Abstract
Narcolepsy type 1 (NT1) is a unique central sleepiness disorder that affects individuals with excessive daytime sleepiness (EDS), cataplexy, sleep paralysis, and hypnagogic hallucinations. The etiology and pathogenesis of NT1 remains unclear, although some viral infections are thought to be related to NT1. This paper reports an unusual case of late-onset NT1 with human immunodeficiency virus (HIV) infection and antiretroviral therapy for five years. The relationship between HIV infection, immune, Immune reconstitution inflammatory syndrome (IRIS) and NT1 should be further investigated, as excessive daytime sleepiness is more common in HIV-infected patients than in the general population.
Keywords: narcolepsy, excessive daytime sleepiness, HIV, immune, Immune reconstitution inflammatory syndrome
Introduction
Narcolepsy type 1 (NT1) is a chronic neurological condition that causes excessive daytime sleepiness (EDS), cataplexy (sudden loss of muscle control), sleep paralysis (inability to move or speak upon waking), hypnagogic hallucinations (vivid and often frightening hallucinations while falling asleep) and disrupted nighttime sleep patterns. The prevalence in Western countries is 0.02–0.05%, and an increase in annual incidence among all age groups has been reported recently, most of which begins at around age 15 and 35 years, but rarely occurs after the age of 45.1–3 The delay period from clinical presentation to clear diagnosis is approximately 8 years. There is strong evidence indicating that NT1 is a result of the interplay between genetic and environmental factors, ultimately leading to the immune-mediated targeted degeneration or impairment of orexin-producing neurons located in the lateral hypothalamus.4 In summary, major histocompatibility complex (MHC) class I and II genes are the main genetic risk factors for NT1. The close relationship between NT1 and HLA-DQB1*06:02 also indicates that the immune system plays a key role in the pathogenesis. In addition, the HLA-DQA1*01:02 allele can bind to DQB1*06:02 to form an αβ-heterodimer, which reacts with receptors on CD4+T lymphocytes through Antigen presenting cells (APCs). Following this genetic susceptibility, exposure to viruses, bacteria, or toxins may activate or trigger an immune reaction that in turn attacks orexin-producing neurons. The current epidemiological and immunological research findings suggest that various infections (such as Streptococcus and H1N1) and vaccinations (including Pandemrix, yellow fever, and tick-borne encephalitis virus vaccines)5–8 may act as potential triggers.
Immune reconstitution inflammatory syndrome (IRIS) is an excessive inflammatory response state that typically occurs in patients infected with human immunodeficiency virus (HIV) during the first six months of treatment and was first reported in the 1990s. We know very little about it, and the exact mechanism of its occurrence is not fully understood. Currently, it is considered one of the potential complications of the use of highly effective antiretroviral therapy (HAART). In HIV infected patients, IRIS is associated with improved immune function as a result of successful antiretroviral therapy. At present, the overall incidence rate of IRIS is still unclear, up to 25% to 30% of HIV patients receiving antiretroviral treatment have IRIS.9,10
However, the link between IRIS and NT1 that occurs after HIV infection has not been clarified. Here, we describe an unusual case of late-onset NT1 with HIV infection and antiretroviral therapy. Moreover, we reviewed and discussed the immunological etiology and pathogenesis of IRIS related narcolepsy.
Case Report
The Clinical Research Ethics Committee of Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, granted approval for this case report. The study was conducted in adherence to the principles of the Declaration of Helsinki. The patient expressed his consent to engage in this study through written informed consent. And written informed consent for publication of details was obtained from the patient.
