Abstract
Background and Aims:
Chronic hepatitis D is the most debilitating form of viral hepatitis frequently progressing to cirrhosis and subsequent decompensation. However, the HDV entry inhibitor bulevirtide is only approved for antiviral treatment of patients with compensated disease. We aimed for the analysis of real-world data on the off-label use of bulevirtide in the setting of decompensated liver cirrhosis.
Approach and Results:
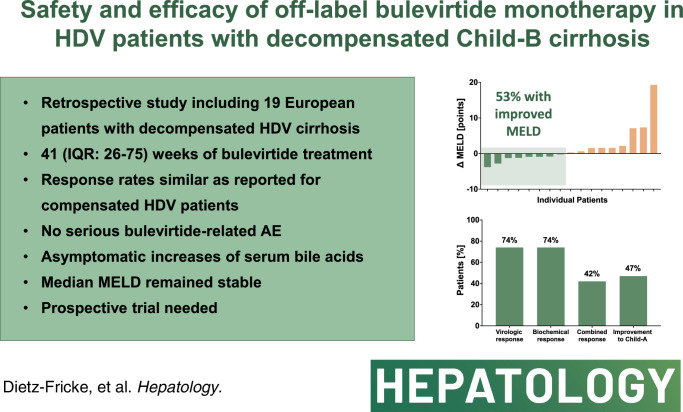
We conducted a retrospective study in patients with HDV with decompensated liver disease at German, Austrian, and Italian centers. We included 19 patients (47% male, mean age: 51 years) with liver cirrhosis Child-Pugh B. The median MELD score was 12 (range 9–17) at treatment initiation. The median observation period was 41 weeks. Virologic response was achieved in 74% and normal alanine aminotransferase was observed in 74%. The combined response was achieved by 42%. The most relevant adverse events included self-limited alanine aminotransferase flares, an asymptomatic increase in bile acids, and the need for liver transplantation. Despite bile acid increases, adverse events were considered unrelated. Clinical and laboratory improvement from Child-Pugh B to A occurred in 47% (n = 9/19). Improvements in the amount of ascites were observed in 58% of the patients initially presenting with ascites (n = 7/12).
Conclusions:
This report on off-label bulevirtide treatment in patients with decompensated HDV cirrhosis shows similar virologic and biochemical response rates as observed in compensated liver disease. Significant improvements were observed in surrogates of hepatic function and portal hypertension. However, this improvement was not seen in all patients. Controlled trials are needed to confirm the safety and efficacy of bulevirtide in decompensated HDV cirrhosis.
INTRODUCTION
Chronic hepatitis D (CHD) is the most debilitating form of viral hepatitis.1 Patients coinfected with the HDV) are at increased risk of developing liver cirrhosis2 and associated complications such as hepatic decompensation.3,4 The progression from compensated (cACLD) to decompensated advanced chronic liver disease (dACLD) is a critical event as mortality risks are substantially elevated once decompensation occurs.5 Referring to the current Baveno VII consensus criteria, decompensation is defined as the development of overt ascites, HE, or variceal bleeding.5 Besides the management of cirrhosis complications, treating the underlying disease is paramount for achieving an improvement in liver function and resolution of cirrhosis complications eventually. While early access to treatment as a preventive measurement in cACLD should be the goal, specific treatment options also for patients that progressed to dACLD are needed in clinical reality. In the context of CHD, the first conditional and now full approval of bulevirtide (BLV) by the European Medicines Agency changed the therapeutic field.6 BLV is an HDV entry inhibitor that blocks sodium taurocholate co-transporting polypeptide—the main transporter for bile acid uptake—which is needed for the entry of HDV but also the HBV.7 In clinical trials, it was shown that BLV leads to viral and biochemical response,8,9 a finding that was reproduced in several real-world studies.10–13 However, according to study inclusion criteria, the approval of BLV is limited to patients with cACLD, leaving the most vulnerable patient collective with dACLD without an option for antiviral treatment. We recently reported anecdotical evidence that in selected cases BLV treatment might be safe and effective despite the presence of hepatic decompensation.10 It was our aim to provide more real-world experience on the so far off-label use of BLV in patients with dACLD. The results can serve as a base for urgently needed controlled trials to overcome the lack of antiviral treatment options for patients with dACLD due to CHD.
METHODS
In collaboration with German, Austrian, and Italian centers, we collected anonymized, retrospective data from patients with dACLD due to CHD. This data collection was approved by the ethics commission at Hannover Medical School (ethical approval number 10161_BO_K_2022) and conducted in accordance with the declaration of Helsinki and Istanbul. Given the anonymized and retrospective data collection no written informed consent was needed. We included patients with BLV monotherapy at a daily dose of 2 mg given subcutaneously. Decompensation was defined according to the Baveno VII consensus5 by significant ascites, HE, or variceal bleeding.
