Abstract
1. The activity of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) synthase (EC 4.1.3.5) in extracts of rapidly frozen rat livers was doubled in animals treated in various ways to increase ketogenic flux. 2. Some 90% of the activity measured was mitochondrial, and changes in mitochondrial activity dominated changes in total enzyme activity. 3. The elevated HMG-CoA synthase activities persisted throughout the isolation of liver mitochondria. 4. Intramitochondrial succinyl-CoA content was lower in whole liver homogenates and in mitochondria isolated from animals treated with glucagon or mannoheptulose. 5. HMG-CoA synthase activity in mitochondria from both ox and rat liver was negatively correlated with intramitochondrial succinyl-CoA levels when these were manipulated artificially. Under these conditions, the differences between mitochondria from control and hormone-treated rats were abolished. 6. These findings show that glucagon can decrease intramitochondrial succinyl-CoA concentration, and that this in turn can regulate mitochondrial HMG-CoA synthase. They support the hypothesis that the formation of ketone bodies from acetyl-CoA may be regulated by the extent of succinylation of mitochondrial HMG-CoA synthase.
Full text
PDF

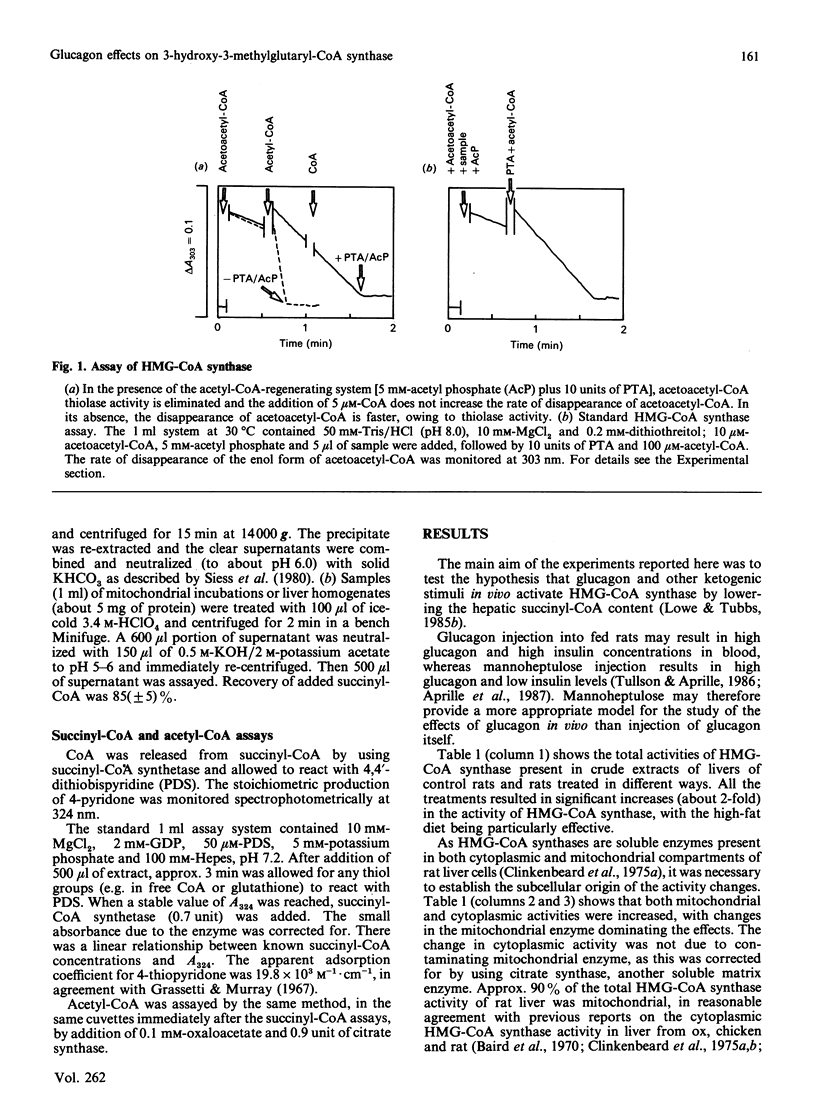
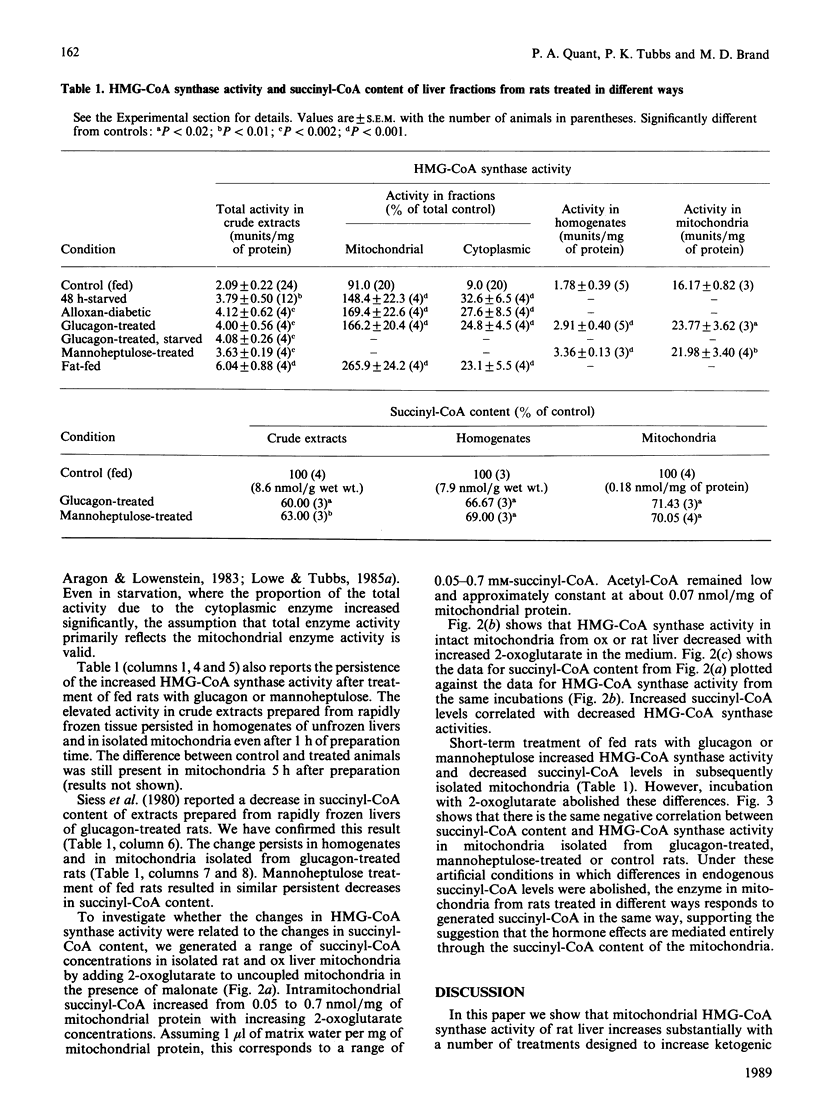
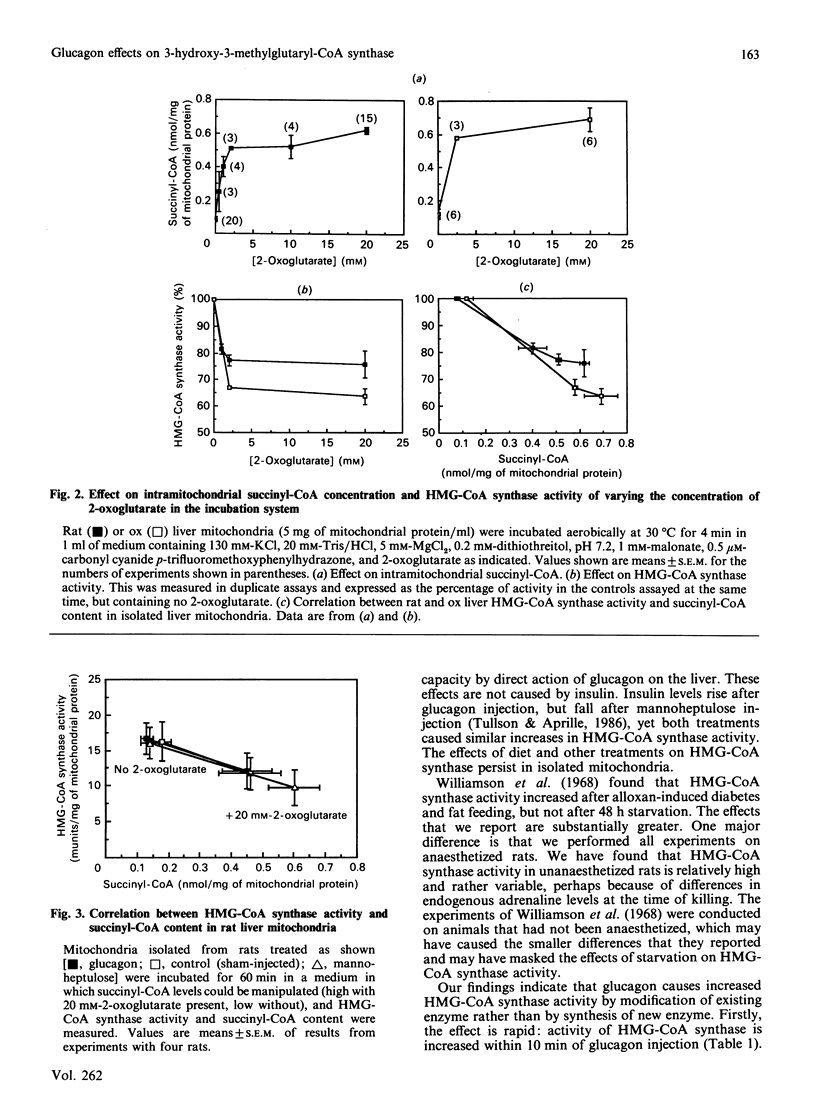

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aprille J. R., Rohweder-Dunn G., Brennan W. A., Jr, Kelley R. T., Nosek M. T. Mitochondrial function after acute alteration of the endogenous insulin-to-glucagon ratio. Biochem Biophys Res Commun. 1987 Jan 30;142(2):315–321. doi: 10.1016/0006-291x(87)90275-0. [DOI] [PubMed] [Google Scholar]
- Aragón J. J., Lowenstein J. M. A survey of enzymes which generate or use acetoacetyl thioesters in rat liver. J Biol Chem. 1983 Apr 25;258(8):4725–4733. [PubMed] [Google Scholar]
- Baird G. D., Hibbitt K. G., Lee J. Enzymes involved in acetoacetate formation in various bovine tissues. Biochem J. 1970 May;117(4):703–709. doi: 10.1042/bj1170703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon M. R., Zammit V. A. Use of a selectively permeabilized isolated rat hepatocyte preparation to study changes in the properties of overt carnitine