y, Villalta JE, Gilbert LA, Horlbeck MA, Hein MY, Pak RA, Gray AN, Gross CA, Dixit A, Parnas O, Regev A, Weissman JS. 2016. A multiplexed single-cell CRISPR screening platform enables systematic dissection of the unfolded protein response. Cell 167:1867–1882. doi: 10.1016/j.cell.2016.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Bock C, Datlinger P, Chardon F, Coelho MA, Dong MB, Lawson KA, Lu T, Maroc L, Norman TM, Song B, Stanley G, Chen S, Garnett M, Li W, Moffat J, Qi LS, Shapiro RS, Shendure J, Weissman JS, Zhuang X. 2022. High-content CRISPR screening. Nat Rev Methods Primers 2:9. doi: 10.1038/s43586-022-00098-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ma P, Amemiya HM, He LL, Gandhi SJ, Nicol R, Bhattacharyya RP, Smillie CS, Hung DT. 2023. Bacterial droplet-based single-cell RNA-seq reveals antibiotic-associated heterogeneous cellular states. Cell 186:877–891. doi: 10.1016/j.cell.2023.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. McNulty R, Sritharan D, Pahng SH, Meisch JP, Liu S, Brennan MA, Saxer G, Hormoz S, Rosenthal AZ. 2023. Probe-based bacterial single-cell RNA sequencing predicts toxin regulation. Nat Microbiol 8:934–945. doi: 10.1038/s41564-023-01348-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Wang B, Lin AE, Yuan J, Novak KE, Koch MD, Wingreen NS, Adamson B, Gitai Z. 2023. Single-cell massively-parallel multiplexed microbial sequencing (M3-seq) identifies rare bacterial populations and profiles phage infection. Nat Microbiol 8:1846–1862. doi: 10.1038/s41564-023-01462-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Elhadi D, Lv L, Jiang X-R, Wu H, Chen G-Q. 2016. CRISPRi engineering E. coli for morphology diversification. Metab Eng 38:358–369. doi: 10.1016/j.ymben.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 172. de Wet TJ, Winkler KR, Mhlanga M, Mizrahi V, Warner DF. 2020. Arrayed CRISPRi and quantitative imaging describe the morphotypic landscape of essential mycobacterial genes. Elife 9:e60083. doi: 10.7554/eLife.60083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Silvis MR, Rajendram M, Shi H, Osadnik H, Gray AN, Cesar S, Peters JM, Hearne CC, Kumar P, Todor H, Huang KC, Gross CA. 2021. Morphological and transcriptional responses to CRISPRi knockdown of essential genes in Escherichia coli. mBio 12:e0256121. doi: 10.1128/mBio.02561-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Arjes HA, Sun J, Liu H, Nguyen TH, Culver RN, Celis AI, Walton SJ, Vasquez KS, Yu FB, Xue KS, Newton D, Zermeno R, Weglarz M, Deutschbauer A, Huang KC, Shiver AL. 2022. Construction and characterization of a genome-scale ordered mutant collection of Bacteroides thetaiotaomicron. BMC Biol 20:285. doi: 10.1186/s12915-022-01481-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Shiver AL, Culver R, Deutschbauer AM, Huang KC. 2021. Rapid ordering of barcoded transposon insertion libraries of anaerobic bacteria. Nat Protoc 16:3049–3071. doi: 10.1038/s41596-021-00531-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Klompe SE, Vo PLH, Halpin-Healy TS, Sternberg SH. 2019. Transposon-encoded CRISPR–Cas systems direct RNA-guided DNA integration. Nature 571:219–225. doi: 10.1038/s41586-019-1323-z [DOI] [PubMed] [Google Scholar]
- 177. Vo PLH, Ronda C, Klompe SE, Chen EE, Acree C, Wang HH, Sternberg SH. 2021. CRISPR RNA-guided integrases for high-efficiency, multiplexed bacterial genome engineering. Nat Biotechnol 39:480–489. doi: 10.1038/s41587-020-00745-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Trujillo Rodríguez L, Ellington AJ, Reisch CR, Chevrette MG. 2023. CRISPR-associated transposase for targeted mutagenesis in diverse proteobacteria. ACS Synth Biol 12:1989–2003. doi: 10.1021/acssynbio.3c00065 [DOI] [PMC free article] [PubMed] [Google Scholar]