Abstract
Background
Emerging evidence suggests initial oral and intravenous (IV) antibiotics have similar efficacy in pediatric community-acquired pneumonia (CAP), but further data are needed. We determined the association between hospital-level initial oral antibiotic rates and outcomes in pediatric CAP.
Methods
This retrospective cohort study included children hospitalized with CAP at 43 hospitals in the Pediatric Health Information System (2016–2022). Hospitals were grouped by whether initial antibiotics were given orally in a high, moderate, or low proportion of patients. Regression models examined associations between high vs. low oral utilizing hospitals and length of stay (LOS, primary outcome), intensive care unit [ICU] transfers, escalated respiratory care, complicated CAP, cost, readmissions, and ED-revisits.
Results
Initial oral antibiotics were used in 16% (IQR 10–20%) of 30,207 encounters, ranging from 1–68% across hospitals. Comparing high vs. low oral utilizing hospitals (oral rate: 32% [27–47%] and 10% [9–11%], respectively), there were no differences in LOS, ICU, complicated CAP, cost, or ED-revisits. Escalated respiratory care occurred in 1.3% and 0.5% of high and low oral utilizing hospitals, respectively (RR 2.96 [1.12, 7.81]) and readmissions in 1.5% and 0.8% (RR 1.68 [1.31, 2.17]).
Conclusion
Initial oral antibiotics varied across hospitals without a difference in LOS. While high oral utilizing hospitals had higher escalated respiratory care and readmission rates, these were rare, the clinical significance of these small differences is uncertain, and there were no differences in other clinically relevant outcomes. This suggests some children may benefit from initial IV antibiotics, but most would probably do well with oral antibiotics.
Introduction
Community-acquired pneumonia (CAP) is the third leading cause of pediatric hospitalizations in the U.S. and is responsible for more antibiotic days than any other infection.1–3 Antibiotic route (i.e., use of intravenous [IV] or oral antibiotics) is an important aspect of antibiotic stewardship. Early transition from IV-to-oral antibiotics in CAP is associated with reduced length of stay (LOS), costs, and risk of peripheral intravenous catheter (PIV)- related harms.4–6 National organizations emphasize the importance of early transitions.7,8 However, less effort has focused on initial antibiotic route, which is important, as reducing initial IV antibiotics may have the greatest potential to decrease overall IV antibiotic use and may spare children the pain of PIV placement and related harms.13,37
Although national CAP guidelines recommend initial IV antibiotics for hospitalized children, the evidence to support this is limited.8 Existing literature, including three randomized controlled trials (RCTs), demonstrate that initial oral antibiotics are as effective as IV antibiotics.9–12 From a pharmacokinetic perspective, oral antibiotics for CAP have good bioavailability (74–92%) and can reach effective antibacterial concentrations.13–15 Given that oral antibiotics can improve patient experience by reducing PIV-associated pain and anxiety and decrease PIV-related harms, particularly if vascular access is avoided altogether, oral therapy would likely be a preferred strategy.16–19 However, only one of the RCTs was conducted in a high-income country and further real-world comparative effectiveness data are needed. Our objectives were to identify national practice patterns of initial antibiotic route and evaluate the association between hospital-level initial oral antibiotic route and outcomes such as LOS for children hospitalized with CAP.
Methods
Study Design, and Data Source
We performed a multicenter retrospective cohort study using the Pediatric Health Information System (PHIS) database (Children’s Hospital Association, Lenexa, KS). The database includes 46 freestanding children’s hospitals and accounts for approximately one-third of all U.S. pediatric hospitalizations.20 PHIS contains patient-level clinical, demographic, and billing data.
Study Population
We included children 3 months to 18 years of age hospitalized with diagnosis of CAP who received at least one dose of antibiotics commonly used for typical bacterial CAP (herein referred to as “CAP” for brevity) on the first or second hospital day from January 2016 to June 2022. A discharge diagnosis of CAP was defined using International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10) codes from a previously validated algorithm (Supplemental Table 1).21 Antibiotics commonly used for CAP included aminopenicillins with or without beta-lactamase inhibitors and cephalosporins (Supplemental Table 2).22,23
We excluded hospitals that did not report LOS in hours (n=3), as this was our primary outcome. To define a cohort of generally healthy children with CAP, we excluded children with complex chronic conditions (such as gastrointestinal malabsorption and total parenteral nutrition dependence, which may have affected antibiotic route choices)24 or any hospitalization in the last 30 days. We excluded patients transferred from other hospitals, as we could not identify antibiotics at the transferring institution. We excluded children who presented with severe pneumonia, for which IV antibiotics are usually indicated, based on the presence of any of the following during the first two days: pleural drainage procedure, mechanical ventilation, intensive care unit (ICU) admission, or death.25 To exclude children with brief hospitalizations whose improvement was unlikely related to initial antibiotic route, we excluded those with LOS <24 hours. To capture children treated specifically for CAP, we excluded children who did not receive antibiotics each day of their hospitalization and those diagnosed with other bacterial infections that may warrant IV antibiotics (e.g., osteomyelitis; Supplemental Table 3). Finally, we excluded children with LOS >7 days, as this is atypical for uncomplicated CAP.22 This study was deemed exempt from human subject’s research by the lead author’s Institutional Review Board.