palmitoyltransferase activity in situ. Biochem J. 1988 Feb 1;249(3):645–652. doi: 10.1042/bj2490645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinkenbeard K. D., Reed W. D., Mooney R. A., Lane M. D. Intracellular localization of the 3-hydroxy-3-methylglutaryl coenzme A cycle enzymes in liver. Separate cytoplasmic and mitochondrial 3-hydroxy-3-methylglutaryl coenzyme A generating systems for cholesterogenesis and ketogenesis. J Biol Chem. 1975 Apr 25;250(8):3108–3116. [PubMed] [Google Scholar]
- Dashti N., Ontko J. A. Rate-limiting function of 3-hydroxy-3-methylglutaryl-coenzyme A synthase in ketogenesis. Biochem Med. 1979 Dec;22(3):365–374. doi: 10.1016/0006-2944(79)90024-3. [DOI] [PubMed] [Google Scholar]
- Decaux J. F., Robin D., Robin P., Ferré P., Girard J. Intramitochondrial factors controlling hepatic fatty acid oxidation at weaning in the rat. FEBS Lett. 1988 May 9;232(1):156–158. doi: 10.1016/0014-5793(88)80407-1. [DOI] [PubMed] [Google Scholar]
- Foster D. W. Banting lecture 1984. From glycogen to ketones--and back. Diabetes. 1984 Dec;33(12):1188–1199. doi: 10.2337/diab.33.12.1188. [DOI] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Restoration of the properties of carnitine palmitoyltransferase I in liver mitochondria during re-feeding of starved rats. Biochem J. 1986 Oct 15;239(2):485–488. doi: 10.1042/bj2390485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Role of carnitine palmitoyltransferase I in the regulation of hepatic ketogenesis during the onset and reversal of chronic diabetes. Biochem J. 1988 Jan 15;249(2):409–414. doi: 10.1042/bj2490409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassetti D. R., Murray J. F., Jr Determination of sulfhydryl groups with 2,2'- or 4,4'-dithiodipyridine. Arch Biochem Biophys. 1967 Mar;119(1):41–49. doi: 10.1016/0003-9861(67)90426-2. [DOI] [PubMed] [Google Scholar]
- Holness M. J., French T. J., Schofield P. S., Sugden M. C. The relationship between fat synthesis and oxidation in the liver after re-feeding and its regulation by thyroid hormone. Biochem J. 1987 Nov 1;247(3):621–626. doi: 10.1042/bj2470621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huth W., Dierich C., von Oeynhausen V., Seubert W. On the mechanism of ketogenesis and its control. I. On a possible role of acetoacetyl-CoA thiolase in the control of ketone body production. Hoppe Seylers Z Physiol Chem. 1973 Jun;354(6):635–649. doi: 10.1515/bchm2.1973.354.1.635. [DOI] [PubMed] [Google Scholar]