Response criteria to antiviral BLV treatment were defined as follows: Biochemical response was assumed when ALT values were or remained within the reference range. Virologic response was defined by either a ≥2 log decline from baseline or if HDV-RNA was undetectable or below the lower limit of quantification. Virologic nonresponse was defined by an HDV-RNA decline by <1 log or an increase. Partial virologic response was assumed when HDV-RNA declined by >1 log but <2 log. Virologic response was assessed at each available treatment week and not at predefined timepoints. Individual MELD values were calculated using the following equation after INR, creatinine, and bilirubin values <1 mg/dL were set to 1, and creatinine levels >4 mg/dL were set to 414:
A substantial change in bilirubin levels was assumed if values changed by more than 5 μmol/L. Changes in platelet count were considered relevant when being >10,000/μL and when this had been confirmed by at least 2 consecutive measurements.
Due to the real-world character of this study, laboratory results were generated at local laboratories. For this reason, absolute HDV-RNA levels cannot be compared. We therefore display relative changes on the log scale to visualize viral kinetics. Under treatment, cirrhosis complications and potential treatment-related adverse events were assessed by the responsible physicians. In the same way, improvements in liver function and portal hypertension were documented and shared in the process of data collection.
Data preparation and analysis were carried out using R version 1.2.1335.15 Due to the retrospective character of this analysis, there was no prior sample size planning, but all available data were included. Figures were created using R version 1.2.1335, Microsoft Office Excel 16.16.5, and GraphPad Prism.
RESULTS
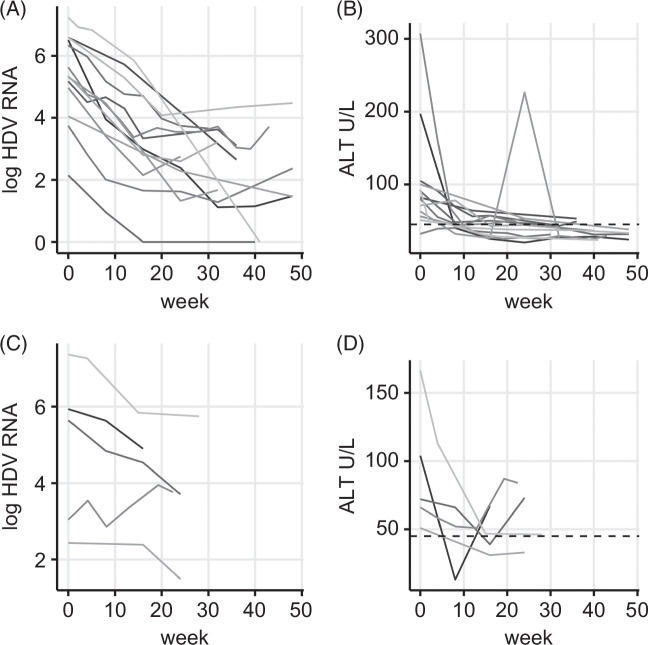
The retrospective analysis included 19 patients (Table 1) with liver cirrhosis Child-Pugh B and clinical signs of hepatic decompensation. Ascites was present in 12 (63%) patients (n = 5 grade 1, n = 6 grade 2, n = 1 grade 3). Liver function parameters showed impaired liver function with albumin levels below 35 g/L in 15 (79%), INR above 1.5 in 5 (26%), and bilirubin levels above 34 μmol/L in 9 (47%) patients. As expected in decompensated liver disease, platelet counts as a surrogate for portal hypertension were decreased in all patients. The median observation period was 41 weeks (16–104, IQR: 26–75). With the exception of one patient who required early liver transplantation at week 16, all patients concluded a minimum of 24 treatment weeks. End points were assessed at each available timepoint and not only at predefined timepoints taking into account the retrospective real-world design of the study. Throughout the observation period, virologic response (Figure 1) was achieved in 14 patients (74%) after a median treatment duration of 17 weeks (IQR: 16–32). Partial response was seen in 3 patients (16%), while 2 cases (11%) were virologic nonresponders at the end of the individual observation period. In those with virologic response, a relapse of HDV-RNA by >1 log was observed in 4 cases. This coincided with a slight increase in ALT in 2 cases and with a significant ALT flare (ALT >3 ULN) in one patient. Following the increase, HDV-RNA levels showed a decline in 2 cases while it stayed elevated in another one. In one case, the relapse occurred at the end of the observational period with no further data on additional follow-up weeks. Biochemical response (Figure 1) with normalization of ALT occurred in 14 patients (74%) after a median of 13 weeks (IQR: 9–16). The combined response was achieved by 8 patients (42%; median treatment week = 19, IQR: 16–27; see Table 2 for individual treatment results). In analogy to clinical trials, treatment response was also evaluated at week 24. The analysis was based on the data acquired between weeks 22 and 26 (n = 11; Table 3). Virologic response was already achieved by 64% while the proportion of biochemical responders was substantially lower than in the overall cohort.
TABLE 1.