Initial Antibiotic Route
Initial antibiotic route was classified into two mutually exclusive groups, oral or IV, according to the antibiotic route of administration during the first calendar day of the hospitalization. Children with intramuscular antibiotics or both oral and IV antibiotics on the first hospital day were included in the IV group. Thus, patients with initial oral antibiotics were defined as those with exclusive oral antibiotics on the first day.
Outcomes
The primary outcome was LOS given its significance to families and hospitals. Secondary outcomes included ICU transfer, escalated respiratory care (i.e., high flow nasal cannula, non-invasive positive pressure ventilation, or intubation and mechanical ventilation), 26 complicated CAP (i.e., discharge diagnosis of complicated CAP or pleural drainage procedure),27 hospital cost (estimated from charges using hospital and year-specific cost to charge ratios), and 7-day CAP-related ED revisit, CAP-related readmission, readmission for complicated CAP, and readmission with pleural drainage procedure. We considered ICU and escalated respiratory care as outcomes if they occurred after and not during the first two calendar days.
Statistical Analyses
We evaluated the proportion of patients who received initial oral vs. IV antibiotics and described the use of oral vs. IV antibiotics throughout the hospitalization. To identify variability across hospitals, we evaluated the proportion of patients with initial oral antibiotics by hospital. We then created high, moderate, and low initial oral antibiotic utilizing hospital groups using an outlier status approach; hospitals were identified as a high (or low) outlier if the 95% confidence interval of their oral antibiotic rate did not contain the overall mean rate across hospitals.28 For brevity, we will refer to hospitals that used initial oral antibiotics in a relatively high (or low) proportion of patients as high (or low) oral hospitals. We compared characteristics across hospital groups using Chi-squared and Wilcoxon rank sum tests.
We evaluated outcomes between the high and low oral hospital groups to compare hospitals on either end of the spectrum of initial oral antibiotic use. We used generalized estimating equations, clustered on hospital, with the low oral hospital group as the reference. We adjusted for potential confounders identified a priori based on clinical significance and prior studies, including the following: patient age; use of narrow- vs. broad-spectrum antibiotics on the first day (narrow spectrum: ampicillin, amoxicillin, or penicillin; broad-spectrum: all other antibiotics used for typical CAP including cephalosporins and ampicillin-sulbactam); diagnostic utilization (i.e., blood culture, blood gas analysis, or advanced imaging with chest ultrasound or computed tomography) on the first two days as an indicator of initial severity of illness; additional respiratory diagnoses including bronchiolitis, asthma, influenza, and atypical CAP (based on discharge diagnosis of bronchiolitis, and receipt of systemic corticosteroids, oseltamivir, and macrolides on first two days, respectively); hospitalization year; winter/fall season; and any hospitalization in the preceding 6 months.25 In a planned sub-analysis, we only included patients with initial amoxicillin or ampicillin to focus on children with likely non-severe initial presentations. Statistical analysis was performed using SAS v9.4 (SAS Institute Inc, Cary, NC), and p-values <0.05 were considered statistically significant.
Results
Study Cohort
We included 30,207 patient encounters across 43 hospitals (Figure 1). The median age was 3 years (interquartile range [IQR] 1–5), 59% received initial narrow-spectrum antibiotics, and most did not have additional respiratory diagnoses (Table 1). The most common initial antibiotics were ampicillin (50%), ceftriaxone (43%), and amoxicillin (33%; Supplemental Table 2).
Figure 1.