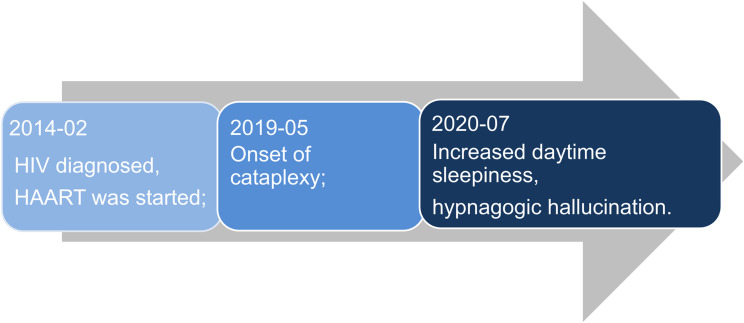
The patient was infected with HIV at the age of 47 years. He accidentally learned that he was infected with HIV due to routine examinations before surgery. When he recalled the past, he had engaged in risky sexual behavior. Based on the clinical presentation and CD4+ T cell count at the time, the patient’s CDC clinical stage was A2. After that, highly active antiretroviral therapy (HAART) was initiated with zidovudine, lamivudine, and nevirapine. About five years passed, the patient presented with episodic facial or tongue muscle weakness mainly triggered by emotional swings, which could be interpreted as cataplexy. These episodes of cataplexy occurred up to four times per day. He also had brief episodes spanning several seconds, characterized by loss of muscle tone in his legs and falling to the ground with preserved consciousness. After experiencing cataplexy for one year, he noticed an increase in daytime somnolence. For a year, he would fall asleep while performing monotonous activities, such as watching TV and car journeys. He occasionally experienced hypnogogic hallucinations, while sleep paralysis and parasomnias were absent (Figure 1). His medical history was unremarkable. No recent vaccinations, head trauma, or family history of narcolepsy were found in the medical records. Additionally, the clinical data did not reveal any evidence of circadian rhythm sleep-wake disorders, sleep deprivation, or psychiatric disorders.
Figure 1.
Schematic diagram of disease progression.
In addition to electrocardiogram (ECG), electroencephalogram (EEG), and routine blood tests, we detected HLA-DQB1*06:02 alleles in the blood and orexin in cerebrospinal fluid (CSF). After cerebral magnetic resonance imaging (MRI), we recorded the patient’s nighttime sleep using polysomnography (PSG) and conducted multiple sleep latency tests (MLST) during the day after PSG.
No significant abnormality was found when the patient received physical examination of nervous system. The Mini-Mental State Examination (MMSE), Epworth Sleepiness Scale (ESS), Pittsburgh Sleep Quality Index (PSQI), Patient Health Questionnaire-9 (PHQ-9), and Generalized Anxiety Disorder-7 (GAD-7) scores were 25, 15, 12, 9, 6 respectively. The initial investigations, including EEG and ECG, were within normal limits. Further blood tests showed no evidence of anemia, thyroid gland dysfunction, Cushing syndrome, or neoplasm. Serum CD4+ and CD8+ cell counts were 629 and 846 per mm3. And the HIV viral load cannot be detected in peripheral blood. The MRI scans showed no signs of HIV encephalopathy. The WBC count and protein level from CSF were within normal range. Besides, the allele of HLA-DQB1*06:02 is positive, and the orexin A in the CSF was as low as 64.28 pg/mL.
Results from the overnight PSG showed the following: total sleep time (TST) of 561.5 minutes, sleep onset latency of 1.5 minutes, REM sleep latency of 72.5 minutes, the periodic limb movement (PLM) during sleep index of 27.89/hour (including 1.1/hour associated with micro-arousals). The mean sleep latency during the MSLT was 1.9 minutes, and four occurrences of sleep onset rapid eye movement Periods (SOREMPs, REM Sleep occurred within 15 minutes of sleep onset) observed across the five naps. Consequently, NT1 was diagnosed based on the criteria outlined in the International Classification of Sleep Disorders (ICSD −3).
After diagnosis of narcolepsy, the patient was prescribed with modafinil (200 mg every morning), the first-line medication for narcolepsy, without terminating HAART. We chose it as the first drug for patient because modafinil was the only choice at that time. However, modafinil was very expensive and not cover by the medical insurance. In addition, modafinil was not effective for cataplexy, although it improved EDS greatly for the patient. Therefore, the patient chose pitolisant to control narcoleptic symptoms when it became available. It was not only because pitolisant was covered by medical insurance, but also it was able to improve sleepiness and cataplexy at the same time. During the following-up, it was true that the EDS and cataplexy of the patient improved by pitolisant (18 mg every morning).
Discussion
Sleep disorders are highly prevalent among patients with HIV infection, with nearly half of patients experiencing sleep disorders such as insomnia, sleep fragmentation, and EDS.1 While most studies investigating sleep disorders and their consequences in patients with HIV infections have mainly relied on self-reported questionnaires, data regarding objective measures are sparse. For example, Faraut et al found that 40% of patients reported EDS, which was assessed solely using ESS.5 To identify the cause of NT1 in a patient with HIV infection and antiretroviral therapy, we performed a full set of sleep-related tests.