Baseline characteristics of patients with Child-Pugh B liver cirrhosis and signs of decompensation at bulevirtide treatment initiation
| Patient characteristics | |
|---|---|
| Patients, n (%) | 19 (100) |
| Age (mean) | 51±10 |
| Male, n (%) | 9 (47) |
| MELD (median, range) | 12 (9–17) |
| Ascites, n (%) | 12 (63) |
| Esophageal varicesa, n (%) | 14 (74) |
| History of variceal hemorrhage, n (%) | 2 (11) |
| HCC, n (%) | 2 (11) |
| Albumin g/L (median, range) | 31 (28–44) |
| INR (median, range) | 1.4 (1.0–1.6) |
| Bilirubin μmol/L (median, range) | 32.5 (8.9–82) |
| Platelet count/μL (median, range) | 65,000 (17,000–140,000) |
| Thrombocytopenia<150,000/μL, n (%) | 19 (100) |
| Alanine transaminase IU/L (median, range) | 82 (32–307) |
| Elevated baseline alanine transaminase >45 U/L, n (%) | 18 (95) |
| History of interferon treatment, n (%) | 5 (26) |
| HBsAg U/Lb (mean, range) | 10,826 (723–25,488) |
| HBeAg negativeb, n (%) | 11 (58) |
An upper gastrointestinal tract endoscopy was available in n = 16 patients.
HBsAg and HBeAg at baseline were available in n = 12 patients.
FIGURE 1.
Viral kinetics and ALT course of n = 19 patients divided by virologic response (A, B) and partial/nonresponse (C, D). Shown are individual kinetics of HDV-RNA on a log scale for patients with virologic response (A) and those with partial or nonresponse (C). Viral response was achieved in n = 14 patients of which in 2 cases HDV-RNA became negative. Individual kinetics of ALT are displayed in (B) for virologic responders and in (D) for partial and nonresponders. The dashed line in (B + D) indicates the threshold above which values are considered elevated.
TABLE 2.
Individual treatment response during the bulevirtide observation period
| Child-Pugh | Ascites | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | Naseline | On treatment | Baseline | On treatment | Change in Diuretic Treatment | Virologic response | Biochemical response/ALT normalization | Liver transplantation, indication | Adverse events, assessment of causal relation to bulevirtide therapy | Observation period (wk) |
| 1 | B7 | A5 | Grade 2 | Resolved week 12 | Discontinued | Week 20 | Week 12 | No | — | 48 |
| 2 | B8 | A6 | Grade 2 | Resolved week 36 | Discontinued | Week 36 | Week 32 | No | — | 75 |
| 3 | B8 | A6 | Grade 2 | Resolved week 64 | Discontinued | Week 20 | Week 12 | No | — | 30 |
| 4 | B7 | A5 | Grade 1 | Resolved week 14 | Discontinued | Week 14 | Week 11 | No | — | 95 |
| 5 | B7 | A6 | Grade 1 | — | — | Week 18 | Week 14 | No | — | 41 |
| 6 | B8 | A6 | No ascites | — | — | Week 52 | ALT reduction (189, 106) | No | — | 104 |
| 7 | B8 | A6 | Grade 2 | Grade 1 week 4 | Reduced dosage | Week 8 | Week 48 | No | — | 48 |
| 8 | B7 | A6 | No ascites | — | — | Week 16 | Week 8 | No | Death after 24 wk of treatment, unrelated | 24 |
| 9 | B7 | B7 | No ascites | De novo (week 60) | — | Week 36 | ALT reduction (82, 61) | No | ALT flare at week 60, unrelated | 85 |
| 10 | B7 | B8 | Grade 1 | Grade 2 | — | Partial response | Week 8 | Yes, decline in liver function | — | 16 |
| 11 | B8 | B7 | Grade 3 | Grade 1 (week 94) | Stable dosage | Week 8 | Week 40 | No | Acute abdomen due to incarcerated hernia, unrelated | 94 |
| 12 | B7 | B9 | Grade 1 | Grade 2 (week 16) | — | Partial response | Week 16 | Yes, decline in liver function | — | 24 |
| 13 | B9 | B9 | Grade 2 | Grade 2 | — | Nonresponse | ALT increase (66, 84) | No | — | 22 |
| 14 | B7 | B9 | No ascites | De novo (week 48) | — | Week 16 | ALT increase (32, 74) | Yes, decline in liver function with de novo ascites | — | 56 |
| 15 | B9 | B7 | No ascites | / | — | Week 16 | Week 8 | No | ALT flare at week 24, unrelated | 32 |
| 16 | B8 | A5 | Grade 2 | Resolved week 24 | — | Week 48 | Week 48 | No | — | 74 |
| 17 | B7 | B7 | No ascites | De novo (week 8) | — | Week 16 | Wek 16 | No | — | 32 |
| 18 | B7 | B8 | No ascites | — | — | Partial response | ALT reduction (166, 46) | No | — | 28 |
| 19 | B8 | B8 | Grade 1 | Grade 1 | Stable dosage | Nonresponse | Week 16 | No | — | 24 |
TABLE 3.
Endpoint analysis at week 24 (n = 11)
| End point | Achieved at week 24 (range weeks 22–26), n (%) |
|---|---|
| Virologic responsea | 7 (64) |
| Biochemical responseb | 5 (46) |
| Combined responsec | 4 (36) |
| Improved Child-Pugh staged | 3 (27) |
Virologic response is defined as either a ≥2 log decline from baseline or HDV-RNA negativity.