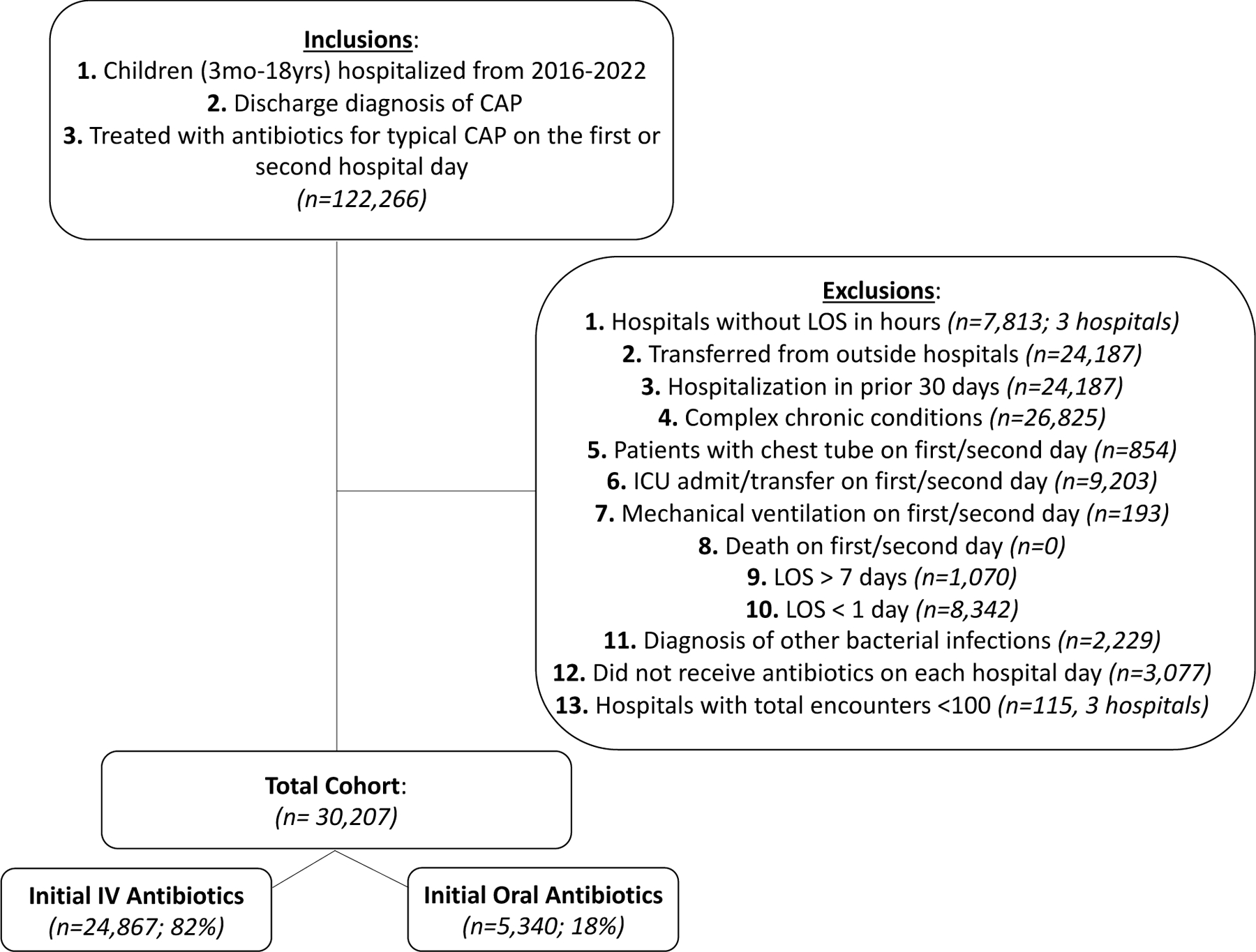
Cohort Flow Diagram
Table 1.
Patient Characteristics by Hospital Group
| Patient Factors | All Patients (n=30,207 at 43 hospitals) | Low Oral Utilizing Hospitals (n=15,609 patients at 16 hospitals) | Moderate Oral Utilizing Hospitals (n=9,929 patients at 20 hospitals) | High Oral Utilizing Hospitals (n=4,669 patients at 7 hospitals) | P-value | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age in years - median, IQR | 3 [1, 5] | 3 [1, 5] | 2 [1, 5] | 3 [1, 5] | <0.001 | |
| Female sex | 14,877 (49.3) | 7,694 (49.3) | 4,913 (49.5) | 2,270 (48.6) | 0.589 | |
| Race/ Ethnicity1 | Non-Hispanic White | 12,949 (42.9) | 5,876 (37.6) | 4,541 (45.7) | 2,532 (54.2) | <0.001 |
| Non-Hispanic Black | 5,721 (18.9) | 2,776 (17.8) | 2,087 (21) | 858 (18.4) | ||
| Hispanic | 8,018 (26.5) | 5,020 (32.2) | 2,217 (22.3) | 781 (16.7) | ||
| Asian | 1,282 (4.2) | 787 (5) | 339 (3.4) | 156 (3.3) | ||
| Other | 2,237 (7.4) | 1,150 (7.4) | 745 (7.5) | 342 (7.3) | ||
| Insurance | Government | 16,085 (53.2) | 8,451 (54.1) | 5,529 (55.7) | 2,105 (45.1) | <0.001 |
| Private | 12,368 (40.9) | 6,298 (40.3) | 3,812 (38.4) | 2,258 (48.4) | ||
| Other | 1,754 (5.8) | 860 (5.5) | 588 (5.9) | 306 (6.6) | ||
| Clinical Characteristics | ||||||
| Initial antibiotics were narrow-spectrum only | 17,923 (59.3) | 8,638 (55.3) | 5878 (59.2) | 3407 (73.0) | <0.001 | |
| Hospitalization in last 6 mo | 1,956 (6.5) | 909 (5.8) | 719 (7.2) | 328 (7) | <0.001 | |
| Diagnostic Utilization in the First Two Days | ||||||
| Blood culture | 10980 (36.3) | 6635 (42.5) | 3344 (33.7) | 1001 (21.4) | <0.001 | |
| Blood gas | 3184 (10.5) | 2029 (13) | 785 (7.9) | 370 (7.9) | <0.001 | |
| Chest CT or ultrasound | 1053 (3.5) | 487 (3.1) | 406 (4.1) | 160 (3.4) | <0.001 | |
| Concurrent Diagnoses | ||||||
| Asthma | 7898 (26.1) | 4365 (28) | 2429 (24.5) | 1104 (23.6) | <0.001 | |
| Atypical pneumonia | 5073 (16.8) | 2840 (18.2) | 1685 (17) | 548 (11.7) | <0.001 | |
| Bronchiolitis | 3473 (11.5) | 1895 (12.1) | 1192 (12) | 386 (8.3) | <0.001 | |
| Influenza | 1437 (4.8) | 718 (4.6) | 495 (5) | 224 (4.8) | <0.001 | |
| Time of Year | ||||||
| Winter/fall (Oct-Mar) | 21430 (70.9) | 11056 (70.8) | 6954 (70) | 3420 (73.2) | <0.001 | |
Values represent N (%) unless otherwise specified. CT, computed tomography; mo, months.