To our knowledge, there is only a single case report of an adolescent who was diagnosed with HIV infection and was undergoing antiretroviral treatment effectively. Subsequently, this individual developed NT1 and initially exhibited symptoms of EDS. As most cases of NT1 onset occur in adolescents, we are the first to report a case of late-onset NT1 following HIV infection worldwide. In our case, the patient was diagnosed two years after the first episode of cataplexy. However, NT1 had a significant impact on mental health, quality of life, and public safety. This finding deserves the attention of clinicians.
The etiology of HIV-related sleep disorders remains unclear. Exposure to specific pathogens can potentially activate or initiate an immune response that depletes of orexin neurons. For instance, CD4+ T cells that respond to streptococcus or influenza may mistakenly target orexin peptides displayed by MHC class II molecules.7 Unlike other infections, HIV infection results in progressive loss of immune function, marked by the depletion of CD4+ T lymphocytes. However, a growing body of evidence suggests that T cell-mediated immune mechanism as the cause of orexin neuron destruction in NT1, highlighting the crucial role of CD4+ T cells.8,9 Thus, it seems unreasonable that the HIV infection itself causes NT1 onset. In addition, no symptoms or signs of neurological infection were observed in our case, and MRI showed no corresponding abnormal changes, which both do not support the claim of direct HIV damage.
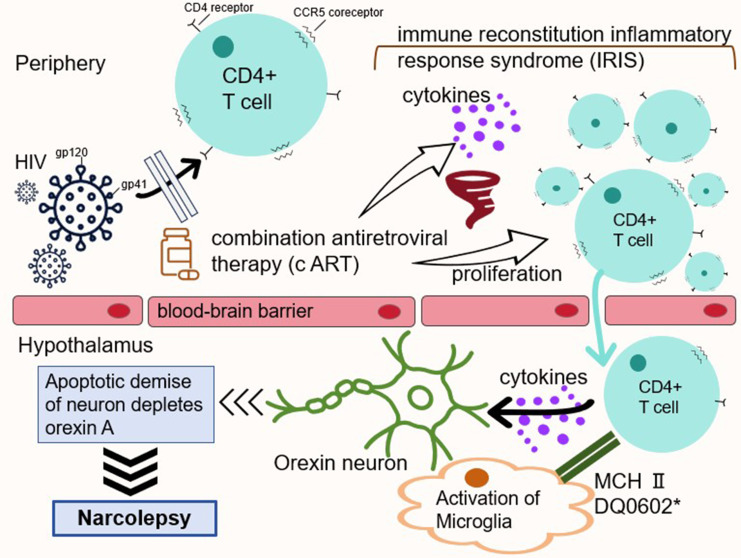
Our case supports the second view that NT1 is associated with the use of antiretroviral drugs (Figure 2). After the initiation of HAART, approximately 10–37% of patients develop opportunistic infections or autoimmune diseases, despite recovery of CD4+ lymphocyte counts and a decline in HIV viral loads.10,11 Known as the IRIS, this paradoxical phenomenon is caused by rapid and uncontrolled reconstitution of the immune system linked to HAART. At present, the incidence rate of IRIS in HIV positive patients has increased sharply. This is caused by an increase in CD4+ T cell count, ultimately leading to an imbalance in the host’s immune response. In the context of ineffective immune modulation, active proliferation, abnormal activation of CD4+ T cells, and massive release of cytokines may result in an abnormally intense immune response. The reason for the selective destruction of orexin-producing neurons remains unclear. Currently, NT1 is considered an autoimmune disease mediated by T cells,1 in which CD4+ T cells play an important role. CD4+ T cells secrete cytokines to trigger T cell receptors (TCR) to regulate immune responses, while CD4+ T cells can recognize antigens bound to MHC class II molecules present on the surface of antigen-presenting cells. It should be noted that CD4+ T cells may not directly damage orexin-producing neurons, but they can release cytokines, thereby stimulating macrophages and natural killer cells to attack orexin neurons.
Figure 2.
A simple schematic diagram of the hypothetical mechanism. *DQ0602, the heterodimer encoded by HLA-DQA1*01:02–DQB1*06:02.