Biochemical response is assumed when alanine transaminase values were or remained within the reference range.
Combined response is assumed when virologic and biochemical responses are achieved.
Percentage of patients that improved from the initial Child-Pugh B stage to Child-Pugh A.
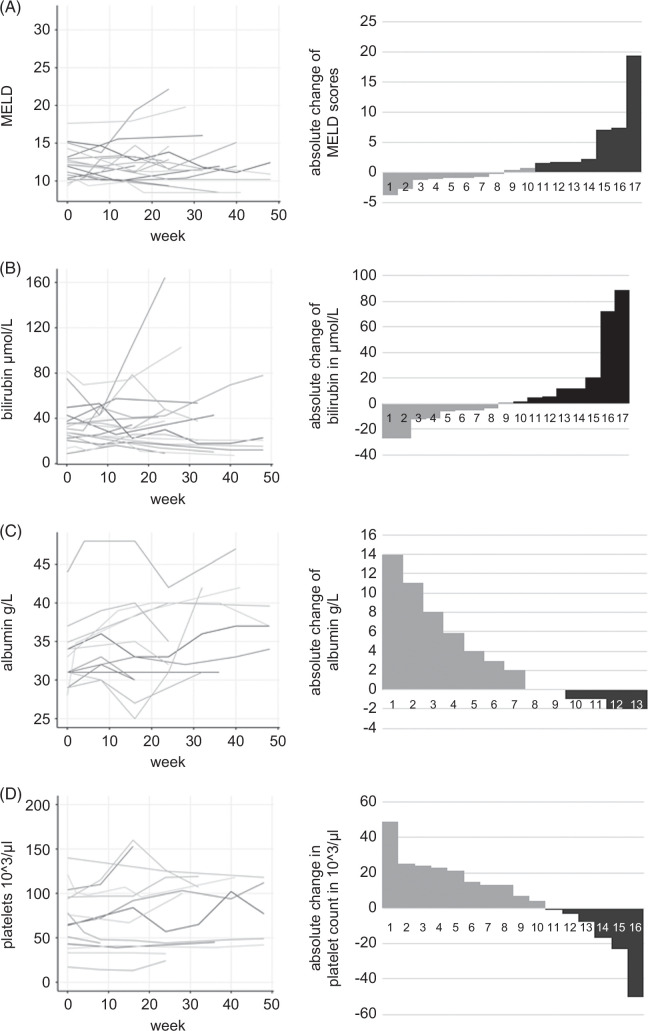
When comparing median Child-Pugh and MELD scores from baseline with the latest treatment week of each patient, stable scores were noted (Child-Pugh B7, MELD 12).
However, when analyzing individual changes in liver function, mixed responses could be described (Figure 2). In some cases, liver function declined with subsequent rises in MELD (Figure 2A) and bilirubin (Figure 2B), finally leading to liver transplantation. Improvements were especially seen in albumin levels (n = 6) while a substantial decrease by more than 1 g/L was noted only in 3 cases (Figure 2C). Rising platelet counts were measured in 10 patients while substantial increases by >10,000/μL confirmed by at least 2 consecutive measurements as surrogate for improvements in portal hypertension were noted in 4 patients (Figure 2D).
FIGURE 2.
Longitudinal development of (A) MELD, (B) bilirubin, (C) albumin, and (D) platelet count. Individual longitudinal courses of the respective parameters of liver function and platelet count as surrogate parameters for portal hypertension are shown. Left-sided line diagrams visualize the longitudinal course of individual parameters. Right-sided waterfall plots show the difference of the respective parameter between the baseline value and the latest follow-up timepoint. Gray bars indicate an improvement, black bars indicate a worsening of each parameter. Values are missing for bilirubin n = 2, albumin n = 6, and platelets n = 3.
Individually, improvements in ascites and liver function led to a downstaging from Child-Pugh B to A in 9 patients (47%). As summarized in Table 2, clinical improvements were mainly due to the resolution of ascites with discontinuation of diuretic treatment in selected cases (n = 4).
Adverse events included the development of ascites in 3 patients of which 2 had already responded virologically (Table 2). Normalization of ALT had not occurred in any of these cases. In more detail, the development of ascites was linked to a rise in the MELD score, which consequently led to the requirement of liver transplantation (n = 1). Another case had HCC as an additional risk factor for decompensation. Liver transplantation was required in a total of 3 patients (Table 2) with declining liver function and worsening of ascites regardless of lowered HDV-RNA levels (n = 3) and improved ALT (n = 2). BLV treatment was terminated at liver transplantation. During BLV therapy, 2 patients developed ALT flares with ALT increases >3 ULN. These flares were self-limited. In one patient, the ALT increase was accompanied by an increase in HDV-RNA of >1 log and the occurrence of ascites, while the other patient showed stable virologic response. During treatment, asymptomatic increases in bile acids were noted (76 μmol/L±76 vs. 170 μmol/L±195). One patient developed an acute abdomen with the need for surgery and subsequent decompensation due to an incarcerated hernia; one patient died from other reasons. Despite the increase in bile acids, recorded adverse events were considered to be unrelated to the BLV treatment.