Self-reported from the electronic medical record
Utilization of Oral vs. IV Antibiotics
Overall, 18% (n=5,437) of patients received initial oral antibiotics. Among patients with initial oral antibiotics, 91% continued exclusive oral antibiotics throughout their hospitalization (Supplemental Figure 1). Thus, 16% of patients overall received exclusive oral antibiotics throughout their stay.
Overall, 5% (n=1,447) received IV and oral antibiotics on the initial day and were included in the IV group. Among children with initial IV antibiotics, 81% received multiple days of IV antibiotics and 19% received IV treatment for just one day prior to transitioning to oral antibiotics. High oral hospitals had a higher proportion of children with just one day of IV treatment prior to switching to orals compared to low oral hospitals (26% vs. 15%, p<0.01).
Variability of Initial Oral Antibiotics Across Hospitals
The median proportion of children who received initial oral antibiotics across hospitals was 16% (IQR 11–21%), with a range of 1–68% (Figure 2). Application of the outlier status approach resulted in the following groupings: low (n=16), moderate (n=20), and high (n=7) oral hospital groups with initial oral antibiotics used in a median of 10% (IQR 9–11%), 19% (IQR 16–20%), and 32% (IQR 27–47%) of encounters, respectively (Supplemental Figure 2). High oral hospitals had a higher proportion of narrow-spectrum antibiotic use and lower proportion of most diagnostics and additional respiratory diagnoses compared with low oral hospitals (Table 1).
Figure 2. Initial Oral Antibiotic Utilization by Hospital.
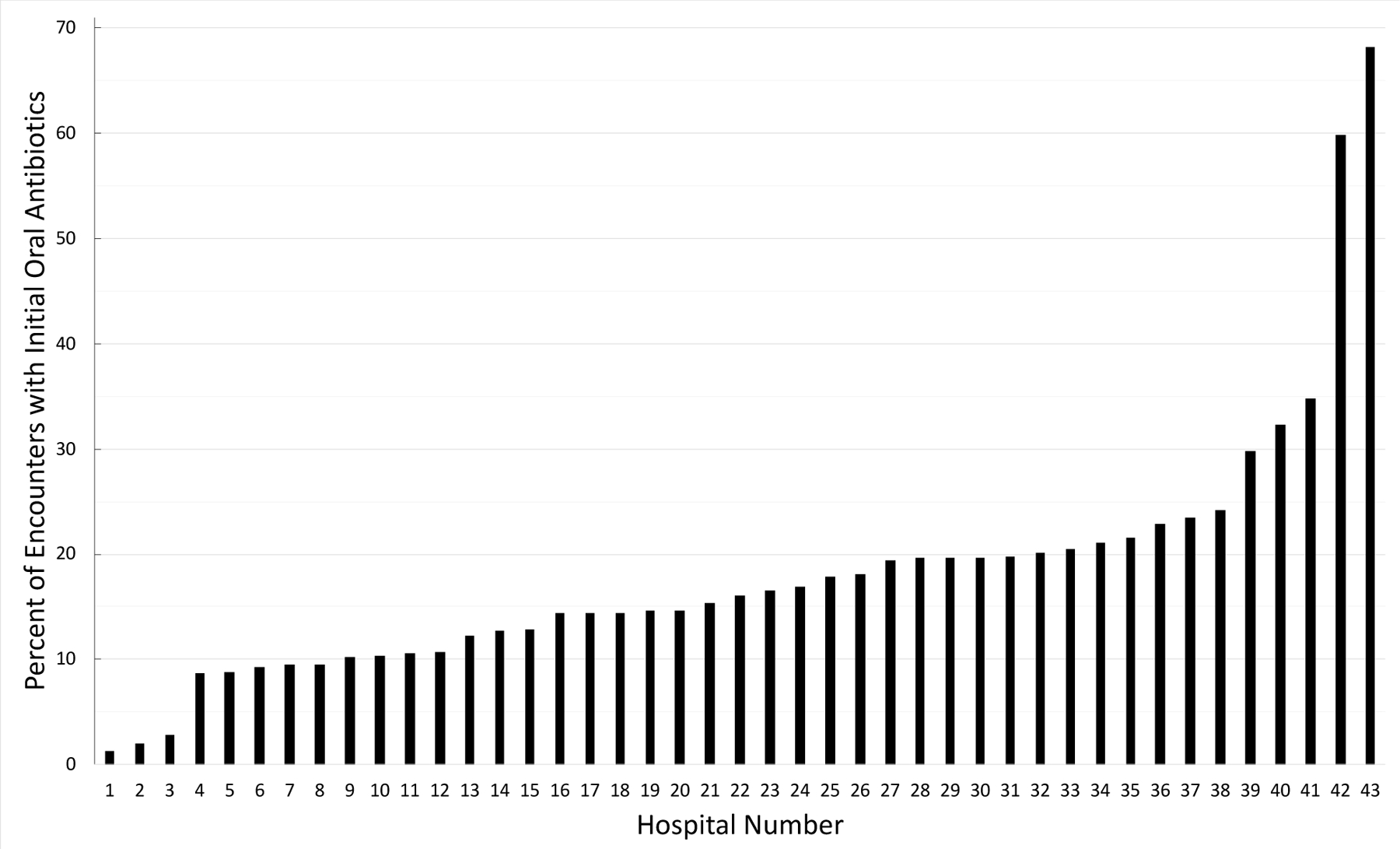
The overall median proportion of children who received initial oral antibiotics was 16%, ranging from 1–68% across hospitals.
Outcomes by Hospital Group