- Ishitani K., Niitsu Y., Listowsky I. Characterization of the different polypeptide components and analysis of subunit assembly in ferritin. J Biol Chem. 1975 Apr 25;250(8):3124–3128. [PubMed] [Google Scholar]
- Lowe D. M., Tubbs P. K. 3-Hydroxy-3-methylglutaryl-coenzyme A synthase from ox liver. Purification, molecular and catalytic properties. Biochem J. 1985 Apr 15;227(2):591–599. doi: 10.1042/bj2270591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. M., Tubbs P. K. Succinylation and inactivation of 3-hydroxy-3-methylglutaryl-CoA synthase by succinyl-CoA and its possible relevance to the control of ketogenesis. Biochem J. 1985 Nov 15;232(1):37–42. doi: 10.1042/bj2320037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. The regulation of ketogenesis from oleic acid and the influence of antiketogenic agents. J Biol Chem. 1971 Oct 25;246(20):6247–6253. [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977 Jul;60(1):265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J., Wright P. H., Foster D. W. Hormonal control of ketogenesis. Rapid activation of hepatic ketogenic capacity in fed rats by anti-insulin serum and glucagon. J Clin Invest. 1975 Jun;55(6):1202–1209. doi: 10.1172/JCI108038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menahan L. A., Hron W. T., Hinkelman D. G., Miziorko H. M. Interrelationships between 3-hydroxy-3-methylglutaryl-CoA synthase, acetoacetyl-CoA and ketogenesis. Eur J Biochem. 1981 Oct;119(2):287–294. doi: 10.1111/j.1432-1033.1981.tb05606.x. [DOI] [PubMed] [Google Scholar]
- Ochs R. S. Glutamine metabolism of isolated rat hepatocytes. Evidence for catecholamine activation of alpha-ketoglutarate dehydrogenase. J Biol Chem. 1984 Nov 10;259(21):13004–13010. [PubMed] [Google Scholar]
- Palmer T. N., Watts D. I., Sugden M. C. Can isolated spans of the tricarboxylic acid cycle operate independently? L-proline, oleate and butyrate metabolism in rat hepatocytes. Biochem Int. 1983 Apr;6(4):433–441. [PubMed] [Google Scholar]