DISCUSSION
To our knowledge, this is so far the largest real-world analysis of the off-label use of BLV in patients with decompensated liver cirrhosis due to CHD. We demonstrate (1) that the use of BLV in this vulnerable patient population is associated with virologic response rates comparable to the results from clinical trials and published real-world studies in compensated patients. Furthermore, (2) ALT normalization as a surrogate for reduced hepatocellular injury is frequently observed. Besides virologic and biochemical responses, we also saw (3) clinical benefits with conversion from Child-Pugh B to Child-Pugh A and improved control of ascites in some patients. Adverse events included the development or worsening of ascites and a decline in liver function with the need for liver transplantation. These (4) adverse events were considered to be not attributed to the use of BLV but rather the consequence of the natural disease course. Consequently, there were no treatment discontinuations due to adverse events. However, liver transplantation was required in three patients. At the timepoint of transplantation, patients had not fully responded to BLV therapy. The post-transplant course of these patients with HDV and their further use of HBV/HDV prophylaxis16 will be followed. Overall, MELD scores remained stable in the majority of patients.
Patients coinfected with HDV are at higher risk of developing liver cirrhosis and subsequent complications.17 So far, there is no approved treatment for patients with decompensated cirrhosis due to CHD. The observed improvements in clinical and laboratory parameters suggest an improvement of hepatic function and/or of portal hypertension potentially linked to the use of BLV.
Besides clinical efficacy, safety considerations are of utmost importance when considering the use of BLV in decompensated cirrhosis. A commonly observed adverse effect of BLV is the elevation of serum bile acids, which is explained by the sodium taurocholate co-transporting polypeptide blockade.8 In line with this observation, increases in bile acids were also noted in this population. Mainly in the context of cholestasis, bile acids were found to confer hepatotoxic effects through the activation of apoptotic signaling pathways.18 However, in clinical trials, the observed increase in bile acids under BLV treatment remained asymptomatic.8,9,19 There are even molecular considerations on potential hepatoprotective effects of the BLV-mediated shielding of hepatocytes from bile acids. In cholestatic mice, it could be shown that the blockage of sodium taurocholate co-transporting polypeptide protects hepatocytes from the toxic effects of bile acids.20 Molecular investigations on the impact of BLV on the hepatic bile acid metabolism in patients have not been performed in patients so far. Regarding safety, one has to consider that once BLV is started in a patient with dACLD, stopping BLV might then bear the risk of severe ALT flares related to an HDV rebound. Recently, it was shown that viral relapses after discontinuation of BLV occur while relevant ALT elevations were rare.21 However, ALT flares might have detrimental effects on the function of an already cirrhotic liver as observed in hepatitis B.22 In fact, in one of our patients, an increase in HDV-RNA was accompanied by an ALT flare and the development of ascites. In HBV monoinfection, the termination of a treatment is not recommended in patients with dACLD.23 Similarly, BLV-associated elevations of transaminases8 might be more dangerous in individuals with dACLD than in those with cACLD or without cirrhosis.
While this case report series gives an insight into the feasibility of BLV treatment in decompensated cirrhosis, controlled trials are urgently needed to thoroughly investigate safety and efficacy. Until then no general recommendation on the use of BLV in dACLD can be made, but clinical case-by-case decisions are necessary. While the use of virologic and biochemical end points was endorsed by the European Medicines Agency (EMA) and Food and Drug Administration (FDA),24 it needs to be evaluated if virologic and biochemical improvements translate into clinical benefits. In this context, the resolution of ascites and discontinuation of diuretics leading to an improvement from Child-Pugh B to A in a considerate number of patients is promising. Importantly, such clinical improvements were also seen prior to virologic response but were accompanied by a decline in ALT as a surrogate for amelioration of hepatic inflammation. This points toward the particular importance of biochemical response in the group of decompensated patients. Thus, the duration of BLV therapy should be determined based on clinical benefits rather than virologic response criteria, especially in patients with decompensated cirrhosis. However, clinical improvements were not seen in all patients and median MELD scores were stable. In a few cases, ALT flares and rises in HDV-RNA occurred. The natural disease progression could not be stopped in all patients and liver transplantation had to be performed in some individuals. We could not find a certain MELD threshold that was associated with clinical nonresponse and further deterioration of the liver function. Taken together, a close monitoring of treatment response and clinical disease course is indicated. For future assessments, the evaluation of transplant-free survival, improvements in portal hypertension, or the impact on MELD scores are clinically of great interest. Based on such results, different treatment modalities (eg, bridge-to-transplant) could be evaluated. While acknowledging fundamental differences in antiviral treatments for HCV, HBV, and HDV, the underlying concept of achieving viral control and thereby less hepatocellular damage is comparable. Experience from the treatment of HCV and HBV might therefore serve as orientation when discussing potential benefits expected from BLV treatment in dACLD. Notably, in patients cured of the HCV by direct-acting antivirals, those with decompensated cirrhosis (ie, Child B/C) did show an improvement in MELD, but this was not associated with improved event-free survival.25 Importantly, antiviral therapy still allowed for the successful delisting of previously decompensated patients with hepatitis C from the liver transplantation waiting list due to clinical improvements.26 This is in line with observations in decompensated patients with HBV cirrhosis—which is likely more similar to the setting of HDV, which showed that prolonged suppression of HBV replication by entecavir therapy may lead to cirrhosis recompensation in 56.2% of the patients.27
This study has limitations. Parts of the patients were included in previous data analysis but were complemented by new data. The results displayed were generated at each participating center and according to local laboratories, which impacts the comparability. Most importantly, this real-world study was carried out retrospectively without a control arm, so observed changes are not necessarily linked to the use of BLV. This has to be overcome by prospective controlled trials. We also cannot rule out a selection bias as patients included here were exclusively treated at tertiary referral centers and might therefore differ in their disease severity from the general population of patients with dACLD due to CHD.