After adjusting for confounders there was no difference in LOS between high vs. low oral hospital groups (mean [standard deviation]: 54 [28] vs. 55 [30] hours; RR 1.00 [0.93, 1.08]; Table 2). There were also no differences in ICU transfers, complicated CAP, cost, ED revisits, or readmissions for complicated CAP. Escalated respiratory care occurred in 1.3% of high oral hospitals and 0.5% of low oral hospitals (RR 2.96 [1.12, 7.81]). CAP-related readmissions occurred in 1.5% and 0.8% of high and low oral hospitals, respectively (RR 1.68 [1.31, 2.17]). Among children with initial amoxicillin or ampicillin (42% of overall cohort), findings aligned with the primary analysis, including no difference in LOS between the groups (RR 1.00 [0.94, 1.07]; Supplemental Table 4).
Table 2.
Clinical Outcomes by Hospital Group
| Outcome | Outcome per Hospital Group | Adjusted1 RR (95% CI) | |
|---|---|---|---|
| Low Oral Hospitals (n=15,609 patients at 16 hospitals) | High Oral Hospitals (n=4,669 patients at 7 hospitals) | High vs. Low | |
| In-hospital outcomes | |||
| LOS, hours – mean (sd) | 55.2 (28.1) | 54.14 (27.52) | 1 (0.93, 1.08) |
| ICU transfer2 | 95 (0.6) | 28 (0.6) | 1.07 (0.57, 1.99) |
| Escalated respiratory care3 | 72 (0.5) | 63 (1.3) | 2.96 (1.12, 7.81) |
| Complicated CAP | 1084 (6.9) | 260 (5.6) | 0.81 (0.59, 1.11) |
| Cost, $ - mean (sd) | 6,435 (3,850) | 6,500 (3988) | 1.06 (0.79, 1.41) |
| Post-discharge outcomes | |||
| 7-day CAP-related ED revisits | 76 (0.5) | 29 (0.6) | 1.21 (0.84, 1.73) |
| 7-day CAP related readmission | 131 (0.8) | 68 (1.5) | 1.68 (1.31, 2.17) |
| Readmission for complicated CAP | 57 (0.4) | 24 (0.5) | 1.42 (0.87, 2.34) |
| Readmission with pleural drainage | 27 (0.2) | 14 (0.3) | Too few to model |
Values represent the number of patients (percentage of total patients) unless otherwise specified.
CI, confidence interval; OR, odds ratio; RR, relative ratio; sd, standard deviation.
Adjusted for patient age, initial use of broad-spectrum antibiotics, initial diagnostic utilization (blood culture, blood gas analysis, chest ultrasound or computed tomography), additional respiratory diagnoses including asthma, influenza, atypical CAP, and viral bronchiolitis, calendar time (hospitalization year and winter/fall season), and hospitalization in the preceding 6 months.