- Schofield P. S., French T. J., Sugden M. C. Ketone-body metabolism after surgical stress or partial hepatectomy. Evidence for decreased ketogenesis and a site of control distal to carnitine palmitoyltransferase I. Biochem J. 1987 Jan 15;241(2):475–481. doi: 10.1042/bj2410475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield P. S., Sugden M. C., Corstorphine C. G., Zammit V. A. Altered interactions between lipogenesis and fatty acid oxidation in regenerating rat liver. Biochem J. 1987 Jan 15;241(2):469–474. doi: 10.1042/bj2410469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess E. A., Fahimi F. M., Wieland O. H. Decrease by glucagon in hepatic succinyl-CoA. Biochem Biophys Res Commun. 1980 Jul 16;95(1):205–211. doi: 10.1016/0006-291x(80)90725-1. [DOI] [PubMed] [Google Scholar]
- Smith C. M., Bryla J., Damon S., LaNoue K. F., Williamson J. R. Fluorometric assays for succinyl-CoA and propionyl-CoA in mitochondrial extracts. Anal Biochem. 1973 Feb;51(2):408–420. doi: 10.1016/0003-2697(73)90494-6. [DOI] [PubMed] [Google Scholar]
- Tullson P. C., Aprille J. R. Increased adenine nucleotides in liver mitochondria after mannoheptulose injection in vivo. Arch Biochem Biophys. 1986 May 1;246(2):611–616. doi: 10.1016/0003-9861(86)90316-4. [DOI] [PubMed] [Google Scholar]
- Unger R. H. Glucagon physiology and pathophysiology in the light of new advances. Diabetologia. 1985 Aug;28(8):574–578. doi: 10.1007/BF00281991. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975 Jan 4;1(7897):14–16. doi: 10.1016/s0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
- Unger R. H. Role of glucagon in the pathogenesis of diabetes: the status of the controversy. Metabolism. 1978 Nov;27(11):1691–1709. doi: 10.1016/0026-0495(78)90291-3. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Krebs H. A. Activity and intracellular distribution of enzymes of ketone-body metabolism in rat liver. Biochem J. 1968 Jul;108(3):353–361. doi: 10.1042/bj1080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki R. K. Glucagon stimulation of mitochondrial respiration. J Biol Chem. 1975 Oct 10;250(19):7924–7930. [PubMed] [Google Scholar]
- Zammit V. A. Mechanisms of regulation of the partition of fatty acids between oxidation and esterification in the liver. Prog Lipid Res. 1984;23(1):39–67. doi: 10.1016/0163-7827(84)90005-5. [DOI] [PubMed] [Google Scholar]