In summary, we report real-world experience from 19 patients with CHD and decompensated cirrhosis treated with BLV. Based on our results, the use of BLV in this vulnerable population seems to be associated with similar virologic and biochemical response rates as in compensated patients. However, clinical improvement did not occur in all patients, and some patients experienced further decompensation including the need for liver transplantation in three patients. However, BLV may still exert beneficial effects on the transplant waiting list by reducing the risk of further decompensation and/or of HBV/HDV reinfection of the liver graft.
Importantly, since the clinical responses under BLV in decompensated patients with HDV cirrhosis were heterogenous, they need to be closely monitored. The use of BLV in decompensated liver disease cannot be generally recommended at this stage. Therefore, controlled trials are urgently needed.
DATA AVAILABILITY STATEMENT
Data can be requested from the authors upon reasonable request.
AUTHOR CONTRIBUTIONS
Christopher Dietz-Fricke, Heiner Wedemeyer, Katja Deterding, Thomas Reiberger, and Pietro Lampertico conceptualized the study; Christopher Dietz-Fricke, Elisabetta Degasperi, and Mathias Jachs were responsible for clinical data acquisition and preparation of the manuscript; Christopher Dietz-Fricke, Elisabetta Degasperi, Mathias Jachs, Heiner Wedemeyer, Katja Deterding, Thomas Reiberger, and Pietro Lampertico wrote the original manuscript; all authors contributed to the data collection and reviewed and edited the manuscript.
CONFLICTS OF INTEREST
Christopher Dietz-Fricke received grants from Gilead. Elisabetta Degasperi consults, is on the speakers’ bureau, and received grants from AbbVie, Gilead, and Intercept. She consults for Roche. She received grants from Advanz. Mathias Jachs consults, is on the speakers’ bureau, and received grants from Gilead. Benjamin Maasoumy consults, is on the speakers’ bureau, and received grants from AbbVie and Roche. He consults and is on the speakers’ bureau for Norgine. He is on the speakers’ bureau and received grants from Fujirebio and Gilead. He consults for Luvos. He is on the speakers’ bureau for W. L. Gore, Medical Tribune and Forum, and MSD. He received grants from Altona and ewimed GmbH. He owns stock in Biontech. Florian P. Reiter advises, is on the speakers’ bureau, and received grants from Gilead. He is on the speakers’ bureau and received grants from AbbVie, AstraZeneca, Falk, Ipsen, and Novartis. Andreas Geier consults, is on the speakers’ bureau, and received grants from AbbVie, Falk, Intercept, and Novartis. He consults and is on the speakers’ bureau for Advanz, Alexion, Bristol Myer Squibb, CSL Behring, Gilead, Merz, MSD, and Roche. He consults for Bayer, Eisai, Heel, Ipsen, Novo Nordisk, Pfizer, Sanofi, and Sequana. He is on the speakers’ bureau for Burgenstein. Kathrin Sprinzl advises, is on the speakers’ bureau, and received grants from Gilead. She is on the speakers’ bureau for AbbVie and Bristol Myers Squibb. Stefan Zeuzem consults and is on the speakers’ bureau for Gilead. He is on the speakers’ bureau and received grants from AbbVie, Gilead, and MSD. He consults for BioMarin, GlaxoSmithKline, Madrigal, and Novo Nordisk. He consults for Ipsen and SoBi. He advises Boehringer Ingelheim. Bernhard Schlevogt advises and received grants from Gilead. He advises Univar. He is on the speakers’ bureau for Alnylam. He received grants from AbbVie. Toni Herta consults and received grants from Albireo. He consults for Advanz. Heiner Wedemeyer consults, advises, and is on the speakers’ bureau for Dr. Falk, MSD, and Gilead. He consults, advises, and received grants from Abbott. He consults and advises Albireo, AstraZeneca, Atea, Bristol Myers-Squibb, Eli Lilly, F. Hoffmann-La Roche, GlaxoSmithKline, Janssen, Mirum, Orphalan, Pfizer, Roche, Sobi, Takeda, and Vir. He is on the speakers’ bureau and received grants from Biotest. He is on the speakers’ bureau for BioMarin, CSL Behring, Falk Foundation, and Olink. He received grants from AbbVie. Katja Deterding advises, is on the speakers’ bureau, and received grants from Gilead. Thomas Reiberger consults and advises AbbVie, AstraZeneca, Bayer, Boehringer-Ingelheim, Gilead, Intercept/Advanz, MSD, Resolution, and Siemens. He received grants from AbbVie, Boehringer-Ingelheim, Dr. Falk Pharma, Gilead, W. L. Gore, Intercept/Advanz, MSD, Myr, Philips Healthcare, Pliant, Roche, and Siemens. He is on the speakers’ bureau for AbbVie, Gilead, W. L. Gore, Intercept/Advanz Pharma, MSD, and Roche. Pietro Lampertico advises and is on the speakers’ bureau for AbbVie, Aligos, Alnylam, Altona, Antios, Arrowhead, Bristol Myer Squibb, Eiger, Gilead, GlaxoSmithKline, Grifols, Janssen, MSD, MYR, Roboscreen, Roche, and Vir. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: BLV, bulevirtide; cACLD, compensated advanced chronic liver disease; CHD, chronic hepatitis D; dACLD, decompensated advanced chronic liver disease; EMA, European Medicines Agency; FDA, Food and Drug Administration.