ICU transfer after the second hospital day
Use of heated high flow oxygen, non-invasive positive pressure ventilation, or intubation and mechanical ventilation after second hospital day
Discussion
In this multicenter retrospective cohort study of non-critically ill children hospitalized with CAP, a minority received initial oral antibiotics (18%) and there was wide variability across hospitals (1–68%). There was no difference in LOS between hospitals that used a high vs. low proportion of initial oral antibiotics. While there were statistical differences in escalated respiratory care and readmissions, these outcomes were rare, the clinical significance of these small absolute differences are uncertain, and other clinically relevant outcomes such as ICU transfers, development of complicated CAP, and readmissions for complicated CAP did not differ between groups. These findings suggest that while a small subset may benefit from initial IV antibiotics, many children can safely receive initial oral therapy.
The infrequent use of initial oral antibiotics suggests that IV is the default treatment route for most hospitalized children. This is consistent with a prior PHIS study which found 78% of children with CAP received IV therapy at some point during their admission.29 Our study adds to existing work by focusing on initial treatment. The preference for initial IV antibiotics is likely related to national guidelines from 2011, which recommend initial IV therapy for hospitalized children, and antiquated notions that IV antibiotics are always superior to oral antibiotics and that IV antibiotics are required to justify hospitalizations to insurance companies.8 However, there has been a concerted effort to reconsider the need for IV antibiotics for many serious infections in both adults and children.2,5,30–32 While IV antibiotics are sometimes needed for CAP (e.g., oral intolerance, critical illness, complicated CAP), these justify only a minority of IV use.33 Given that RCTs have demonstrated non-inferiority of oral antibiotics, admission criteria may be unrelated to the need for IV antibiotics (e.g., hospitalized for oxygen), and oral antibiotics commonly used for CAP have good bioavailability, can reduce cost and PIV-related harms, and can improve patient experience by offering a noninvasive treatment option, it is also reasonable to reconsider IV antibiotics as the default route for CAP.9–12,14,19,29,34
We found large variability across hospitals in the use of initial oral antibiotics. This suggests that some hospitals promote IV antibiotics and others promote oral antibiotics as standard initial treatment for CAP. This may be driven by local culture, stewardship programs, and clinical pathways. Hospitals that used more initial oral antibiotics also transitioned more children on initial IV therapy to oral antibiotics after just one day, suggesting that this culture likely spans the ED and inpatient settings. The variability in initial oral antibiotic use highlights the need to identify best practices regarding antibiotic route, including best candidates for oral antibiotics, and translate this evidence into practice.
Hospitals with high initial IV antibiotic use were more likely to use broad-spectrum antibiotics and obtain diagnostics, potentially suggesting an overall culture of overuse at some hospitals. Given that broad-spectrum antibiotics are associated with increased resistance and labs are not routinely recommended, this is an important consideration for IV therapy.8,35
Most patients continued the antibiotic route they were started on. Specifically, most children (91%) who received initial oral antibiotics remained on oral therapy. This suggests that for most patients, clinicians were not concerned about treatment failure with oral antibiotics. Similarly, most children (81%) with initial IV antibiotics continued IV therapy in subsequent days. This highlights the potential role of therapeutic momentum, which has been described as a facilitator of antibiotic overuse for pediatric CAP and may also facilitate overuse of IV antibiotics by preventing early IV-to-oral transitions.36
Conversely, nearly 1 in 5 children who received initial IV therapy switched to oral antibiotics on the second day and remained on orals throughout the hospitalization. This small but significant proportion of patients raises the question of the utility of one day, and potentially one dose, of IV antibiotics. If PIVs are placed predominantly due to the perceived need for IV antibiotics, these patients could potentially receive oral therapy and avoid vascular access, its associated harms, and the “cascade effect”, in which PIV placement and IV antibiotics leads to further testing and interventions (e.g., non-recommended blood tests, intravenous fluids).37,38
There was no difference in LOS between high and low oral hospitals, suggesting that both routes were able to facilitate clinical improvement and discharge from the hospital within similar time frames. Prior literature on this topic includes three RCTs outside of the U.S. that randomized children to initial oral vs. IV antibiotics.9,11,12 Two RCTs, which were in low resource countries, found no difference in LOS and the third, in the United Kingdom, found shorter LOS with oral antibiotics. Differences between our findings and the study in the United Kingdom may be related to retrospective vs. prospective study design, hospital- vs. patient-level analysis, different settings, and different pneumonia definitions. One retrospective study found that U.S. hospitals with earlier IV-to-oral transitions had a shorter LOS.6 It is reasonable to hypothesize that if earlier transitions to oral antibiotics can facilitate quicker discharges, then so should initial treatment with oral antibiotics. Further prospective work is needed to better understand whether initial oral antibiotics can reduce LOS. However, all together, prior work and this study suggest that LOS is similar or decreased with oral antibiotics.