Our patient developed NT1 after 2 years of receiving HAART with a normal CD4+ cell counts of 629.00 cells/mm3, which is consistent with the characteristics of IRIS. Therefore, we guess that it is precisely because the patient received HAART treatment after being infected with HIV that CD4+ T cells quickly recovered from an exhausted state to a higher level and developed IRIS. The probability of NT1 mediated by CD4+ T cells significantly increase in the internal environment of immune storm like conditions.
However, the relationship between HIV infection and IRIS after HIV treatment and the incidence of NT1 has not been elucidated yet. We hope that this case can attract more attention from experts and scholars, and conduct large-scale research in the future to evaluate its pathogenic mechanism.
Acknowledgments
We would like to express our gratitude to Dr. Lingzhi Li for her valuable suggestions and guidance on this project, and for her help with editing the manuscript.
Abbreviations
NT1, narcolepsy type 1; HIV, human immunodeficiency virus; IRIS, immune reconstitution inflammatory syndrome; EDS, excessive daytime sleepiness; HAART, highly active antiretroviral therapy; HLA, human leukocyte antigen; ECG, electrocardiogram; EEG, electroencephalogram; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; PSG, polysomnography; TST, total sleep time; PLM, periodic limb movement; MLST, multiple sleep latency test; REM, rapid eye movement; SOREMPs, sleep-onset rapid eye movement periods; ICSD-3, International Classification of Sleep Disorders, Third Edition; ESS, Epworth Sleepiness Scale; MHC, major histocompatibility complex.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Mahoney CE, Cogswell A, Koralnik IJ, Scammell TE. The neurobiological basis of narcolepsy. Nat Rev Neurosci. 2019;20(2):83–93. doi: 10.1038/s41583-018-0097-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassetti CLA, Adamantidis A, Burdakov D, et al. Narcolepsy - clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol. 2019;15(9):519–539. doi: 10.1038/s41582-019-0226-9 [DOI] [PubMed] [Google Scholar]
- 3.Voss JG, Barroso J, Wang T. A Critical Review of Symptom Management Nursing Science on HIV-Related Fatigue and Sleep Disturbance. Int J Environ Res Public Health. 2021;18(20):10685. doi: 10.3390/ijerph182010685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allavena C, Guimard T, Billaud E, et al. Prevalence and Risk Factors of Sleep Disturbance in a Large HIV-Infected Adult Population. AIDS Behav. 2016;20(2):339–344. doi: 10.1007/s10461-015-1160-5 [DOI] [PubMed] [Google Scholar]
- 5.Faraut B, Tonetti L, Malmartel A, et al. Sleep, Prospective Memory, and Immune Status among People Living with HIV. Int J Environ Res Public Health. 2021;18(2):438. doi: 10.3390/ijerph18020438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scherrer KS, Relly C, Hackenberg A, Berger C, Paioni P. Case report: narcolepsy type 1 in an adolescent with HIV infection-coincidence or potential trigger? Medicine. 2018;97(30):e11490. doi: 10.1097/MD.0000000000011490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brigham EP, Patil SP, Jacobson LP, et al. Association between systemic inflammation and obstructive sleep apnea in men with or at risk for HIV infection. Antivir Ther. 2014;19(8):725–733. doi: 10.1038/s41582-019-0226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latorre D, Kallweit U, Armentani E, et al. T cells in patients with narcolepsy target self-antigens of hypocretin neurons. Nature. 2018;562(7725):63–68. doi: 10.1038/s41586-018-0540-1 [DOI] [PubMed] [Google Scholar]
- 9.Vishnu P, Dorer RP, Aboulafia DM. Immune reconstitution inflammatory syndrome-associated Burkitt lymphoma after combination antiretroviral therapy in HIV-infected patients. Clin Lymphoma Myeloma Leuk. 2015;15(1):e23–e29. doi: 10.1016/j.clml.2014.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson T, Nath A. Immune reconstitution inflammatory syndrome and the central nervous system. Curr Opin Neurol. 2011;24(3):284–290. doi: 10.1097/WCO.0b013e328346be57 [DOI] [PubMed] [Google Scholar]
- 11.Novak RM, Richardson JT, Buchacz K, et al. Immune reconstitution inflammatory syndrome: incidence and implications for mortality. AIDS. 2012;26(6):721–730. doi: 10.1097/QAD.0b013e3283511e91 [DOI] [PMC free article] [PubMed] [Google Scholar]