Preliminary data were presented at EASL Congress 2023.
Christopher Dietz-Fricke, Mathias Jachs, and Elisabetta Degasperi contributed equally and share the first authorship.
Katja Deterding, Thomas Reiberger, and Pietro Lampertico contributed equally and share the last authorship.
Contributor Information
Christopher Dietz-Fricke, Email: Dietz-Fricke.Christopher@mh-hannover.de.
Elisabetta Degasperi, Email: elisabetta.degasperi@policlinico.mi.it.
Mathias Jachs, Email: mathias.jachs@meduniwien.ac.at.
Benjamin Maasoumy, Email: Maasoumy.Benjamin@mh-hannover.de.
Florian P. Reiter, Email: Reiter_F@ukw.de.
Andreas Geier, Email: geier_a2@ukw.de.
Julia M. Grottenthaler, Email: julia.grottenthaler@med.uni-tuebingen.de.
Christoph P. Berg, Email: christoph.berg@med.uni-tuebingen.de.
Kathrin Sprinzl, Email: Sprinzl@med.uni-frankfurt.de.
Stefan Zeuzem, Email: zeuzem@em.uni-frankfurt.de.
Juliana Gödiker, Email: Juliana.Goediker@ukmuenster.de.
Bernhard Schlevogt, Email: Bernhard.Schlevogt@klinikum-os.de.
Toni Herta, Email: Toni.Herta@medizin.uni-leipzig.de.
Johannes Wiegand, Email: johannes.wiegand@medizin.uni-leipzig.de.
Roberta Soffredini, Email: roberta.soffredini@policlinico.mi.it.
Heiner Wedemeyer, Email: wedemeyer.heiner@mh-hannover.de.
Katja Deterding, Email: deterding.katja@mh-hannover.de.
Thomas Reiberger, Email: thomas.reiberger@meduniwien.ac.at.
Pietro Lampertico, Email: pietro.lampertico@unimi.it.
REFERENCES
- 1.Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. The Lancet. 2011;378:73–85. [DOI] [PubMed] [Google Scholar]
- 2.Jachs M, Binter T, Schmidbauer C, Hartl L, Strasser M, Laferl H, et al. Hepatitis D virus (HDV) prevalence in Austria is low but causes considerable morbidity due to fast progression to cirrhosis. United European Gastroenterol J. 2021;9:1119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bockmann JH, Grube M, Hamed V, von Felden J, Landahl J, Wehmeyer M, et al. High rates of cirrhosis and severe clinical events in patients with HBV/HDV co-infection: Longitudinal analysis of a German cohort. BMC Gastroenterol. 2020;20:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asselah T, Rizzetto M. Hepatitis D virus infection. N Engl J Med. 2023;389:58–70. [DOI] [PubMed] [Google Scholar]
- 5.de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Abraldes JG, et al. Baveno VII—renewing consensus in portal hypertension. J Hepatol. 2022;76:959–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hepcludex epar product information. Accessed August 7, 2022. https://www.ema.europa.eu/en/documents/product-information/hepcludex-epar-product-information_de.pdf
- 7.Urban S, Neumann-Haefelin C, Lampertico P. Hepatitis D virus in 2021: Virology, immunology and new treatment approaches for a difficult-to-treat disease. Gut. 2021;70:1782–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wedemeyer H, Schöneweis K, Bogomolov P, Blank A, Voronkova N, Stepanova T, et al. Safety and efficacy of bulevirtide in combination with tenofovir disoproxil fumarate in patients with hepatitis B virus and hepatitis D virus coinfection (MYR202): A multicentre, randomised, parallel-group, open-label, phase 2 trial. Lancet Infect Dis. 2023;23:117–29. [DOI] [PubMed] [Google Scholar]
- 9.Wedemeyer H, Aleman S, Brunetto MR, Blank A, Andreone P, Bogomolov P, et al. A phase 3, randomized trial of bulevirtide in chronic hepatitis D. N Engl J Med. 2023;389:22–32. [DOI] [PubMed] [Google Scholar]
- 10.Dietz-Fricke C, Tacke F, Zöllner C, Demir M, Schmidt HH, Schramm C, et al. Treating hepatitis D with bulevirtide—real-world experience from 114 patients. JHEP Reports. 2023;5:100686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degasperi E, Anolli MP, Lampertico P. Bulevirtide-based treatment strategies for chronic hepatitis delta: A review. J Viral Hepat. 2023;30:27–33. [DOI] [PubMed] [Google Scholar]