We found no clinically relevant differences in secondary outcomes between high and low oral hospitals. This aligns with the RCTs which found no differences in treatment failure, time to symptom resolution, oxygen duration, development of empyema, readmissions, or further antibiotic courses.9,11,12 We did find higher proportion of escalated respiratory support and readmissions at high oral hospitals compared to low oral hospitals. However, both outcomes were very rare (0.5–1.5%) and these small absolute differences (all <1%) are likely not clinically significant. Furthermore, there were no differences in ICU transfers or LOS, which are clinically meaningful outcomes, and no differences in complicated CAP or readmissions for complicated CAP, a main complication of untreated CAP. Thus, our results demonstrate that most children did very well, regardless of the antibiotic route they received.
Our study has several limitations. First, while we adjusted for potential confounding, there is always the possibility of residual confounding due to characteristics that we could not identify (e.g., chest radiograph results). Second, because we captured antibiotics per calendar day (rather than doses) and patients with IV and oral antibiotics on the same day were classified as IV, there is potential for misclassification that underestimated initial oral antibiotics and early transitions. Third, because poor outcomes were rare, the statistical significance of our findings may have changed if misclassification occurred. However, the clinical significance of our findings would not have differed and supports that most did well regardless of antibiotic route. Fourth, we categorized exposure at the hospital rather than patient-level because we hypothesized that differences between patients with oral vs. IV antibiotics were greater than between patients at different hospitals. However, this may have identified hospital-level variation in outcomes related to characteristics for which we could not control (e.g., discharge processes). Fifth, the difference in escalated respiratory support was likely driven by high flow oxygen (given no difference in ICU transfers), which is limited because high flow is a non-specific marker of clinical worsening and may be related to underlying viral illnesses rather than improperly treated bacterial CAP. Sixth, given that it often takes 24–48 hours to observe clinical response to antibiotics, we defined outcomes occurring after day two to avoid capturing events that reflected severity of illness upon presentation rather than response to antibiotics.39 This may have decreased the overall proportion of outcomes but would not have differentially impacted the two groups. Additionally, while we excluded many concurrent bacterial infections that require IV antibiotics, we did not exclude children with concurrent otitis media, who may have received oral antibiotics for otitis media rather than presumed bacterial CAP. Thus, high oral hospitals may have had a higher proportion of viral respiratory infections which could have impacted outcomes. However, this limitation is likely small given that concurrent diagnoses of viral bronchiolitis at the high oral hospitals were found in a lower (not higher) proportion of patients. Finally, because we included children at freestanding children’s hospitals with non-severe CAP, results may not be generalizable to other settings or to children with severe CAP, and future studies in these specific populations are needed.
In conclusion, we found that most children hospitalized with CAP received initial IV antibiotics, although wide variability across hospitals highlights the need to gather more evidence to inform best practices. Limited clinically significant differences in outcomes between hospitals that used initial oral antibiotics in a high vs. low proportion of patients suggests that a small subset of children may benefit from initial IV antibiotics, but for most, initial oral antibiotics are likely equally successful. Given the potential benefits of oral antibiotics and heterogenous nature of pediatric CAP, there is an opportunity to depart from the one-size-fits-all approach to a more personalized approach to initial antibiotic route decisions. Further prospective and patient-level research could identify which patients can safely receive initial oral antibiotics and which would benefit from IV treatment.
Supplementary Material
Funding sources:
Dr. Cotter’s effort contributing to this article was in part funded by a career development award from Children’s Hospital Colorado. The other authors received no external funding that contributed to this work.
Footnotes
Conflicts of interest/disclosures: Drs. Cotter and Ambroggio receive grant support from Pfizer Inc for a study unrelated to this manuscript. Dr. Blaschke has intellectual property in BioFire Diagnostics through the University and receives royalties through the University of Utah related to this IP. The authors have no other conflicts of interest or financial disclosures to disclose.
References
- 1.Gill PJ, et al. Identifying Conditions With High Prevalence, Cost, and Variation in Cost in US Children’s Hospitals. JAMA Netw Open 4, e2117816 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keren R, et al. Comparative Effectiveness of Intravenous vs Oral Antibiotics for Postdischarge Treatment of Acute Osteomyelitis in Children. JAMA Pediatrics 169, 120–128 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Gerber JS, et al. Identifying targets for antimicrobial stewardship in children’s hospitals. Infect Control Hosp Epidemiol 34, 1252–1258 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Jumani K, Advani S, Reich NG, Gosey L & Milstone AM Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr 167, 429–435 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMullan BJ, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis 16, e139–152 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Christensen EW, Spaulding AB, Pomputius WF & Grapentine SP Effects of Hospital Practice Patterns for Antibiotic Administration for Pneumonia on Hospital Lengths of Stay and Costs. Journal of the Pediatric Infectious Diseases Society 8, 115–121 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Barlam TF, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 62, e51–77 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley JS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 53, e25–76 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkinson M, et al. Comparison of oral amoxicillin and intravenous benzyl penicillin for community acquired pneumonia in children (PIVOT trial): a multicentre pragmatic randomised controlled equivalence trial. Thorax 62, 1102–1106 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rojas MX & Granados C Oral antibiotics versus parenteral antibiotics for severe pneumonia in children. Cochrane Database Syst Rev 2006, Cd004979 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Addo-Yobo E, et al. Oral amoxicillin versus injectable penicillin for severe pneumonia in children aged 3 to 59 months: a randomised multicentre equivalency study. Lancet 364, 1141–1148 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Hazir T, et al. Ambulatory short-course high-dose oral amoxicillin for treatment of severe pneumonia in children: a randomised equivalency trial. The Lancet 371, 49–56 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Fonseca W, Hoppu K, Rey LC, Amaral J & Qazi S Comparing pharmacokinetics of amoxicillin given twice or three times per day to children older than 3 months with pneumonia. Antimicrob Agents Chemother 47, 997–1001 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gras-Le Guen C, et al. Therapeutic amoxicillin levels achieved with oral administration in term neonates. Eur. J. Clin. Pharmacol. 63, 657–662 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Lexicomp Online®, Lexi-Drugs® [database on Internet]. Hudson (OH): Lexicomp Inc; ©1978–2014 [cited 2022 Sept 2]. Available from: www.lexi.com/. [Google Scholar]
- 16.Wieczorek B, Burke C, Al-Harbi A & Kudchadkar SR Early mobilization in the pediatric intensive care unit: a systematic review. J Pediatr Intensive Care 2015, 129–170 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hignell P Peripheral Intravenous Initiation, Self Learning Module. FH Vascular Access Regional Shared Work Team (2012). [Google Scholar]
- 18.Friedrichsdorf SJ, et al. Pain Outcomes in a US Children’s Hospital: A Prospective Cross-Sectional Survey. Hospital pediatrics 5, 18–26 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Bamford KB, et al. Patients’ views and experience of intravenous and oral antimicrobial therapy: room for change. Injury 42 Suppl 5, S24–27 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Colvin JD, et al. Financial Loss for Inpatient Care of Medicaid-Insured Children. JAMA Pediatr 170, 1055–1062 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Williams DJ, et al. Identifying pediatric community-acquired pneumonia hospitalizations: Accuracy of administrative billing codes. JAMA Pediatr 167, 851–858 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brogan TV, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J 31, 1036–1041 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Queen MA, et al. Comparative effectiveness of empiric antibiotics for community-acquired pneumonia. Pediatrics 133, e23–29 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feudtner C, Feinstein JA, Zhong W, Hall M & Dai D Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC pediatrics 14, 199–199 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams DJ, et al. Narrow vs broad-spectrum antimicrobial therapy for children hospitalized with pneumonia. Pediatrics 132, e1141–1148 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shanahan KH, Monuteaux MC, Nagler J & Bachur RG Early Use of Bronchodilators and Outcomes in Bronchiolitis. Pediatrics 148(2021). [DOI] [PubMed] [Google Scholar]
- 27.Gross CJ, et al. Variation in Management and Outcomes of Children With Complicated Pneumonia. Hosp Pediatr 11, 207–214 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Jacobs JP, et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database Mortality Risk Model: Part 2-Clinical Application. Ann. Thorac. Surg 100, 1063–1068; discussion 1068–1070 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kronman MP, Hersh AL, Newland JG & Gerber JS Getting Over Our Inpatient Oral Antibiotic Aversion. Pediatrics 142, e20181634 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Shah SS, et al. Intravenous Versus Oral Antibiotics for Postdischarge Treatment of Complicated Pneumonia. Pediatrics 138(2016). [DOI] [PubMed] [Google Scholar]
- 31.Li HK, Agweyu A, English M & Bejon P An unsupported preference for intravenous antibiotics. PLoS Med 12, e1001825 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunha BA Oral or intravenous-to-oral antibiotic switch therapy for treating patients with community-acquired pneumonia. The American Journal of Medicine 111, 412–413 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Cotter JM, et al. Opportunities for Stewardship in the Transition From Intravenous to Enteral Antibiotics in Hospitalized Pediatric Patients. J Hosp Med 16, 70–76 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Odom B, Lowe L & Yates C Peripheral Infiltration and Extravasation Injury Methodology: A Retrospective Study. J Infus Nurs 41, 247–252 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huttner A, et al. Antimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob Resist Infect Control 2, 31 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cotter JM, et al. Factors Associated With Antibiotic Use for Children Hospitalized With Pneumonia. Pediatrics 150(2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deyo RA Cascade effects of medical technology. Annual review of public health 23, 23–44 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Tchou MJ, et al. Patterns of Electrolyte Testing at Children’s Hospitals for Common Inpatient Diagnoses. Pediatrics 144(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlossberg D Clinical approach to antibiotic failure. Med Clin North Am 90, 1265–1277 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