- 12.Jachs M, Schwarz C, Panzer M, Binter T, Aberle SW, Hartl L, et al. Response-guided long-term treatment of chronic hepatitis D patients with bulevirtide-results of a “real world” study. Aliment Pharmacol Ther. 2022;56:144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Degasperi E, Anolli MP, Uceda Renteria SC, Sambarino D, Borghi M, Perbellini R, et al. Bulevirtide monotherapy for 48 weeks in patients with HDV-related compensated cirrhosis and clinically significant portal hypertension. J Hepatol. 2022;77:1525–31. [DOI] [PubMed] [Google Scholar]
- 14.Kamath P. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. [DOI] [PubMed] [Google Scholar]
- 15.rstatix: Pipe-Friendly Framework for Basic Statistical Tests [computer program]. 2021.
- 16.Ferenci P, Reiberger T, Stadlbauer V, Zoller H. Transplantation of hepatitis D virus patients: Lifelong hepatitis B immunoglobulins? Liver Int. 2023;43:96–100. [DOI] [PubMed] [Google Scholar]
- 17.Kamal H, Westman G, Falconer K, Duberg AS, Weiland O, Haverinen S, et al. Long-term study of hepatitis delta virus infection at secondary care centers: The impact of viremia on liver-related outcomes. Hepatology. 2020;72:1177–90. [DOI] [PubMed] [Google Scholar]
- 18.Wei S, Ma X, Zhao Y. Mechanism of hydrophobic bile acid-induced hepatocyte injury and drug discovery. Front Pharmacol. 2020;11:1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deterding K, Xu C, Port K, Dietz‐Fricke C, Xun J, Maasoumy B, et al. Bile acid increase during bulevirtide treatment of hepatitis D is not associated with a decline in HDV RNA. J Viral Hepat. 2023;30:597–606. [DOI] [PubMed] [Google Scholar]
- 20.Slijepcevic D, Roscam Abbing RLP, Fuchs CD, Haazen LCM, Beuers U, Trauner M, et al. Na(+) -taurocholate cotransporting polypeptide inhibition has hepatoprotective effects in cholestasis in mice. Hepatology. 2018;68:1057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jachs M, Panzer M, Hartl L, Schwarz M, Balcar L, Camp JV, et al. Long-term follow-up of patients discontinuing bulevirtide treatment upon long-term HDV-RNA suppression. JHEP Rep. 2023;5:100751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu YC, Lin YH, Lee TY, Nguyen MH, Tseng CH, Ho HJ, et al. Severe hepatitis B flares with hepatic decompensation after withdrawal of nucleos(t)ide analogues: A population-based cohort study. Aliment Pharmacol Ther. 2023;58:463–73. [DOI] [PubMed] [Google Scholar]
- 23.Cornberg M, Sandmann L, Protzer U, Niederau C, Tacke F, Berg T, et al. S3-Leitlinie der Deutschen Gesellschaft für Gastroenterologie,Verdauungs- und Stoffwechselkrankheiten (DGVS) zur Prophylaxe, Diagnostik und Therapie der Hepatitis-B-Virusinfektion. Z Gastroenterol. 2021;59:691–776. [DOI] [PubMed] [Google Scholar]
- 24.(FDA) FDA . Chronic Hepatitis D Virus Infection: Developing Drugs for Treatment Guidance for Industry. Food and Drug Administration; 2019. [Google Scholar]
- 25.Krassenburg LAP, Maan R, Ramji A, Manns MP, Cornberg M, Wedemeyer H, et al. Clinical outcomes following DAA therapy in patients with HCV-related cirrhosis depend on disease severity. J Hepatol. 2021;74:1053–63. [DOI] [PubMed] [Google Scholar]
- 26.Pascasio JM, Vinaixa C, Ferrer MT, Colmenero J, Rubin A, Castells L, et al. Clinical outcomes of patients undergoing antiviral therapy while awaiting liver transplantation. J Hepatol. 2017;67:1168–76. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Zhao H, Deng Y, Zheng H, Xiang H, Nan Y, et al. Validation of Baveno VII criteria for recompensation in entecavir-treated patients with hepatitis B-related decompensated cirrhosis. J Hepatol. 2022;77:1564–72. [DOI] [PubMed] [Google Scholar]